Abstract
Recent studies reported that smoking cessation leads to higher short-term risk of type 2 diabetes than continuing to smoke. However, the duration of increased diabetes risk following smoking cessation needs further investigation. We followed 135,906 postmenopausal women aged 50–79 years enrolled in the Women's Health Initiative between September 1, 1993, and December 31, 1998, over an average of 11 years to examine the association between smoking cessation and risk of diabetes using Cox proportional hazard multivariable-adjusted regression models. Compared with that for never smokers, the risk for diabetes was significantly elevated in current smokers (hazard ratio = 1.28, 95% confidence interval: 1.20, 1.36) but was even higher in women who quit smoking during the first 3 years of follow-up (hazard ratio = 1.43, 95% confidence interval: 1.26, 1.63). Among former smokers, the risk of diabetes decreased significantly as the time since quitting increased and was equal to that of never smokers following a cessation period of 10 years. In new quitters with low cumulative exposure (<20 pack-years), diabetes risk was not elevated following smoking cessation. In conclusion, the risk of diabetes in former smokers returns to that in never smokers 10 years after quitting, and even more quickly in lighter smokers.
Keywords: risk factors, smoking, smoking cessation, type 2 diabetes
A body of literature shows that cigarette smoking is associated with an increased risk of type 2 diabetes (1). Therefore, smoking cessation should reduce the risk of diabetes among current smokers. However, smoking cessation can also be accompanied by substantial weight gain (2, 3), which may subsequently increase the risk of diabetes (4).
Three epidemiologic studies have prospectively investigated smoking cessation in relation to diabetes risk (5–7). All of them observed that, within the first 5 years, individuals who stopped smoking had a higher short-term risk of type 2 diabetes than those who continued to smoke (5–7). However, 2 of these studies were conducted only among middle-aged men (5, 6). Another recent study (7) that included both men and women did not perform separate analyses by gender. Thus, it is uncertain whether a similar pattern between smoking cessation and risk of diabetes is present among women. Women tend to gain more weight than men after quitting smoking (8). Hence, the increased short-term diabetes risk associated with smoking cessation might be more pronounced in women.
Two previous studies have looked at the impact of weight gain after quitting smoking on the relationship between smoking cessation and diabetes risk, with mixed findings (6, 7). Yeh et al. (7) found that adjustment for weight gain partially explained the excess diabetes risk in new quitters. Wannamethee et al. (6) reported that a significant increased risk of diabetes associated with smoking cessation was seen both in men who gained weight and in men who did not. Therefore, it is unclear whether the increased risk of diabetes associated with smoking cessation is due to the residual cumulative effects of smoking, substantial weight gain, or a combination of both.
In this study, we assessed the relationship between smoking cessation and risk of developing type 2 diabetes in the Women's Health Initiative (WHI)—a large prospective study, with detailed information on smoking status, cumulative exposure, weight changes, and potential confounders. Our brief report on this subject (9) found that there was an increased risk of diabetes in new quitters and that weight gain was 1 factor contributing to the elevated risk. In this paper, we conducted additional analyses examining whether the risk of diabetes differs by the cumulative amount of smoking and whether the duration of increased risk following smoking cessation differs by cumulative exposure.
MATERIALS AND METHODS
Women's Health Initiative
Designed to address the major causes of morbidity and mortality in postmenopausal women (10), the WHI included both clinical trials and an observational study. Details of the scientific rationale, eligibility requirements, and baseline characteristics of the participants in the WHI have been published elsewhere (11–15). Briefly, a total of 161,808 women aged 50–79 years were recruited at 40 clinical centers throughout the United States between September 1, 1993, and December 31, 1998. The WHI clinical trial includes 4 overlapping components: 2 hormone therapy trials (27,347 women), a dietary modification trial (48,835 women), and a calcium/vitamin D supplementation trial (36,282 women). Participants in the observational study were 93,676 women who were screened for the clinical trial but proved to be ineligible or unwilling to participate or were recruited through a direct invitation for the observational study. The study was overseen by ethics committees at all 40 clinical centers and at the coordinating center, as well as by a data and safety monitoring board. All participants in the WHI gave informed consent and were followed prospectively.
Study population
We included participants in both the WHI clinical trial and WHI observational study. The associations between smoking cessation and the risk of incident diabetes were assessed at 2 different time points. First, at baseline we compared never smokers, former smokers and current smokers. Second, starting at the year 3 follow-up, we also looked at the group of new quitters (women who smoked at baseline but had quit smoking at the year 3 follow-up). The following participants were excluded from the original cohort of 161,808: 14,849 women who had a history of cancer (except nonmelanoma skin cancer) at baseline; 783 women who had no follow-up time; 8,441 women who had diabetes at baseline; and 1,829 women who had missing values for smoking and diabetes at baseline. This left 135,906 women for our first set of analyses on the risk of diabetes among former and current smokers at baseline. In the second set of analyses assessing the association between smoking cessation and the risk of diabetes in subsequent years, we further excluded 752 never or former smokers who reported newly initiating smoking at the year 3 follow-up; 15,836 women who had no follow-up information at year 3; 1,414 women who had no follow-up observation time close to the year 3 scheduled visit (defined as follow-up time shorter than 2 years or longer than 4 years since baseline); 690 women who had missing values for smoking at the year 3 follow-up; and 2,122 women who developed diabetes between baseline and the year 3 follow-up. Thus, 115,092 women remained for this analysis and were followed for an average of about 8.5 years after year 3.
