Abstract
We have recently demonstrated that in C57/Bl6 mice long term voluntary wheel running is anxiogenic, and focal hippocampal irradiation prevents the increase in anxiety-like behaviors as well as neurobiological changes in the hippocampus induced by wheel running. Evidence supports a role of hippocampal 5-HT1A receptors in anxiety. Therefore, we investigated hippocampal binding and function of 5-HT1A receptors in this mouse model of anxiety. Four weeks of voluntary wheel running resulted in hippocampal subregion-specific changes in 5-HT1A receptor binding sites and function, as measured by autoradiography of [3H]8-OH-DPAT binding and agonist-stimulated binding of [35S]GTPγS to G proteins, respectively. In the dorsal CA1 region, 5-HT1A receptor binding and function were not altered by wheel running or irradiation. In the dorsal dentate gyrus and CA2/3 region, 5-HT1A receptor function was decreased by running, but also by irradiation. In the ventral pyramidal layer, wheel running resulted in a decrease of 5-HT1A receptor function, which was prevented by irradiation. Neither irradiation nor wheel running affected 5-HT1A receptors in medial prefrontal cortex, or in the dorsal or median raphe nuclei. Our data indicate that down-regulation of 5-HT1A receptor function in ventral pyramidal layer may play a role in anxiety-like behavior induced by wheel running.
Keywords: exercise, serotonin, neurogenesis, raphe nucleus, hippocampus
INTRODUCTION
We have previously demonstrated that long-term voluntary wheel running increases levels of anxiety-like behavior in approach-avoidance tests in mice (Fuss et al., 2010b). In this mouse model of anxiety, individual levels of anxiety were highly correlated with genesis of new neurons in the hippocampus (Fuss et al., 2010b), a brain region of particular interest involved in emotion (Fanselow and Dong, 2010; Gray and McNaughton, 2003). Moreover, ablation of hippocampal neurogenesis by focalized irradiation was sufficient to prevent the development of increased anxiety-like behaviors in dark-light box, o-maze and open field test (Fuss et al., 2010a), indicating that hippocampal neurogenesis may be a crucial factor in the increase in anxiety-like behavior after long-term running. This finding was recently corroborated by others using a molecular approach to block neurogenesis (Onksen et al., 2012). In our model running mice also have altered cellular activity, as measured by c-Fos activation, in hippocampal subfields in anxiogenic situations (Fuss et al., 2010a). These effects of wheel running on anxiety-induced cell activation in hippocampus are prevented by focalized irradiation (Fuss et al., 2010a).
Given the elevated levels of hippocampal neurogenesis and cell activation in this established model of exercise-induced anxiety, we hypothesized that additional neurochemical changes occur. The hippocampus receives a dense plexus of serotonergic innervation arising from serotonergic cell bodies in the dorsal and median raphe nuclei (see Molliver 1987). In order to begin to unravel mechanisms for the involvement of the serotonergic system in this animal model of anxiety, we focused our attention on the serotonin-1A (5-HT1A) receptor.
The unique distribution of 5-HT1A receptors in brain is consistent with its role in cognitive or integrative functions, as well as in emotional states. 5-HT1A receptors are present in high density in cortical and limbic areas, including the hippocampus (Hensler et al., 1991, Kia et al., 1996, Verge et al., 1986). In these terminal field areas of serotonergic innervation, the 5-HT1A receptor is located postsynaptically to serotonergic neurons (Hensler et al., 1991, Riad et al., 2000, Verge et al., 1986). Hippocampal 5-HT1A receptors play an important role in the maintenance of normal neuronal function (Sarnyai et al., 2000), neurogenesis, and neuronal survival (Gould 1999, Fricker et al., 2005, Santarelli et al., 2003). While the activation of 5-HT1A receptors promotes neurogenesis in the adult hippocampus (Gould 1999, Banasr et al., 2004), antagonists of the receptor inhibit cell proliferation in the dentate gyrus (Radley & Jacobs 2002).
Human and animal studies indicate that forebrain 5-HT1A receptor function is closely linked to anxiety symptoms (Andrews et al., 1994, Spindelegger et al., 2009). A number of studies in humans show an inverse correlation between 5-HT1A receptor binding and anxiety traits in healthy humans (Tauscher et al., 2001) as well as reduced 5-HT1A binding in patients suffering from panic disorder (Nash et al., 2008, Neumeister et al., 2004) and social anxiety disorder (Lanzenberger et al., 2007). Local injections of 5-HT1A receptor agonists into the hippocampus of rats reduces anxiety-like behavior in open field and elevated plus-maze tests (Kostowski et al., 1989). In 5-HT1A knockout mice the absence of 5-HT1A receptors in all brain regions induces an animal model of increased anxiety (Ramboz et al., 1998). However, a region-specific rescue of forebrain 5-HT1A receptors is sufficient to restore normal behaviour in these mice, further highlighting the important role of 5-HT1A receptor function in anxiety (Gross et al., 2002).
