Current therapeutic approaches to restore blood flow in stenotic blood vessels involve the use of percutaneous devices and coronary bypass surgery. In all procedures that disrupt the normal integrity of the blood vessels, there is an increased incidence of vessel luminal narrowing, termed restenosis. Restenosis, arbitrarily defined as greater than 50% narrowing of vessel diameter compared with the reference vessel, is modulated by genetic background and diseases that affect the cardiovascular system, including diabetes, hypertension, and hypercholesterolemia. In the 1970s, Andreas Gruntzig pioneered the use of transluminal dilatation of coronary arteries for symptomatic coronary artery disease and reported a 19% rate of restenosis (6 of 32 patients).1–3 Subsequent studies demonstrated a restenosis rate of approximately 33%.4 More than 25 years later, despite pharmacological and mechanical approaches to reduce the incidence of restenosis, it remains a significant problem, especially in high-risk patient groups, limiting overall success.
Restenosis after percutaneous intervention is characterized by platelet aggregation, release of growth factors, inflammatory cell infiltration, medial smooth muscle cell proliferation and migration, and extracellular matrix remodeling. The vascular response to injury depends not only on the cells within the vessels but is also modulated by circulating bone marrow–derived cells. Understanding the molecular mechanisms underlying the physiological healing response and the pathological restenosis response has been the focus of extensive investigations, which have led to the development of novel approaches to control the pathological formation of the neointima. In this review, we will focus on some of the molecular mechanisms responsible for the abnormal neointimal hyperplasia, specifically focusing on cell cycle and microRNA (miRNA) in the vascular smooth muscle.
Pathophysiology of Restenosis
Acute occlusion at the percutaneous intervention site within hours to days after the procedure is usually caused by an intimal flap, thrombus formation, subintimal hemorrhage extending into the media, or elastic recoil of the overstretched vessel wall. The incidence of acute occlusion after percutaneous intervention has been minimized by the use of intravascular stents and aggressive anticoagulation/antiplatelet treatment during the periprocedural period. Nevertheless, the overall rate of restenosis with bare-metal stents is approximately 20%.5 Drug-eluting stents have reduced the rate of restenosis, in most studies to <10%.
Animal models and human postmortem studies have shown that the mechanism of intimal hyperplasia is similar to wound healing.6 The wound-healing process can be divided into 3 phases: inflammatory phase (hours to days), granulation or cellular proliferation phase (days to weeks), and an extracellular matrix remodeling phase (months).6,7 After injury, there is deposition of platelets that release platelet-derived growth factor (PDGF) and other mitogenic factors that penetrate the vascular wall and trigger medial vascular smooth muscle cell proliferation and migration. The platelets also release chemokines, which initiate an inflammatory response. Over the first 2 weeks after injury, the vascular smooth muscle cells multiply 3 to 5 times, accounting for 90% of the ultimate intimal proliferation.
Endothelial Cells in Restenosis
A normal functioning endothelium is very important because it promotes vasodilatation and suppresses intimal hyperplasia by inhibiting thrombus formation, inflammation, and smooth muscle proliferation and migration. The endothelium provides a selectively permeable barrier that protects against circulating growth factors.8 Endothelial denudation and medial wall injury are the initial effects of balloon- and/or stent-induced injury and are important triggers of the wound “healing” program.