Measurement of exposures, confounders, and outcomes
Smoking information at baseline
We considered all available information on smoking at enrollment, including smoking status (never, former, and current), the age of smoking initiation, the number of cigarettes smoked per day, the duration of smoking in years, and age at the time of quitting among former smokers. Duration of smoking for former smokers at baseline was calculated in years by using baseline age minus age at the time of quitting.
New quitters between baseline and the year 3 follow-up
Study participants changing smoking habits between baseline and the year 3 follow-up were classified as never smokers, former smokers, continuing smokers, and new quitters. The never smokers were those women who were never smokers at both baseline and the year 3 follow-up. The former smokers were defined as women who classified themselves as former smokers both at baseline and the year 3 follow-up. The continuing smokers were defined as women who smoked at baseline and the year 3 follow-up. The new quitters were defined as women who smoked at baseline but were abstinent at the year 3 follow-up. A small proportion of women (0.6%) who changed smoking status from never or former smokers at baseline to current smokers in year 3 were excluded.
Confounders
The potential confounders considered in multivariable analyses included age at enrollment (<55, 55–59, 60–64, 65–69, 70–74, ≥75 years), ethnicity (American Indian or Alaska Native, Asian or Pacific Islander, black or African American, Hispanic/Latino, non-Hispanic white, and other), education (high school or less, some college/technical training, college or some postcollege, and master's degree or higher), body mass index (<18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35.0–39.9, ≥40), waist circumference (continuous), physical activity as metabolic equivalent tasks per week: <5, 5–<10, 10–<20, 20–<30, ≥30), alcohol intake (nondrinker, past drinker, <1 drink/month, from 1 drink/month to <1 drink/week, 1–<7 drinks/week, ≥7 drinks/week), hypertension (yes, no), and high cholesterol requiring pills (yes, no). All these potential confounders measured at baseline were used. In addition, we also calculated weight gain from baseline to year 3 of follow-up and used 4 categories selected a priori on the basis of conventional cutpoints (weight gain stayed within 2.5 kg, weight gain was 2.5–<5 kg, weight gain was ≥5 kg, weight loss was 2.5–<5 kg, and weight loss was ≥5 kg).
Ascertainment of incident diabetes and follow-up
The definition of incident diabetes was a positive answer to the questions regarding “newly prescribed treatment for diabetics with pills or insulin shots” or a positive answer to the question using “diet and/or exercise for diabetes” on any of the semiannual or annual follow-up questionnaires. The date of diabetes onset was assigned as the midpoint between the dates between the survey when diabetes was self-reported and the previous survey. As indicated above, when we assessed the association between smoking status at baseline and the risk of diabetes, all women with prevalent diabetes at baseline were excluded. When we assessed the association between the changes of smoking status between baseline and year 3 follow-up and the risk of diabetes, all incident diabetes cases before the year 3 follow-up were excluded. Self-reported diabetes in the WHI has been found to be a reliable indicator of diagnosed diabetes based on medication inventories, fasting glucose levels, and medical record review (16, 17).
In the analyses of baseline smoking status and risk of diabetes, all participants were followed from baseline to diabetes diagnosis, date of death, loss to follow-up, or September 30, 2010, whichever occurred first. As of September 30, 2010, with an average 11 years of follow-up from baseline, 15,076 women developed type 2 diabetes. This first set of analyses included information available only at baseline. In the second set of analyses of changes in smoking status from baseline to year 3 follow-up, participants were followed from year 3 follow-up to diabetes diagnosis, date of death, loss to follow-up, or September 30, 2010, whichever occurred first. From baseline to the year 3 visit, 5,335 women continued to smoke, and 2,054 women newly quit smoking. Over an average 8.5 years of follow-up since year 3, 535 women developed diabetes among women who continued to smoke and 254 women developed diabetes among women who newly quit smoking.
Statistical analysis
We described the distribution of demographic characteristics and factors related to diabetes and tobacco behavior by changes of smoking status between baseline and the year 3 follow-up in Table 1. A χ2 test was used to evaluate differences for categorical covariates, and analysis of variance was used for continuous variables.
Table 1.
Baseline Characteristics of Participants and Weight Changes by Changes of Smoking Status Between Baseline and Year 3 Follow-up, the Women's Health Initiative, United States, 1993–1998
| Variable | Never Smokers (n = 59,904) |
Former Smokers (n = 47,799) |
Continuing Smokers (n = 5,335) |
New Quitters (n = 2,054) |
P Valuea | ||||
|---|---|---|---|---|---|---|---|---|---|
| % | Mean | % | Mean | % | Mean | % | Mean | ||
| Age at baseline, years | 63.4 | 63.0 | 61.0 | 61.2 | 0.3 | ||||
| White, non-Hispanic ethnicity | 83.2 | 87.9 | 80.2 | 81.8 | 0.3 | ||||
| College graduate or above education | 41.1 | 42.7 | 30.7 | 35.3 | 0.0002 | ||||
| Body mass indexb | 27.5 | 27.7 | 26.5 | 27.0 | 0.002 | ||||
| Waist circumference, cm | 84.6 | 85.9 | 84.8 | 85.2 | 0.2 | ||||
| Physical activity, METs/week | 12.4 | 13.9 | 9.0 | 10.5 | <0.0001 | ||||
| Alcohol intake, ≥7 drinks/week | 66.6 | 79.5 | 78.3 | 79.4 | 0.5 | ||||
| Hypertension, yes | 30.9 | 30.8 | 26.4 | 27.1 | 0.8 | ||||
| High cholesterol requiring pills, yes | 12.1 | 12.7 | 12.5 | 11.7 | 0.005 | ||||
| Smoking metrics | |||||||||
| Age at smoking initiation, years | 20.4 | 20.9 | 21.8 | <0.0001 | |||||
| Average no. of cigarettes/day | 15.3 | 16.2 | 12.2 | <0.0001 | |||||
| No. of smoking years | 19.9 | 37.8 | 33.5 | <0.0001 | |||||
| No. of smoking pack-years | 18.5 | 31.8 | 23.0 | <0.0001 | |||||
| Type of weight change between baseline and year 3 visit | <0.0001 | ||||||||
| Weight stable (within 2.5 kg) | 49.7 | 47.4 | 42.4 | 30.6 | |||||
| Weight gain | |||||||||
| 2.5–<5 kg | 15.0 | 14.9 | 14.6 | 18.2 | |||||
| ≥5 kg | 10.2 | 11.6 | 12.3 | 30.5 | |||||
| Weight loss | |||||||||
| 2.5–<5 kg | 10.0 | 9.9 | 11.0 | 5.1 | |||||
| ≥5 kg | 9.1 | 9.2 | 11.5 | 5.9 | |||||
Abbreviation: MET, metabolic equivalent task.