Here, we measured both 5-HT1A receptor binding sites and function in forebrain regions, specifically medial prefrontal cortex and hippocampus, after voluntary wheel running. We also examined 5-HT1A receptor binding and function in serotonergic cell body areas, particularly the dorsal and median raphé nuclei, where these receptors function as somatodendritic autoreceptors (Aghajanian et al., 1990, de Montigny et al., 1984) and therefore control serotonergic neurotransmission. In our earlier experiments hippocampal irradiation prevented the increase in anxiety after wheel running. Hence, we also studied the effects of focalized irradiation on wheel running-induced changes in these 5-HT1A receptor populations. 5-HT1A receptor function was measured at the level of receptor-G protein interaction using quantitative autoradiography of [35S]GTPγS binding stimulated by the 5-HT1A receptor agonist 8-OH-DPAT (Hensler, 2002; Hensler and Durgam, 2001). The coupled, high-affinity agonist state of the 5-HT1A receptor was measured by the binding of the agonist radioligand [3H]8-OH-DPAT (Chamberlain, et al., 1993;Verge et al., 1986).
MATERIALS AND METHODS
Animals
C57Bl/6J male mice (n = 40) were obtained at the age of 4 weeks from Charles River (Sulzfeld, Germany). Half of the mice (n=20) received hippocampus-focalized irradiation at the age of 4 weeks with a dose of 10 Gy (at a rate of 1 Gy/min). Irradiation was performed through a small stereotactically-defined opening (3.7 × 11 mm) sparing other brain regions from irradiation and allowing an even distribution of X-rays in the irradiated field (Fuss et al., 2010a). Cells expressing neural differentiation marker doublecortin and proliferation marker Ki67 are evenly reduced in ventral and dorsal hippocampus after hippocampal irradiation (Fuss et al., 2010a). Controls (n = 20) received a sham-treatment with the same anesthesia consisting of ketamine and xylazine (Fuss et al., 2010a). Mice were housed in a light-dark cycle with lights on at 7 p.m. and provided with standard chow and water ad libitum. Following a recovery period of 4 weeks, irradiated and non-irradiated mice were single housed and 1 week later divided into runners and sedentary controls, the latter equipped with blocked wheels. After 4 weeks of daily running mice were sacrificed between 10 a.m. and 2 p.m. by decapitation and brain tissue prepared for autoradiographic studies. All procedures complied with the regulations covering animal experimentation within the EU (European Communities Council Directive 86/609/EEC) and Germany (Deutsches Tierschutzgesetz). Experiments were approved by the German animal welfare authorities (Regierungspräsidium Karlsruhe) and comply with the “Animal Research: Reporting In Vivo Experiments” (ARRIVE) guidelines. All efforts were made to minimize the number of animals used and the severity of procedures applied in this study.
Tissue Preparation
Brains were rapidly removed, frozen on powdered dry ice and then stored at −80°C until sectioning. Coronal sections of 20 μm thickness were cut at −17°C in a cryostat microtome at the level of the medial prefrontal cortex (plates 13-14), dorsal hippocampus (plates 46-47), ventral hippocampus (plate 55) and dorsal raphe nucleus (plates 68-70) according to the atlas of the mouse brain by (Paxinos & Franklin 2001). Sections were thaw-mounted onto gelatin-coated glass slides, desiccated at 4°C for 18 hours under vacuum and then stored at −80°C until they were used in the autoradiographic experiments.
[3H]8-OH-DPAT autoradiography
Autoradiography of the binding of [3H]8-OH-DPAT to 5-HT1A receptors in brain sections was performed as described with minor modification (Hensler et al., 1991). Briefly, slide-mounted sections were thawed and desiccated at 4° C for 1 hour. Sections were pre-incubated for 30 minutes at 30° C in assay buffer (170 mM Tris–HCl; pH 7.6), and then incubated in assay buffer containing 2 nM [3H]8-OHDPAT for 60 minutes at room temperature. Non-specific binding was defined by incubating adjacent sections in the presence of 10 μM WAY 100635. Incubation was terminated by two washes for 5 minutes each in ice-cold 170 mM Tris–HCl buffer (pH 7.6), followed by a dip in ice-cold de-ionized water. Sections were dried on a slide warmer and exposed to Kodak BioMax MR Film for a period of 9 week to generate autoradiograms.
[35S]GTPγS autoradiography
Autoradiography of 8-OH-DPAT-stimulated [35S]GTPγS binding in brain sections was performed as previously described (Hensler 2002; Hensler & Durgam 2001; Rossi et al., 2006) with slight modifications. Briefly, slide-mounted sections were incubated in HEPES buffer containing GDP (2 mM), adenosine A1 receptor antagonist 1,3-Dipropyl-8-cyclopentylxanthine (DPCPX, 1 μM) and 40 pM [35S]GTPγS, either in the absence or presence of racemic 8-OH-DPAT (1 μM), for 60 minutes at 25°C. This concentration of 8-OH-DPAT produces maximal stimulation of [35S]GTPγS binding (Hensler & Durgam 2001). 8-OH-DPAT, which has high affinity for 5-HT1A receptors (Ki=1 nM) (e.g Sprouse et al., 2004), also has moderate affinity for and agonist activity at 5-HT7 receptors (Ki=250 nM) (Sprouse et al., 2004, Duncan & Franklin 2007, Hagan et al., 2000, Hedlund et al., 2004). However, the stimulation of [35S]GTPγS binding by (1 μM) 8-OH-DPAT is completely blocked by the 5-HT1A receptor antagonist WAY 100635 (100 nM) in all areas of brain examined (Hensler & Durgam 2001), and is not altered by the selective 5-HT7 receptor antagonist SB 269970 (100 nM) (Rossi et al., 2006). Basal [35S]GTPγS binding was defined by incubating adjacent sections in the absence of 8-OH-DPAT. Nonspecific [35S]GTPγS binding was defined in the absence of 8-OH-DPAT and in the presence of 10 μM GTPγS. The incubation was stopped by two washes for 5 minutes each in ice-cold 50 mM HEPES buffer (pH 7.4), followed by a brief immersion in ice-cold de-ionized water. Sections were dried on a slide-warmer and exposed to Kodak Biomax MR film for 48 hours.