Vascular Smooth Muscle Cells
Vascular smooth muscle cells retain remarkable plasticity during postnatal development and can undergo dedifferentiation to a synthetic phenotype.9 This probably represents a survival advantage because it enables the efficient repair of the vasculature after injury. As in many evolutionarily conserved processes, these properties can be disadvantageous and can predispose to abnormal responses after injury, contributing to restenosis. Vascular smooth muscle progenitor cells have been identified in the bone marrow (multipotent vascular stem cell progenitors and mesenchymal stem cells), in the circulation (circulating progenitor cells), in the vessel wall (resident progenitors cells and mesangioblasts), and in extravascular sites.10
The normally quiescent smooth muscle cells within the medial layer of the vessel wall are activated to migrate and proliferate in response to increased stimulatory growth factors and cytokines (PDGF, interleukin-1, interleukin-6, and tumor necrosis factor-α) and reduced endothelium-derived inhibitory factors (nitric oxide, heparin sulfate proteoglycan). Some of the neointimal smooth muscle cells may also originate from the adventitial fibroblasts, which migrate to the intima and differentiate in myofibroblasts.11–13 Recent studies have challenged the concept14 that neointimal formation is dependent on the proliferation and migration of local endothelial and smooth muscle cells. It has been proposed that in models of postangioplasty restenosis, graft vasculopathy, and hyperlipidemia-induced atherosclerosis, smooth muscle progenitor cells are mobilized from the bone marrow and hone to sites of vascular injury, differentiating into smooth muscle cells.15,16 In these studies, a bone marrow or circulating cell marker has been found to colocalize with vascular smooth muscle cell markers in vascular lesions after arterial injury or atherosclerosis. Based on the species, type of injury, and the method of labeling, the percentage of bone marrow–derived cells within the neointima is between 20% and 66%. Although the selective control of vascular smooth muscle progenitors in the vessel wall may be an attractive therapeutic target, the concept is controversial, and more studies are required to define the role of progenitor cells in mediating vascular diseases.
Mechanisms of Smooth Muscle Cell Proliferation: The Cell Cycle
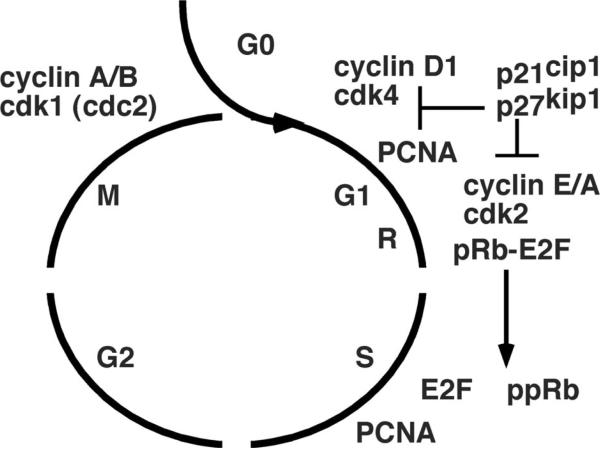
Smooth muscle cells progress through DNA replication and mitosis in a regulated series of cell-cycle events.17–20 Under normal conditions, quiescent smooth muscle cells are maintained in a nonproliferative phase (G0) (Figure 1). After vessel injury, smooth muscle cells enter a gap phase (G1), at which time factors necessary for DNA replication in the subsequent synthetic phase (S) are produced. After S phase, the cells enter another gap phase (G2), when proteins are synthesized for mitosis (M phase). Restriction points (R) at the G1-to-S and G2-to-M junctions ensure orderly progression through the cell cycle.
Figure 1.
Schematic of cell cycle. Cell cycle progression is dependent on the regulated expression and activation of a set of kinases (cyclin-dependent kinases, cdk) and cyclins, which phosphorylate proteins that regulate cell growth including the retinoblastoma protein (pRb indicates unphosphorylated; ppRb, phosphorylated form) at the restriction point (R) at the G1/S junction. Cdk inhibitors p27Kip1 and p21Cip1 delay cell cycle progression by inhibiting cdk activity.
Growth factors, such as PDGF, basic fibroblast growth factor, and insulin-like growth factor-1, stimulate cells to enter the cell cycle and propel them to reach the R point in the late G1 phase. Beyond this, cell cycle progression from the G2 phase to the M phase does not require further growth factor stimulation. On binding to their respective cell surface tyrosine kinase receptors, growth factors trigger cell cycle entry by transactivating nuclear factors such as c-fos and c-myc. These nuclear factors act as transcriptional factors and increase the expression of various cell cycle regulatory proteins.