a There is a significant difference across the 4 groups for all listed variables; all P values were less than 0.01. The P value is testing the difference between continuing smokers and new quitters.
b Body mass index: weight (kg)/height (m)2.
Cox proportional hazards regression models were used to estimate hazard ratios and 95% confidence intervals of 1) risk of diabetes among former smokers and current smokers, relative to never smokers at baseline; 2) risk of diabetes among former smokers and current smokers, relative to never smokers at baseline, stratified by different years since quitting in former smokers (<5, 5–<10, 10–<20, 20–<30, and ≥30 years) and cumulative amount of smoking in former and current smokers (<10, 10–<20, 20–<30, 30–<40, 40–<50, and ≥50 years); 3) risk of diabetes among former smokers, continuing smokers, and new quitters, relative to never smokers at year 3; and 4) risk of diabetes among continuing smokers and new quitters at year 3 stratified by different cumulative amount of smoking. For the combined effect of smoking category and cumulative amount of smoking, we created a new variable coded as never smokers, former smokers at baseline, continuing smokers, <20 pack-years; new quitters, <20 pack-years; continuing smokers, 20–<40 pack-years; new quitters, 20–<40 pack-years; continuing smokers, ≥40 pack-years; and new quitters, ≥40 pack-years. In the multivariable models, we adjusted for potential baseline confounders including age, ethnicity, education, body mass index, waist circumference, alcohol consumption, physical activity, hypertension, and high cholesterol requiring pills. Four dietary variables, including total energy intake, dietary total fat, daily fruit consumption, and daily vegetable consumption, were further added into models but did not change results substantially; thus, they were not included in our final model. Because we combined cases from both the WHI observational study and the clinical trial, different study cohorts (participation in the observational study or clinical trials and different treatment assignments for all 4 clinical trials) were treated as strata in the model in order to take account of possible different baseline hazards in different subgroups.
Tests for trend were performed by treating ordinal variables as continuous variables in the proportional hazard models without the reference group included. The proportionality assumption was satisfied for all exposure variables of interest, and potential confounding variables were based on graphs of scaled Schoenfeld residuals (18).
Finally, we performed a cubic spine function to investigate the nonlinear relationship between years since quitting through the year 3 follow-up (including both new quitters and former smokers) and the risk of diabetes (19) after adjustment for the same potential confounders as mentioned above. All tests were 2 sided, and a significance level of 0.05 was used. All statistical analyses were conducted by using SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Baseline characteristics
Baseline characteristics by changes of smoking status between baseline and the year 3 follow-up are shown in Table 1. Compared with continuing smokers, new quitters were significantly more likely to have a college level or above education, have higher body mass index, be more physically active, have a lower prevalence of high cholesterol requiring pills, have initiated smoking at a later age, smoke fewer cigarettes per day, have shorter duration of smoking, and have fewer smoking pack-years. However, new quitters were more likely to gain substantial weight (all P < 0.05). There were no significant differences in the other baseline characteristics between continuing smokers and new quitters, including age, race, waist circumference, alcohol intake, hypertension history, and total energy intake (Table 1).
Smoking status at baseline and risk of diabetes
Compared with the risk among never smokers, the overall risk of diabetes in former smokers was not elevated (hazard ratio (HR) = 1.00, 95% confidence interval (CI): 0.97, 1.04), after adjustment for potential confounders (Table 2). However, within the group of former smokers at baseline, the risk of diabetes decreased significantly as the time since quitting increased, and it was equal to that of never smokers following a cessation period of 10 years. In addition, the risk of diabetes was significantly greater with higher pack-years of cigarette smoking. Although the overall risk of diabetes was significantly elevated in current smokers (HR = 1.28, 95% CI: 1.20, 1.36), we did not observe that the risk of diabetes was significantly associated with pack-years of cigarette smoking.
Table 2.