Image Analysis
Analysis of the digitized autoradiograms was performed using the image analysis program ImageJ, version 1.42q (National Institutes of Health, Bethesda, MD). Tissue sections were stained with thionin and the brain areas identified using the atlas of the mouse brain by (Paxinos & Franklin 2001).
Autoradiograms of [3H]8-OH-DPAT binding were quantified by the use of simultaneously exposed [3H] standards (ART-123, American Radiochemicals, St. Louis, MO, USA), which had been calibrated using brain-mash sections according to the method of Geary and colleagues (Geary et al., 1985, Geary & Wooten 1983). This allowed the conversion of optical density measurements to femtomoles per milligram of protein. Specific binding was calculated by subtracting non-specific binding from total binding on adjacent sections.
Autoradiograms of 8-OH-DPAT-stimulated [35S]GTPγS binding were quantified by the use of simultaneously exposed [14C] standards (ARC-146, American Radiochemicals). Standard curves were fitted to pixel data obtained from [14C] standards and tissue equivalent values (nCi/g) provided by American Radiochemicals, and were used to transform the actual regional densitometric values into relative radioactivity measures. Non-specific binding of [35S]GTPγS was subtracted from basal binding and from binding in the presence of 8-OH-DPAT. Specific 8-OH-DPAT-stimulated binding was expressed as % above basal.
Basal [35S]GTPγS binding was determined in tissue sections adjacent to those used to determine agonist-stimulated [35S]GTPγS binding. Determination of basal binding in these sections serves as a control for any potential differences in tissue handling (e.g. dissection, freezing, sectioning). We and others commonly express agonist stimulated [35S]GTPγS binding as % above basal, which serves not only to control for potential differences in tissue handling, but also controls for brain region-specific differences in basal [35S]GTPγS binding. It is important to note that neither irradiation nor exercise resulted in changes in basal binding (Table 1).
Table 1.
Basal binding of [35S]GTPγS in specific brain regions of sham-treated (SS and SR) and irradiated, sedentary or running mice (IRS and IRR). Basal binding is expressed as tissue equivalent values (nCi/g). Values represent mean ± S.E.M. n = 9 per experimental group.
| Basal binding of [35S]GTPγS | ||||
|---|---|---|---|---|
| SS | SR | IRS | IRR | |
| Dorsal Hippocampus | ||||
|
| ||||
| CA1 region | 169 ± 11.1 | 172 ± 8.4 | 176 ± 10.6 | 176 ± 7.3 |
| CA2/3 region | 173 ± 11.6 | 180 ± 7.9 | 177 ± 10.4 | 183 ± 6.8 |
| Dentate gyrus | 160 ± 11.9 | 156 ± 8.5 | 162 ± 11.3 | 150 ± 7.1 |
|
| ||||
| Ventral Hippocampus | ||||
|
| ||||
| Pyramidal layer | 265 ± 13.9 | 252 ± 11.1 | 251 ± 9.9 | 253 ± 12.1 |
Statistical Analyses
Statistical analysis was carried out using SPSS 16.0 (SPSS Inc., Chicago, IL). All data are reported as means ± S.E.M.. Differences between groups were detected using two-factorial analysis of variance followed by Fischer’s LSD post hoc analysis. Significance was evaluated at a probability of 5% or less (<0.05).
Materials
[35S]GTPγS (1250 Ci/mmol) was purchased from PerkinElmer (Waltham, MA). [3H]8-OH-DPAT (210 Ci/mmol) was purchased from Amersham Biosciences (Piscataway, NJ). Racemic 8-OH-DPAT hydrobromide, and 1,3-Dipropyl-8-cyclopentylxanthine (DPCPX) were purchased from Tocris (Ellisville, MO). WAY 100635 maleate, GDP (disodium salt) were purchased from Sigma/RBI (St. Louis, MO). GTPγS (tetralithium salt) was purchased from Roche/Boehringer-Mannheim (Indianapolis, IN).
RESULTS
First, our objective was to discover the impact of voluntary wheel running on 5-HT1A receptors in the dorsal and median raphé nuclei, where 5-HT1A receptors function as somatodendritic autoreceptors. Alterations in 5-HT1A receptor function or binding sites in these serotonergic cell body areas would be expected to directly alter the serotonergic tone in brain regions that receive projections from these raphé nuclei. Neither voluntary wheel running, nor focal hippocampal irradiation affected the 5-HT1A receptor binding in the dorsal and median raphé nuclei as measured by the binding of [3H]8-OH-DPAT (Table 2a). Moreover, we did not find significant changes in 5-HT1A receptor function as indicated by 8-OH-DPAT (1 μM)-stimulated [35S]GTPγS binding in either of the raphé nuclei (Table 2b).