The cell cycle is coordinated by the expression and activities of regulatory proteins. Cyclins and their respective cyclin-dependent kinases (CDK) form distinct complexes and are positive regulators of cell cycle progression. CDK phosphorylation of the retinoblastoma gene product (Rb) at the R point at G1-S junction is an important step in the progression through the cell cycle.18 Rb ordinarily binds to and inactivates the transcription factor E2F to maintain the cell in a quiescent state. Phosphorylation of Rb in late G1 releases E2F, which in turn enhances the expression of genes encoding regulatory proteins necessary for cell cycle progression through S, G2, and M.21
The CDK inhibitors (CDKIs) are critical negative regulators of the cell cycle.22 The CDKIs are structurally divided into 2 different families: the INK4 family (p14, p15, p16, p18 and p19) and the KIP/CIP family (p21, p27, and p57). For example, p27Kip1, which binds to and inhibits phase G1 cyclin/CDK complexes, is maintained at high levels in quiescent cells and declines to permit cell cycle progression. In the later phases of arterial healing, p27Kip1 is upregulated, which is associated with inhibition of cellular proliferation.23 Another CDKI, p21Cip1, is present at low levels in G0, accumulates in late G1, and provides counterbalance to increased cyclin/CDK activities. Apart from CDKIs, other indirect cell cycle inhibitors also contribute to the regulation of mitosis.
Overexpression of p27Kip1 results in cell cycle arrest in the G1 phase.24 Gene transfer of p27Kip1 or p21Cip1 into balloon-injured vessels significantly reduced vascular smooth muscle cell proliferation and neointimal formation.23,25 Conversely, a reduction in p27Kip1 levels (caused by gene deletion in mice) resulted in increased neointimal formation and inflammatory cell accumulation after mechanical vascular injury.26
Mechanisms of Smooth Muscle Cell Migration
In the noninjured vessel, vascular smooth muscle cells are nonmigratory because of a combination of several factors including the relative absence of stimulatory factors, their quiescence from a proliferative standpoint, and because the matrix is highly adhesive.27 There are many promigratory and antimigratory molecules, including small biogenic amines, peptides growth factors, cytokines, and extracellular matrix components.27 Blood flow, sheer stress, and matrix stiffness can also affect the migration of vascular smooth muscle. Vascular smooth muscle cell migration begins with the stimulation of cell surface receptors that activate signal transduction pathways, which trigger remodeling of the cytoskeleton, changes in the adhesiveness of the smooth muscle cell to the matrix, and activation of the motor proteins. Vascular smooth muscle cells extend lamellipodia toward the stimulus through actin polymerization.27 Focal contacts form just behind the leading edge to increase adhesion of the cell membrane to the matrix. Vascular smooth muscle cells also must degrade the focal contacts in the trailing edge. The actin cytoskeleton is regulated by numerous signaling pathways and molecules, including trimeric G proteins, small G proteins, lipid kinases, Ca2+-dependent kinases, Rho kinase,28 and mitogen-activated protein kinases.27,29 Promigratory stimuli that increase myoplasmic Ca2+ concentration activate myosin light chain kinase and myosin II.30
Vascular smooth muscle cell migration is also linked to the cell cycle and proliferation. Smooth muscle cells can migrate when in the G1 phase but not in later phases of the cell cycle.31 p21Cip1 and p27Kip1 are not only negative regulators of the cell cycles but also have important inhibitory effects on vascular smooth muscle migration. Overexpression of p27Kip1 and p21Cip1 reduced human vein endothelial cell and vascular smooth muscle cell migration in vitro.32,33 p27Kip1 inhibits the cellular changes such as lamellipodia formation and reorganization of actin filaments and focal adhesions that are associated with vascular smooth muscle cell migration.34 A p27Kip1 mutant that lacks CDK inhibitory activity failed to inhibit the proliferation and migration of vascular smooth muscle cells.34 p27Kip1 also affects the sensitivity to rapamycin's antimigratory effects in both vascular smooth muscle and endothelial cells (see below).