Hazard Ratios and 95% Confidence Intervals for Incident Diabetes Since Baseline in Relation to Smoking Status and Cumulative Exposure at Baseline, Women's Health Initiative, United States, 1993–2010a
| Exposure | No. of Cases | Multivariable Adjusted |
|
|---|---|---|---|
| HR | 95% CI | ||
| Smoking status | |||
| Never smokers | 7,727 | 1 | |
| Former smokers | 6,188 | 1.00 | 0.97, 1.04 |
| Years since quit smoking | |||
| <5 | 620 | 1.15 | 1.06, 1.25 |
| 5–<10 | 850 | 1.14 | 1.06, 1.22 |
| 10–<20 | 1,608 | 1.01 | 0.96, 1.07 |
| 20–<30 | 1,384 | 0.94 | 0.89, 1.00 |
| ≥30 | 1,344 | 0.94 | 0.88, 0.99 |
| Ptrendb | <0.0001 | ||
| No. of smoking pack-years among former smokers | |||
| <10 | 2,538 | 0.97 | 0.93, 1.02 |
| 10–<20 | 1,068 | 0.97 | 0.91, 1.03 |
| 20–<30 | 655 | 0.97 | 0.89, 1.05 |
| 30–<40 | 683 | 1.09 | 1.00, 1.18 |
| 40–<50 | 172 | 1.25 | 1.08, 1.46 |
| ≥50 | 725 | 1.10 | 1.02, 1.19 |
| Ptrendb | <0.0001 | ||
| Current smokers | 1,161 | 1.28 | 1.20, 1.36 |
| No. of smoking pack-years among current smokers | |||
| <10 | 268 | 1.20 | 1.06, 1.36 |
| 10–<20 | 230 | 1.35 | 1.18, 1.55 |
| 20–<30 | 183 | 1.20 | 1.03, 1.39 |
| 30–<40 | 119 | 1.12 | 0.93, 1.35 |
| 40–<50 | 134 | 1.28 | 1.07, 1.52 |
| ≥50 | 215 | 1.48 | 1.29, 1.70 |
| Ptrendb | 0.17 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a In the multivariable models, we adjusted for potential confounders including age, ethnicity, education, body mass index, waist circumference, alcohol consumption, physical activity, hypertension, and high cholesterol requiring pills.
b The trend test did not include the reference group.
After stratifying by pack-years of smoking among former smokers, we found that the risk of diabetes among women with low (<20 pack-years) previous cumulative smoking exposure who were abstinent less than 10 years was similar to that in never smokers, while the increased risk of diabetes did not return to that in never smokers until after 10 years of smoking cessation in former smokers with medium and high previous cumulative exposures (Table 3).
Table 3.
Effect of Duration Since Quitting (Years) on Diabetes Risk Stratified by Number of Smoking Pack-Years in Former Smokers at Baseline, Women's Health Initiative, United States, 1993–2010
| <20 Smoking Pack-Years |
20–<40 Smoking Pack-Years |
≥40 Smoking Pack-Years |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | HR | 95% CI | No. of Cases | HR | 95% CI | No. of Cases | HR | 95% CI | |
| Never smokers | 7,727 | 1 | 7,727 | 1 | 7,727 | 1 | |||
| Years since quit smoking | |||||||||
| <5 | 160 | 0.92 | 0.79, 1.08 | 201 | 1.19 | 1.04, 1.38 | 233 | 1.30 | 1.14, 1.48 |
| 5–<10 | 285 | 1.08 | 0.96, 1.22 | 272 | 1.15 | 1.02, 1.30 | 265 | 1.19 | 1.05, 1.35 |
| 10–<20 | 790 | 1.04 | 0.97, 1.12 | 457 | 0.96 | 0.87, 1.05 | 280 | 1.00 | 0.88, 1.13 |
| 20–<30 | 989 | 0.94 | 0.88, 1.003 | 254 | 0.94 | 0.83, 1.06 | 63 | 0.86 | 0.67, 1.10 |
| ≥30 | 1,150 | 0.94 | 0.88, 1.00 | 81 | 0.92 | 0.73, 1.14 | 23 | 1.51 | 0.99, 2.30 |
| Ptrenda | 0.08 | 0.005 | 0.02 | ||||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a The Ptrend was tested among former smokers.
New quitters and risk of diabetes
Compared with the hazard ratio for women who never smoked, the hazard ratios of diabetes were 1.20 (95% CI: 1.09, 1.31) among continuing smokers and 1.43 (95% CI: 1.26, 1.63) among new quitters, after adjustment for age, ethnicity, education, body mass index, waist circumference, alcohol consumption, physical activity, hypertension, and high cholesterol requiring pills (Table 4). After further adjustment for weight gain, the hazard ratio for diabetes remained similar among continuing smokers (HR = 1.20, 95% CI: 1.10, 1.32) but attenuated slightly among new quitters (HR = 1.36, 95% CI: 1.19, 1.54) (Table 4). However, the hazard ratio reduction among new quitters was not statistically significant comparing before and after adjustment for weight gain. Analysis stratified by the number of smoking pack-years at baseline revealed that there was a significant graded association between smoking pack-years and incidence of diabetes in new quitters before adjustment for weight gain in the model (P = 0.03), but the dose-response trend became statistically nonsignificant after adjustment for weight gain (P = 0.13). In addition, we performed a sensitivity analysis by removing the first 2 years of follow-up to check the possibility of reverse causality. After further adjustment for weight gain, the hazard ratios for diabetes were 1.17 (95% CI: 1.06, 1.29) among continuing smokers and 1.28 (95 CI: 1.11, 1.47) among new quitters, which results were similar to those of the main analysis.
Table 4.