Table 2.
5-HT1A receptor binding sites and function are not altered in the dorsal raphe nucleus (DRN), median raphe nucleus (MRN) or medial prefrontal cortex (mPFCTx) by voluntary wheel running or hippocampus-focalized irradiation. Specific binding of [3H]8-OH-DPAT (2 nM) (a) is expressed as fmol/mg protein. [35S]GTPγS binding (b) was stimulated by the 5-HT1A receptor agonist 8-OH-DPAT (1 μM), and is expressed as % above basal.
| a 5-HT1A receptor binding sites (fmol/mg protein) | |||||||
|---|---|---|---|---|---|---|---|
| SS | SR | IRS | IRR | Running | Irradiation | Interaction | |
| DRN | 218 ± 16 | 215 ± 16 | 217 ± 16 | 218 ± 16 | F < 1 | F < 1 | F < 1 |
| MRN | 45.5 ± 6.9 | 49.8 ± 7.3 | 38.3 ± 7.3 | 44.8 ± 6.9 | F < 1 | F < 1 | F < 1 |
| mPFCTx | 120 ± 9 | 120 ± 9 | 122 ± 10 | 120 ± 9 | F < 1 | F < 1 | F < 1 |
| b 5-HT1A receptor function (% above basal) | |||||||
|---|---|---|---|---|---|---|---|
| SS | SR | IRS | IRR | Running | Irradiation | Interaction | |
| DRN | 36.9 ± 4.6 | 29.3 ± 4.4 | 45.5 ± 4.4 | 37.1 ± 4.4 | F = 3.2 p=.08 | F = 3.5 p=.07 | F < 1 |
| MRN | 29.1 ± 3.4 | 21.7 ± 3.4 | 20.1 ± 3.4 | 20.2 ± 3.4 | F = 1.2 p=.29 | F = 2.4 p=.13 | F = 1.2 p=.28 |
| mPFCTx | 52.1 ± 6.2 | 56.5 ± 6.2 | 49.6 ± 6.2 | 58.7±6.2 | F = 1.1 p=.29 | F < 1 | F < 1 |
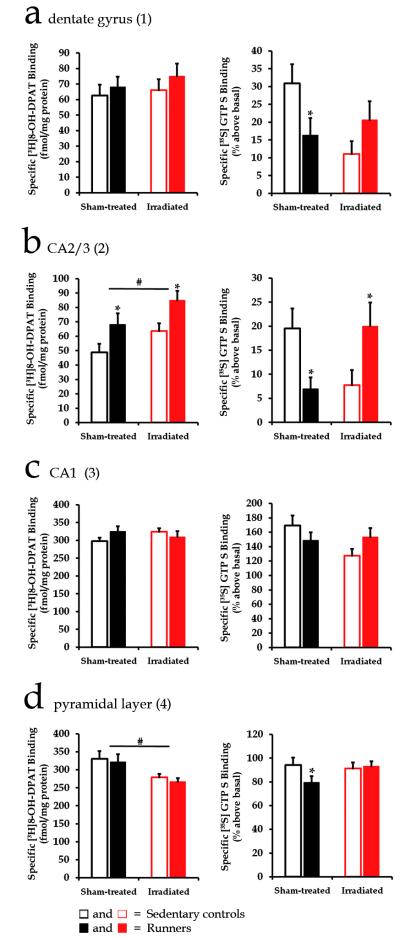
The main focus of our the present work, however, was on the hippocampal formation (Fig. 1), since our previous studies had demonstrated a specific role of the hippocampus in voluntary exercise-induced anxiety, with a plethora of cellular and neurochemical alterations occurring specifically in the hippocampus following wheel running (Biedermann et al., 2012, Fuss et al., 2010b). In dentate gyrus of dorsal hippocampus (Fig. 1a), [35S]GTPγS binding stimulated by 8-OH-DPAT (1 μM) was decreased by long term wheel running, but also by irradiation (Fig 2a). Two-way ANOVA revealed an interaction effect of the factors running and irradiation (F(1,35) = 6.41; p = 0.01). Post-hoc comparisons showed a significant reduction in 8-OH-DPAT -stimulated [35S]GTPγS binding in sham-treated runners compared to sham-treated sedentary mice (p = 0.04). 8-OH-DPAT-stimulated [35S]GTPγS binding was also decreased by focal irradiation in sedentary mice (p = 0.006). [3H]8-OH-DPAT binding to 5-HT1A receptors was not significantly altered by voluntary wheel running or by focal irradiation (Fig. 2a). Our data indicate that 5-HT1A receptor function, at the level of receptor-G protein interaction is reduced in dentate gyrus following long term wheel running, but also by irradiation.
Figure 1.
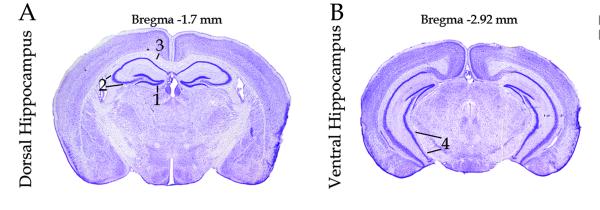
Representative sections of hippocampus illustrating regions of interest. A. Dorsal hippocampus, (1) dentate gyrus, (2) CA2/3 regions, (3) CA1 region. B. Ventral hippocampus, (4) pyramidal layer. (adapted from Paxinos and Franklin, 2001)
Figure 2.
5-HT1A receptor function and binding in the dorsal (panels A, B and C) and ventral (panel D) hippocampal subregions. [35S]GTPγS binding was stimulated by the 5-HT1A receptor agonist 8-OH-DPAT (1 μM), and is expressed as % above basal. Specific binding of [3H]8-OH-DPAT (2 nM) is expressed as fmol/mg protein. * indicates a significant post-hoc difference between runners and sedentary mice within groups of sham-treated or irradiated mice. # indicates significant differences between groups of sham-treated and irradiated mice in two-way ANOVA. Columns present means ± SEM.
In CA2/3 region of the dorsal hippocampus (Fig. 1a) [35S]GTPγS binding stimulated by 8-OH-DPAT (1 μM) was decreased by long term wheel running, but also by irradiation (Fig 2b). Two-way ANOVA revealed an interaction effect of the factors running and irradiation (F(1,35) = 10.88; p = 0.002). Post-hoc comparisons showed a significant reduction in 8-OH-DPAT-stimulated [35S]GTPγS binding in sham-treated runners compared to sham-treated sedentary mice (p = 0.02). 8-OH-DPAT-stimulated [35S]GTPγS binding was also decreased by focal irradiation in sedentary mice (p = 0.04). These decreases in 5-HT1A receptor function were accompanied by an increase in 5-HT1A receptor binding. [3H]8-OH-DPAT binding to 5-HT1A receptors was increased by voluntary wheel running in both sham-treated and irradiated mice (F(1,36) = 10.06; p = 0.003), and by irradiation in running as well as in sedentary mice (F(1,36) = 6.02; p = 0.01) (Fig. 2b). 5-HT1A receptor function was restored in irradiated runners compared to irradiated sedentary mice (p = 0.02). This increase in 5-HT1A receptor function in irradiated runners was accompanied by an increase in [3H]8-OH-DPAT binding.
In CA1 region of dorsal hippocampus (Fig. 1a), [35S]GTPγS binding stimulated by 8-OH-DPAT (1 μM) was not significantly altered by voluntary wheel running or by focal irradiation (Fig 2c). [3H]8-OH-DPAT binding to 5-HT1A receptors was also not altered (Fig 2c). Two-way ANOVA revealed an interaction effect of the factors running and irradiation for [35S]GTPγS binding (F(1,35) = 3.90; p = 0.05), Post-hoc comparisons indicated no significant reduction in dorsal CA1 (p = 0.23). These data indicate that in the dorsal CA1 region, 5-HT1A receptor binding and function were not altered by wheel running or irradiation.
In the pyramidal layer of ventral hippocampus (Fig. 1b), [3H]8-OH-DPAT binding to 5-HT1A receptors was decreased by irradiation (F(1,30) = 10.52; p = 0.003), but not by wheel running (Fig. 2d). Although irradiation caused a reduction in 5-HT1A receptor binding in ventral pyramidal layer of (Fig 2d) there was no change in 8-OH-DPAT-stimulated [35S]GTPγS binding, suggesting a compensatory increase in 5-HT1A receptor function. [35S]GTPγS binding stimulated by 8-OH-DPAT (1 μM) however was decreased by wheel running (Fig 2d). Two-way ANOVA revealed an interaction effect of the factors running and irradiation (F(1,30) = 5.47; p = 0.02). Post-hoc comparisons showed a significant reduction in [35S]GTPγS binding stimulated by 8-OH-DPAT (1 μM) in sham-treated runners compared to sham-treated sedentary mice (p = 0.005). These data indicate that 5-HT1A receptor function, at the level of receptor-G protein interaction is reduced in ventral hippocampus following long term wheel running. Focal irradiation prevented the decrease in 5-HT1A receptor function observed in this brain region of runners.
To demonstrate the regional selectivity of our findings we measured 5-HT1A receptor binding sites and function in another serotonergic projection region, namely the medial prefrontal cortex (mPFCtx). Here, neither 5-HT1A receptor function (Table 2b) nor the number of 5-HT1A receptor binding sites as measured by the binding of [3H]8-OH-DPAT (Table 2a) were affected by wheel running or by focal hippocampal irradiation. In conclusion, our observations seem to be restricted to the hippocampus as a core structure influenced by long-term voluntary wheel running.
DISCUSSION
Our objective in the present study was to investigate 5-HT1A receptor binding and function in an animal model of anxiety. In this mouse model, long term voluntary wheel running is anxiogenic. Focal hippocampal irradiation prevents the increase in anxiety-like behaviors in a variety of tests as well as neurobiological changes in the hippocampus induced by wheel running (Fuss et al., 2010a, b). In hippocampus, we observed subregion-specific changes in 5-HT1A receptor binding sites and function, as measured by [3H]8-OH-DPAT binding and agonist-stimulated binding of [35S]GTPγS to G proteins. In the ventral pyramidal layer, wheel running resulted in a decrease of 5-HT1A receptor function, which was prevented by irradiation. Our data suggest an association between decreased 5-HT1A receptor function in ventral hippocampus and anxiety-like behavior in this model of anxiety.