Hazard Ratios and 95% Confidence Intervals for Incident Diabetes Since Year 3 Follow-up in Relation to Changes in Smoking Status Between Baseline and Year 3 Follow-up, Women's Health Initiative, United States, 1993–2010
| Exposure | No. of Cases | Model 1a |
Model 2a |
Model 3a |
Model 4a |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Never smokers | 5,735 | 1 | 1 | 1 | 1 | ||||
| Former smokers at baseline | 4,532 | 1.00 | 0.96, 1.04 | 0.99 | 0.95, 1.03 | 1.00 | 0.96, 1.04 | 0.99 | 0.95, 1.03 |
| Continuing smokers | 535 | 1.20 | 1.09, 1.31 | 1.20 | 1.10, 1.32 | ||||
| No. of smoking pack-years at baseline | |||||||||
| <20 | 202 | 1.20 | 1.04, 1.38 | 1.20 | 1.04, 1.39 | ||||
| 20–<40 | 158 | 1.14 | 0.97, 1.34 | 1.14 | 0.98, 1.34 | ||||
| ≥40 | 169 | 1.23 | 1.06, 1.44 | 1.24 | 1.06, 1.45 | ||||
| Ptrendb | 0.76 | 0.87 | |||||||
| New quitters | 254 | 1.43 | 1.26, 1.63 | 1.36 | 1.19, 1.54 | ||||
| No. of smoking pack-years at baseline | |||||||||
| <20 | 131 | 1.29 | 1.09, 1.54 | 1.25 | 1.05, 1.49 | ||||
| 20–<40 | 64 | 1.57 | 1.22, 2.01 | 1.46 | 1.14, 1.87 | ||||
| ≥40 | 58 | 1.75 | 1.35, 2.27 | 1.59 | 1.23, 2.07 | ||||
| Ptrendb | 0.03 | 0.13 | |||||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a In model 1 and model 2, the main exposure was coded as never smokers, former smokers at baseline, continuing smokers, and new quitters. In model 3 and model 4, the main exposure was coded as never smokers, former smokers at baseline, continuing smokers, <20 pack-years; new quitters, <20 pack-years; continuing smokers, 20–<40 pack-years; new quitters, 20–<40 pack years; continuing smokers, ≥40 pack-years; new quitters, ≥40 pack-years. For model 1 and model 3, we adjusted for potential confounders, including age, ethnicity, education, body mass index, waist, alcohol consumption, physical activity, hypertension, and high cholesterol requiring pills. For model 2 and model 4, we further adjusted for weight gain between baseline and the year 3 visit.
b The trend test did not include the reference group.
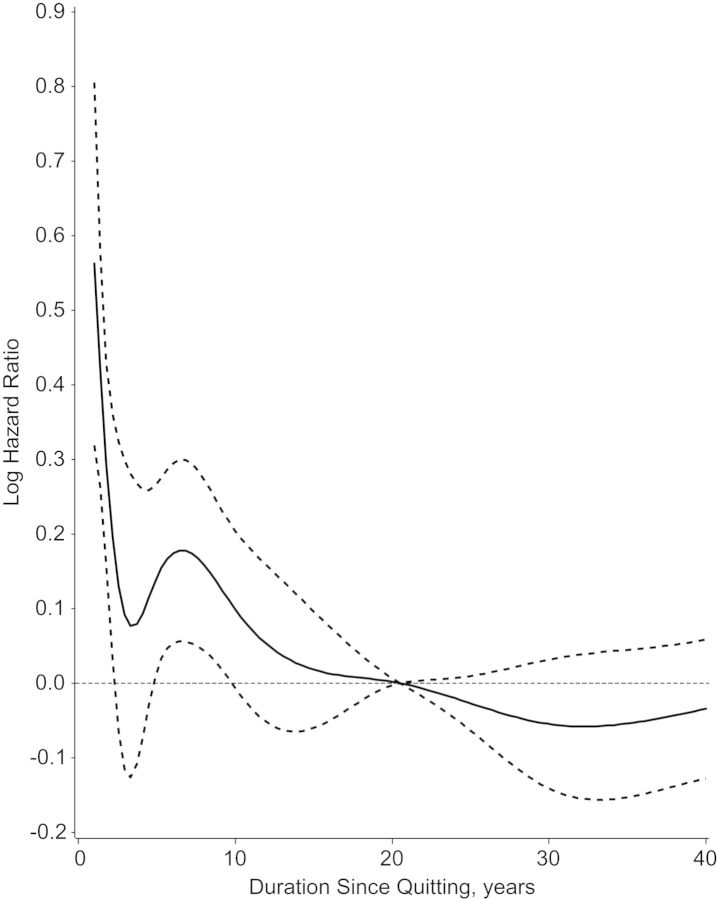
Cubic spline analysis showed a nonlinear relationship between years since quitting and the risk of diabetes, confirming the categorical analysis of years since quitting. The risk of diabetes was highest among new quitters, and then the risk reduced to that in never smokers after 10 years of smoking cessation (Figure 1).
Figure 1.
Effects of duration since quitting by year 3 follow-up on diabetes risk among 115,092 women, the Women's Health Initiative, United States, 1993–1998. The log hazard ratio is the natural logarithm of the hazard ratio of former smokers by duration since quitting, compared with never smokers. Solid curve, point estimator; dashed curves, 95% confidence interval.
DISCUSSION
In this large, prospective study in postmenopausal women, we observed that the risk of diabetes was significantly elevated in current smokers. Among former smokers, years since quitting and low cumulative smoking exposure were both significantly inversely associated with diabetes risk, and the risk of diabetes decreased to that in never smokers after 10 years of smoking cessation. In former smokers with low cumulative previous exposure, the increased risk of diabetes dissipated soon after quitting. In addition, we observed that new quitters had the highest risk of diabetes, especially among heavy smokers. After adjustment for weight gain, the increased risk of diabetes remained higher than that of nonsmokers, although it did not differ significantly from that of continuing smokers. These results suggest that weight gain may not be the only factor contributing to the elevated risk of diabetes in new quitters and that the residual effects of smoking may be important as well.
There is an extensive body of literature reporting on the association between cigarette smoking and the incidence of diabetes (1, 7, 20, 21). Our findings of an elevated risk of diabetes in postmenopausal women who are current smokers is consistent with the results from a recent meta-analysis of 25 prospective cohort studies on active smoking and diabetes (1) and results from recent cohort studies published after the review (7, 20, 21). The magnitude of the hazard ratio we observed for current smoking (HR = 1.28, 95% CI: 1.20, 1.36) was lower than the recent pooled adjusted relative risk (HR = 1.44, 95% CI: 1.31, 1.58) but was similar to that of the pooled adjusted relative risk of 1.25 (95% CI: 1.06, 1.46) among women from stratified analyses of studies that included both men and women (1). Although the exact mechanism has not been fully established, it is proposed that the potential biological mechanisms by which smoking increases risk of diabetes may be through the general inflammation caused by smoking (22, 23), increased insulin resistance or inadequate compensatory insulin secretion responses, and greater abdominal obesity in smokers (23–28) or through a direct toxic effect on pancreatic tissue (29, 30).
Several longitudinal studies investigated the association of smoking cessation on the risk of diabetes. Most studies (5–7, 20, 31), but not all (32), observed that the risk of diabetes decreased gradually as the time since quitting increased, although the years since quitting required for the risk to return to that in never smokers were inconsistent. The study by Will et al. (31) reported that smoking cessation–reduced risk of diabetes was equivalent to that of nonsmokers after 5 years for women and 10 years for men. The study by Wannamethee et al. (6) reported that the risk of diabetes following a cessation period of 20 years was equal to that of never smokers, and the Nurses' Health Study (20) reported that the risk of diabetes went back to that of never smokers following a cessation period of 30 years. To our knowledge, no previous study has examined the association between cumulative exposure and the duration of increased diabetes risk.
Taken together, these data suggest that, despite undesirable weight gain that often follows smoking cessation, stopping smoking could decrease the risk of developing diabetes and other metabolic perturbations in the long run. Studies have demonstrated that stopping smoking can improve blood lipid levels by increasing high density lipoprotein cholesterol and reducing low density lipoprotein cholesterol, regardless of any weight gain (33). Over time, smoking cessation increases insulin sensitivity and improves the lipoprotein profile (34, 35), although these improvements are not reported consistently (23, 36, 37).
Our results among former smokers are consistent with a reduction in risk for former smokers who quit 20 or more years ago, although there was an elevated diabetes risk among new quitters. It suggests that perhaps there is no overall increase in lifetime risk of diabetes associated with former smoking but just an acceleration in the onset of diabetes in this group of susceptible individuals. However, any increase in the average duration of diabetes and the higher risk of associated complications among former smokers appear to be greatly offset by the other health benefits of quitting. Recent research highlights the loss of a decade of life among smokers and confirms that quitting smoking at any age dramatically lowers mortality (38, 39).
Strengths of our study include the prospective design, the large size and broad geographical distribution of the cohort, the large number of diabetes cases, and detailed information on smoking, weight change, and potential confounders. The large number of cases enabled us to thoroughly assess how the excess risk of diabetes among new quitters was affected by cumulative smoking before quitting or weight gain after quitting. However, several limitations deserve mention. First, we used self-reported diabetes status. This may reduce the specificity of diabetes classification and lead to some degree of case misclassification. However, the misclassification bias is likely to be nondifferential, which may have led us to underestimate the strength of the association we observed. In addition, a validation study in the WHI has shown a high concordance of self-report with a “gold standard” based on medical record review and with medication inventories (16, 17). Second, given the strong relationship between type 2 diabetes and adiposity, even the most careful adjustment for body mass index and waist circumference may leave some possibility of residual confounding. Third, we lacked information on treatments, such as nicotine replacement, that women used for quitting. It is possible that nicotine may have direct toxic effects on the pancreas and insulin receptor sensitivity (40, 41). Fourth, it is possible that some of the observed association may be due to detection bias. For example, in some women, smoking cessation may have been triggered by a diagnosis of another disease, such as cardiovascular disease, pulmonary embolism, or cancer. These women may have more contact with the medical system that, in turn, led to detection of undiagnosed diabetes. However, the proportion of women who quit smoking in proximity to the incidence of these types of conditions was low, suggesting that the observed association is unlikely to have been substantially affected by detection bias. In addition, the WHI has a low rate of smoking relative to this age group in the general population, and thus, it is possible that results could differ in populations that include more smokers.
In conclusion, our prospective analysis suggests that the increased risk of diabetes associated with quitting is confined to heavy smokers. Despite the risk that smoking cessation can result in weight gain and subsequently a short-term increased risk of diabetes among heavy smokers, the risk of diabetes diminishes over the long term and returns to that in never smokers 10 years after quitting.