In the dentate gyrus and CA2/3 region of dorsal hippocampus, 5-HT1A receptor function was decreased by running, and also by irradiation. In the CA2/3 region, the decrease in 5-HT1A receptor function induced by running was accompanied by an increase of the binding of [3H]8-OH-DPAT to the high affinity agonist state (i.e. the coupled state) of the receptor (Chamberlain, et al., 1993; Verge et al., 1986). As we are using [3H]8-OH-DPAT at a concentration near the Kd, this could be interpreted as an increase in the affinity 5-HT1A receptors for agonist, or an increase in the number of 5-HT1A receptors in the coupled state. Taken together, these data suggest that the running-induced decrease in 5-HT1A receptor function in the dorsal CA2/3 region may not be at the level of the receptor (e.g. receptor affinity for agonist or receptors coupled to G protein), but may be due to changes in G protein function (e.g. the capacity of G protein to be activated by agonist binding to the receptor). Interestingly, running reversed the deficit in 5-HT1A receptor function in the CA2/3 region caused by irradiation and resulted in an up-regulation of [3H]8-OH-DPAT binding in irradiated mice. These data suggest that in irradiated mice, running induces an increase in the affinity of the receptor for agonist or increases the number of 5-HT1A receptors coupled to G proteins, which is accompanied by an increase in receptor function.
Voluntary exercise promotes neuronal activity in the hippocampus and increases the differentiation and proliferation of progenitor cells in the subgranular layer of the dentate gyrus (Matsumoto et al., 2011, van Praag et al., 1999). We and others have previously shown a strong correlation of running-induced hippocampal neurogenesis and anxiety-like behavior of mice (Fuss et al., 2010 a,b; Onksen et al., 2012). However, some investigators have found no relation of physical exercise and increased anxiety-like behavior (Dubreucq et al., 2011), or have found a decrease in anxiety-like behavior after exercise (Salam et al., 2009; Duman et al., 2008; Brocardo et al., 2012). A possible explanation for these discrepant findings might be differing degrees of running, and whether wheel running is voluntary or forced (Leasure and Jones, 2008). In our model C57Bl/6J mice performed excessive levels of running at an average of 10 km / active phase (Fuss et al., 2010a, b). Differing levels of running might underlie motivational aspects mediated for example by the endocannabinoid system (Dubreucq et al., 2010; Fuss et al., 2010c). Furthermore strain, housing conditions (group versus single) and cage enrichment of control groups (blocked wheel versus no wheel) appear to impact anxiety-like behavior of mice (Chourbaji et al., 2008; Dubreucq et al., 2011).
When hippocampal neurogenesis is eliminated by focal irradiation or molecular approaches, running mice no longer exhibit an increase in anxiety-like behavior, indicating that hippocampal neurogenesis may be an important factor for the development of this anxiety-like phenotype (Fuss et al., 2010a; Onksen et al., 2012). Irradiation specifically targets cells with high proliferation rate like neural progenitors in the dentate gyrus (Jenrow et al., 2010). Interestingly the production of other cell lines like astrocytes and oligodendrocytes seems to be less affected by irradiation (Mizumatsu et al., 2003). We have previously reported a dramatic reduction in proliferating cells and newborn neurons 5 and 10 weeks after irradiation (Fuss et al., 2010a). Running was not sufficient to compensate for this decrease. Therefore only sham-treated runners have increased numbers of newborn neurons following 4 weeks of exercise.
In dentate gyrus and CA 2/3 region of dorsal hippocampus, irradiation as well as wheel running decreased 5-HT1A receptor function. The reduction in 5-HT1A receptor function in these subregions of dorsal hippocampus of sedentary mice as a result of irradiation was comparable to that seen in sham-treated, wheel running mice. We and others have previously demonstrated that wheel running increases hippocampal neurogenesis (Onksen et al., 2012; Fuss et al., 2010a,b). Data from multiple anxiety tests indicate that mice with increased neurogenesis exhibit heightened anxiety (Onksen et al., 2012; Fuss et al., 2010a,b). Blockade of neurogenesis by irradiation (Fuss et al., 2010a,b) or molecular approaches (Onksen et al., 2012) prevents this anxiety-like phenotype. Taken together these data suggest that in the dorsal hippocampus decreases in 5-HT1A receptor function occur independently of neurogenesis, and may be unrelated to the anxiety-like phenotype in this model.
Hippocampus-focalized irradiation is widely used to generate control groups to dissect neurogenesis-dependent and -independent mechanisms in emotional behaviors (compare e.g. Surget et al., 2011). And it is argued that irradiation specifically targets proliferating cells. Yet in the present experiment we demonstrate that irradiation also affects 5-HT1A receptor function in dorsal hippocampus. However, we cannot conclude from our data if this effect is due to the reduction of newborn neurons, or a neurogenesis-independent effect of irradiation that affects 5-HT1A receptors directly. Given that earlier studies also employing an irradiated control group have demonstrated a neurogenesis-dependent effect of serotonergic drugs (namely selective serotonin reuptake-inhibitors) on emotional behavior (Santarelli et al., 2003), our findings of attenuated 5-HT1A receptor function should be taken into consideration in future experimental interventions. To understand how irradiation blocks emotional consequences of treatments stimulating neurogenesis, the role of 5-HT1A receptors needs to be evaluated in future experiments.