ACKNOWLEDGMENTS
Author affiliations: Department of Community Medicine, Mary Babb Randolph Cancer Center, West Virginia University, Morgantown, West Virginia (Juhua Luo); National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland (Jacques Rossouw); Division of General Internal Medicine, University of California, Davis, Medical Center, Sacramento, California (Elisa Tong); Department of Community Health and Health Behavior, School of Public Health and Health Professions, University at Buffalo, The State University of New York, Buffalo, New York (Gary A. Giovino); Division of Geriatrics, Department of Internal Medicine, David Geffen School of Medicine at University of California, and Veterans Affairs Greater Los Angeles Healthcare System Geriatrics Research Education and Clinical Center, Los Angeles, California (Cathy C. Lee); Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington (Chu Chen); Division of Preventive and Behavioral Medicine, Department of Medicine, University of Massachusetts Medical School, Worcester, Massachusetts (Judith K. Ockene); Division of Biostatistics, Department of Public Health Sciences, School of Medicine, University of California, Davis, Davis, California (Lihong Qi); and HealthPartners Research Foundation, Minneapolis, Minnesota (Karen L. Margolis).
The Women's Health Initiative program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services, through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221, and by HHSN268201100003C.
Program Office: National Heart, Lung, and Blood Institute, Bethesda, Maryland—Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: Fred Hutchinson Cancer Research Center, Seattle, Washington—Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles Kooperberg, and Chu Chen; Medical Research Labs, Highland Heights, Kentucky—Evan Stein; University of California at San Francisco, San Francisco, California—Steven Cummings. Clinical Centers: Albert Einstein College of Medicine, Bronx, New York—Sylvia Wassertheil-Smoller; Baylor College of Medicine, Houston, Texas—Haleh Sangi-Haghpeykar; Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts—JoAnn E. Manson; Brown University, Providence, Rhode Island—Charles B. Eaton; Emory University, Atlanta, Georgia—Lawrence S. Phillips; Fred Hutchinson Cancer Research Center, Seattle, Washington—Shirley Beresford; George Washington University Medical Center, Washington, DC—Lisa Martin; Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California—Rowan Chlebowski; Kaiser Permanente Center for Health Research, Portland, Oregon—Erin LeBlanc; Kaiser Permanente Division of Research, Oakland, California—Bette Caan; Medical College of Wisconsin, Milwaukee, Wisconsin—Jane Morley Kotchen; MedStar Research Institute/Howard University, Washington, DC—Barbara V. Howard; Northwestern University, Chicago/Evanston, Illinois—Linda Van Horn; Rush Medical Center, Chicago, Illinois—Henry Black; Stanford Prevention Research Center, Stanford, California—Marcia L. Stefanick; State University of New York at Stony Brook, Stony Brook, New York—Dorothy Lane; The Ohio State University, Columbus, Ohio—Rebecca Jackson; University of Alabama at Birmingham, Birmingham, Alabama—Cora E. Lewis; University of Arizona, Tucson/Phoenix, Arizona—Cynthia A. Thomson; University at Buffalo, Buffalo, New York—Jean Wactawski-Wende; University of California at Davis, Sacramento, California—John Robbins; University of California at Irvine, Irvine, California—F. Allan Hubbell; University of California at Los Angeles, Los Angeles, California— Lauren Nathan; University of California at San Diego, LaJolla/Chula Vista, California—Robert D. Langer; University of Cincinnati, Cincinnati, Ohio—Margery Gass; University of Florida, Gainesville/Jacksonville, Florida—Marian Limacher; University of Hawaii, Honolulu, Hawaii—J. David Curb; University of Iowa, Iowa City/Davenport, Iowa—Robert Wallace; University of Massachusetts/Fallon Clinic, Worcester, Massachusetts—Judith Ockene; University of Medicine and Dentistry of New Jersey, Newark, New Jersey—Norman Lasser; University of Miami, Miami, Florida—Mary Jo O'Sullivan; University of Minnesota, Minneapolis, Minnesota—Karen Margolis; University of Nevada, Reno, Nevada—Robert Brunner; University of North Carolina, Chapel Hill, North Carolina—Gerardo Heiss; University of Pittsburgh, Pittsburgh, Pennsylvania—Lewis Kuller; University of Tennessee Health Science Center, Memphis, Tennessee—Karen C. Johnson; University of Texas Health Science Center, San Antonio, Texas—Robert Brzyski; University of Wisconsin, Madison, Wisconsin—Gloria E. Sarto; Wake Forest University School of Medicine, Winston-Salem, North Carolina—Mara Vitolins; and Wayne State University School of Medicine/Hutzel Hospital, Detroit, Michigan—Michael S. Simon. Women's Health Initiative Memory Study: Wake Forest University School of Medicine, Winston-Salem, North Carolina—Sally Shumaker.
Conflict of interest: none declared.
REFERENCES
- 1.Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Willett WC, Rotnitzky A, et al. Weight-gain as a risk factor for clinical diabetes-mellitus in women. Ann Intern Med. 1995;122(7):481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Williamson DF, Madans J, Anda RF, et al. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324(11):739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 4.Wild SH, Byrne CD. ABC of obesity. Risk factors for diabetes and coronary heart disease. BMJ. 2006;333(7576):1009–1011. doi: 10.1136/bmj.39024.568738.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hur NW, Kim HC, Nam CM, et al. Smoking cessation and risk of type 2 diabetes mellitus: Korea Medical Insurance Corporation Study. Eur J Cardiovasc Prev Rehabil. 2007;14(2):244–249. doi: 10.1097/01.hjr.0000239474.41379.79. [DOI] [PubMed] [Google Scholar]
- 6.Wannamethee SG, Shaper AG, Perry IJ. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care. 2001;24(9):1590–1595. doi: 10.2337/diacare.24.9.1590. [DOI] [PubMed] [Google Scholar]
- 7.Yeh HC, Duncan BB, Schmidt MI, et al. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2011;152(1):10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegal KM, Troiano RP, Pamuk ER, et al. The influence of smoking cessation on the prevalence of overweight in the United States. N Engl J Med. 1995;333(18):1165–1170. doi: 10.1056/NEJM199511023331801. [DOI] [PubMed] [Google Scholar]
- 9.Luo J, Rossouw J, Tong E, et al. Smoking cessation, weight gain, and risk of type 2 diabetes mellitus among postmenopausal women. Arch Intern Med. 2012;172(5):438–440. doi: 10.1001/archinternmed.2012.24. [DOI] [PubMed] [Google Scholar]
- 10.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 11.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 suppl):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 12.Jackson RD, LaCroix AZ, Cauley JA, et al. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 suppl):S98–S106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 13.Langer RD, White E, Lewis CE, et al. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 suppl):S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 14.Ritenbaugh C, Patterson RE, Chlebowski RT, et al. The Women's Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 suppl):S87–S97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 15.Stefanick ML, Cochrane BB, Hsia J, et al. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 suppl):S78–S86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 16.Margolis KL, Lihong Q, Brzyski R, et al. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5(3):240–247. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson JM, DeFor TA, Crain AL, et al. Validity of diabetes self-reports in the Women's Health Initiative. J Clin Epidemiol. 2013;66(3):349–350. doi: 10.1016/j.jclinepi.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14(15):1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 19.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54(3):201–208. doi: 10.1016/s0169-2607(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang LX, Curhan GC, Hu FB, et al. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care. 2011;34(4):892–897. doi: 10.2337/dc10-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jee SH, Foong AW, Hur NW, et al. Smoking and risk for diabetes incidence and mortality in Korean men and women. Diabetes Care. 2010;33(12):2567–2572. doi: 10.2337/dc10-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncalves RB, Coletta RD, Silverio KG, et al. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. 2011;60(5):409–424. doi: 10.1007/s00011-011-0308-7. [DOI] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, US Department of Health and Human Services;; 2010. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. http://www.surgeongeneral.gov/library/tobaccosmoke/report/full_report.pdf. (Accessed March 15, 2012) [Google Scholar]
- 24.Berlin I. Smoking-induced metabolic disorders: a review. Diabetes Metab. 2008;34(4):307–314. doi: 10.1016/j.diabet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Chiolero A, Faeh D, Paccaud F, et al. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 26.Attvall S, Fowelin J, Lager I, et al. Smoking induces insulin resistance—a potential link with the insulin resistance syndrome. J Intern Med. 1993;233(4):327–332. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 27.Facchini FS, Hollenbeck CB, Jeppesen J, et al. Insulin resistance and cigarette smoking. Lancet. 1992;339(8802):1128–1130. doi: 10.1016/0140-6736(92)90730-q. [DOI] [PubMed] [Google Scholar]
- 28.Canoy D, Wareham N, Luben R, et al. Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obes Res. 2005;13(8):1466–1475. doi: 10.1038/oby.2005.177. [DOI] [PubMed] [Google Scholar]
- 29.Spector TD, Blake DR. Effect of cigarette smoking on Langerhans’ cells [letter] Lancet. 1988;2(8618):1028. doi: 10.1016/s0140-6736(88)90794-5. [DOI] [PubMed] [Google Scholar]
- 30.Wittel UA, Pandey KK, Andrianifahanana M, et al. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am J Gastroenterol. 2006;101(1):148–159. doi: 10.1111/j.1572-0241.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 31.Will JC, Galuska DA, Ford ES, et al. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int J Epidemiol. 2001;30(3):540–546. doi: 10.1093/ije/30.3.540. [DOI] [PubMed] [Google Scholar]
- 32.Beziaud F, Halimi JM, Lecomte P, et al. Cigarette smoking and diabetes mellitus. Diabetes Metab. 2004;30(2):161–166. doi: 10.1016/s1262-3636(07)70102-7. [DOI] [PubMed] [Google Scholar]
- 33.Mikhailidis DP, Papadakis JA, Ganotakis ES. Smoking, diabetes and hyperlipidaemia. J R Soc Health. 1998;118(2):91–93. doi: 10.1177/146642409811800209. [DOI] [PubMed] [Google Scholar]
- 34.Eliasson B, Attvall S, Taskinen MR, et al. Smoking cessation improves insulin sensitivity in healthy middle-aged men. Eur J Clin Invest. 1997;27(5):450–456. doi: 10.1046/j.1365-2362.1997.1330680.x. [DOI] [PubMed] [Google Scholar]
- 35.Campbell SC, Moffatt RJ, Stamford BA. Smoking and smoking cessation—the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis. 2008;201(2):225–235. doi: 10.1016/j.atherosclerosis.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 36.Balkau B, Vierron E, Vernay M, et al. The impact of 3-year changes in lifestyle habits on metabolic syndrome parameters: the D.E.S.I.R study. Eur J Cardiovasc Prev Rehabil. 2006;13(3):334–340. doi: 10.1097/01.hjr.0000214614.37232.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda K, Noguchi Y, Fukui T. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Prev Med. 2003;37(4):283–290. doi: 10.1016/s0091-7435(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 38.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 39.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowdhury P, MacLeod S, Udupa KB, et al. Pathophysiological effects of nicotine on the pancreas: an update. Exp Biol Med (Maywood) 2002;227(7):445–454. doi: 10.1177/153537020222700708. [DOI] [PubMed] [Google Scholar]
- 41.Chowdhury P, Rayford PL, Chang LW. Pathophysiological effects of nicotine on the pancreas. Proc Soc Exp Biol Med. 1998;218(3):168–173. doi: 10.3181/00379727-218-44284. [DOI] [PubMed] [Google Scholar]