5-HT1A receptor function and binding in medial prefrontal cortex and serotonergic cell body areas (i.e. dorsal and median raphé nuclei) were not altered by wheel running or by focal hippocampal irradiation. This is in marked contrast to what we observed in hippocampus, and may be related to region-specific differences in the coupling of 5-HT1A receptors to subtypes of G protein belonging to the Gi/Go family (Mannoury la Cour et al., 2006). In hippocampus, 5-HT1A receptors interact mainly with Gαo and weakly with Gαi3. This is in contrast with what is found in the cortex, where 5-HT1A receptors interact equally with Gαo and Gαi3. By contrast, in the anterior raphe, the 5-HT1A receptor couples exclusively with Gαi3 (Mannoury la Cour et al., 2006). The differences in G protein coupling between hippocampus, cortex and anterior raphe may be relevant to the region-specific regulation of 5-HT1A receptor function.
In conclusion, we found a region-specific modulation of hippocampal 5-HT1A receptor function in this mouse model of anxiety. In the dorsal dentate gyrus and CA2/3 region, changes in 5-HT1A receptor function appear to be unrelated to neurogenesis and the anxiety-like phenotype associated with this model of anxiety. In the ventral pyramidal layer however, wheel running resulted in a decrease of 5-HT1A receptor function, which was prevented by irradiation. These data suggest an association between decreased 5-HT1A receptor function in ventral hippocampus with anxiety-like behavior in this model. Gene expression studies and anatomical data support a functional distinction between the dorsal and ventral hippocampus. The dorsal hippocampus performs primarily cognitive functions, whereas the ventral hippocampus plays a critical role in emotion and the regulation of stress responses (Fanselow and Dong, 2010). Future pharmacological studies will help to further clarify the role of post-synaptic 5-HT1A receptors in the hippocampus in anxiety. For example, local injection of 5-HT agonist in ventral and dorsal hippocampus (Jolas et al., 1995) may clarify the role of post-synaptic 5-HT1A receptors in hippocampal subregions in the development of running-induced anxiety-like behavior in this model.
ACKNOWLEDGEMENTS
This work was supported by grants from the Deutsche Forschungsgemeinschaft (GA427/9-1 and SFB636-B3 to P.G.) and National Institute of Mental Health (MH 083300 to J.G.H.).
ABBREVIATIONS
- 5-HT1A
serotonin-1A
- GTPγS
guanosine 5′-O-[gamma-thio]triphosphate
- 8-OH-DPAT
8-Hydroxy-2-(di-n-propylamino)tetralin
Footnotes
CONFLICT OF INTEREST None
REFERENCES
- Aghajanian GK, Sprouse JS, Sheldon P, Rasmussen K. Electrophysiology of the central serotonin system: receptor subtypes and transducer mechanisms. Ann N Y Acad Sci. 1990;600:93–103. doi: 10.1111/j.1749-6632.1990.tb16875.x. discussion 103. [DOI] [PubMed] [Google Scholar]
- Andrews N, Hogg S, Gonzalez LE, File SE. 5-HT1A receptors in the median raphe nucleus and dorsal hippocampus may mediate anxiolytic and anxiogenic behaviours respectively. Eur J Pharmacol. 1994;264:259–264. doi: 10.1016/0014-2999(94)00473-0. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Biedermann S, Fuss J, Zheng L, Sartorius A, Falfan-Melgoza C, Demirakca T, Gass P, Ende G, Weber-Fahr W. In vivo voxel based morphometry: Detection of increased hippocampal volume and decreased glutamate levels in exercising mice. Neuroimage. 2012;61:1206–1212. doi: 10.1016/j.neuroimage.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise. Neuropharmacology. 2012;62:1607–1618. doi: 10.1016/j.neuropharm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Chamberlain J, Offord SJ, Wolfe BB, Tyau LS, Wang HL, Frazer A. Potency of 5-hydroxytryptamine1a agonists to inhibit adenylyl cyclase activity is a function of affinity for the ‘low-affinity’ state of [3H]8-hydroxy-N,Ndipropylaminotetralin ([3H]8-OH-DPAT) binding. J Pharmacol ExpTher. 1993;266:618–625. [PubMed] [Google Scholar]
- Chourbaji S, Brandwein C, Vogt MA, Dormann C, Hellweg R, Gass P. Nature vs. nurture: can enrichment rescue the behavioural phenotype of BDNF heterozygous mice? Behav Brain Res. 2008;192:254–258. doi: 10.1016/j.bbr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- de Montigny C, Blier P, Chaput Y. Electrophysiologically-identified serotonin receptors in the rat CNS. Effect of antidepressant treatment. Neuropharmacology. 1984;23:1511–1520. doi: 10.1016/0028-3908(84)90095-9. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Marsicano G, Chaouloff F. Emotional consequences of wheel running in mice: Which is the appropriate control? Hippocampus. 2011;21:239–242. doi: 10.1002/hipo.20778. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviors and hippocampal neurogenesis. Exp. Neurol. 2010;224:106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Franklin KM. Expression of 5-HT7 receptor mRNA in the hamster brain: effect of aging and association with calbindin-D28K expression. Brain Res. 2007;1143:70–77. doi: 10.1016/j.brainres.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci. 1996;16:4810–4815. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker AD, Rios C, Devi LA, Gomes I. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res Mol Brain Res. 2005;138:228–235. doi: 10.1016/j.molbrainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS One. 2010a:5. doi: 10.1371/journal.pone.0012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, Witzemann V, Hellweg R, Gass P. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010b;20:364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- Fuss J, Gass P. Endocannabinoids and voluntary activity in mice: Runner’s high and long-term consequences in emotional behaviors. Exp Neurol. 2010c;224:103–105. doi: 10.1016/j.expneurol.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Geary WA, 2nd, Toga AW, Wooten GF. Quantitative film autoradiography for tritium: methodological considerations. Brain Res. 1985;337:99–108. doi: 10.1016/0006-8993(85)91613-0. [DOI] [PubMed] [Google Scholar]
- Geary WA, 2nd, Wooten GF. Quantitative film autoradiography of opiate agonist and antagonist binding in rat brain. J Pharmacol Exp Ther. 1983;225:234–240. [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety. second edition Oxford University Press; 2003. [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, et al. Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Hensler J, Durgam H. Regulation of 5-HT(1A) receptor-stimulated [35S]-GtpgammaS binding as measured by quantitative autoradiography following chronic agonist administration. Br J Pharmacol. 2001;132:605–611. doi: 10.1038/sj.bjp.0703855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology. 2002;26:565–573. doi: 10.1016/S0893-133X(01)00395-5. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Kovachich GB, Frazer A. A quantitative autoradiographic study of serotonin1A receptor regulation. Effect of 5,7-dihydroxytryptamine and antidepressant treatments. Neuropsychopharmacology. 1991;4:131–144. [PubMed] [Google Scholar]
- Jenrow KA, Brown SL, Liu J, Kolozsvary A, Lapanowski K, Kim JH. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat Oncol. 2010;5:6. doi: 10.1186/1748-717X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolas T, Schreiber R, Laporte AM, Chastanet M, De Vry J, Glaser T, Adrien J, Hamon M. Are postsynaptic 5-HT1A receptors involved in the anxiolytic effects of 5-HT1A receptor agonists and in their inhibitory effects on the firing of serotonergic neurons in the rat? J Pharmacol Exp Ther. 1995;272:920–929. [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Plaznik A, Stefanski R. Intra-hippocampal buspirone in animal models of anxiety. Eur J Pharmacol. 1989;168:393–396. doi: 10.1016/0014-2999(89)90803-0. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Lucki I. Behavioral studies of serotonin receptor agonists as antidepressant drugs. J Clin Psychiatry. 1991;(52 Suppl):24–31. [PubMed] [Google Scholar]
- Mannoury la Cour C, El Mestikawy S, Hanoun N, Hamon M, Lanfumey L. Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol Pharmacol. 2006;70:1013–1021. doi: 10.1124/mol.106.022756. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Tsunekawa Y, Nomura T, Suto F, Matsumata M, Tsuchiya S, Osumi N. Differential proliferation rhythm of neural progenitor and oligodendrocyte precursor cells in the young adult hippocampus. PLoS One. 2011;6:e27628. doi: 10.1371/journal.pone.0027628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- Molliver ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, Grasby PM, Nutt DJ. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A. The importance of 5-HT1A receptor agonism in antipsychotic drug action: rationale and perspectives. Curr Opin Investig Drugs. 2010;11:802–812. [PubMed] [Google Scholar]
- Onksen JL, Briand LA, Galante RJ, Pack AI, Blendy JA. Running-induced anxiety is dependent on increases in hippocampal neurogenesis. Genes Brain Behav. 2012;11:529–38. doi: 10.1111/j.1601-183X.2012.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic, San Diego, Calif.; London: 2001. [Google Scholar]
- Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955:264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Rossi DV, Valdez M, Gould GG, Hensler JG. Chronic administration of venlafaxine fails to attenuate 5-HT1A receptor function at the level of receptor-G protein interaction. Int J Neuropsychopharmacol. 2006;9:393–406. doi: 10.1017/S1461145705005754. [DOI] [PubMed] [Google Scholar]
- Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57mice is anxiolytic across severalmeasures of anxiety. Behav. Brain Res. 2009;197:31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci U S A. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindelegger C, Lanzenberger R, Wadsak W, et al. Influence of escitalopram treatment on 5-HT 1A receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry. 2009;14:1040–1050. doi: 10.1038/mp.2008.35. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A. 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology. 2004;46:52–62. doi: 10.1016/j.neuropharm.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5-HT(1A) receptor binding and anxiety: a [(11)C]WAY-100635 PET investigation in healthy volunteers. Am J Psychiatry. 2001;158:1326–1328. doi: 10.1176/appi.ajp.158.8.1326. [DOI] [PubMed] [Google Scholar]
- Taylor DP. Buspirone, a new approach to the treatment of anxiety. Faseb J. 1988;2:2445–2452. doi: 10.1096/fasebj.2.9.2836252. [DOI] [PubMed] [Google Scholar]
- Traber J, Glaser T. 5-HT1A receptor-related anxiolytics. Trends in Pharmacological Sciences. 1987;8:432–437. [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H, Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]