Role of MicroRNAs in Regulation of Vascular Smooth Muscle Cell Differentiation
Recent studies have demonstrated that microRNAs (miRNAs) are expressed in the vascular system and are involved in control of proliferation and differentiation of vascular smooth muscle cells.35 Each miRNA is able to regulate the expression of multiple target genes, frequently involved in the same cellular pathway. Conversely, target genes may be affected by more than 1 miRNA. It is presumed that thousands of human genes are targeted by miRNAs.36
Mature miRNAs are short, noncoding ribonucleic acid molecules, typically 22 nucleotides long, which bind to complementary sequences in the 3′ untranslated regions of target mRNA transcripts. PremiRNAs are exported from the nucleus to the cytoplasm, where they are processed by an RNAse II enzyme, Dicer. They are then bound to the miRNA-induced silencing complex, which contains 2 key proteins, argonaute 2 and transactivation-responsive RNA-binding protein, to form mature miRNA.37 The mature miRNA and miRNA-induced silencing complex binds to complementary sites in the mRNA transcripts and negatively regulate gene expression. MiRNA binding leads to either mRNA degradation or inhibition of translation, depending on whether the miRNAs bind to their mRNA targets with perfect complementarity or imperfect complementarity, respectively.38,39 An important characteristic of miRNA expression is tissue- and cell-specific expression patterns.40
Overexpression of miR-145 or miR-143 is sufficient to promote differentiation and inhibit proliferation of cultured vascular smooth muscle cells.41,42 Deficiency of miR-145 or miR-143 promoted the synthetic phenotype of vascular smooth muscle, with increased migration to PDGF.43,44 miR-221 and miR-222, expression of which can be transcriptionally induced by PDGF, are also implicated in vascular smooth muscle cell differentiation.45,46 Overexpression of miR-221 reduced differentiation and increased proliferation and migration of vascular smooth muscle. Reduction of miR-221 increased expression of smooth muscle differentiation markers and blocked the effects of PDGF on proliferation and migration. miR-221 targets the 3′ untranslated regions of c-Kit and p27Kip1 mRNA.
In response to vascular injury, the expression of miRNA is dynamically regulated. At 7 days after balloon injury of the rat carotid artery, 113 of 140 artery miRNAs were differentially expressed; 60 were upregulated and 53 were downregulated.47 At 14 days after injury, 110 of 140 miRNAs were differentially expressed and at 28 days after injury, 102 of the 140 artery miRNAs were differentially expressed. In particular, miR-21 had more than 5-fold increase compared with control. Depletion of miR-21 caused decreased cell proliferation and increased cell apoptosis. Local delivery of an antisense oligonucleotide to knockdown miR-21 inhibited neointima formation in rat carotid artery after angioplasty.47 Adenoviral-mediated gene transfer of miR-145, which is downregulated after injury,47 inhibited neointimal lesion formation in injured rat carotid arteries.42 Surprisingly, neointimal formation was significantly impaired in miR-143 knockout, mir-145 knockout, or double knockout mice, which is contrary to other reports.48 Knockdown of miR-221 and miR-222, which are both increased in proliferative vascular smooth muscle, inhibits vascular smooth muscle cell proliferation and neointimal formation in rat carotid artery after injury.45
In endothelial cells, miR-126 has been shown to be required for migration and proliferation of human umbilical vein endothelial cells in response to vascular endothelial growth factor stimulation.49 miR-126 functions in part by directly repressing negative regulators of the vascular endothelial growth factor pathway, including the Sprouty-related protein SPRED1 and phosphoinositol-3 kinase regulatory subunit 2 (PIK3R2/p85-beta),49 and by regulating vascular cell adhesion molecule 1 expression, which mediates leukocyte adherence to endothelial cells.50
Current Therapies Targeting Vascular Smooth Muscle Proliferation and Migration
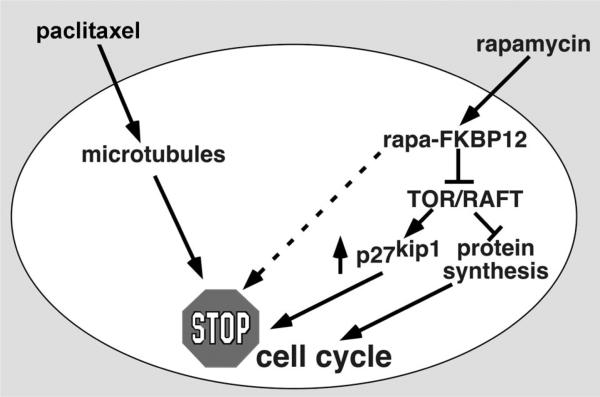
Currently available drug-eluting stent formulations deliver high concentrations of rapamycin, rapamycin analogues, or paclitaxel into the vessel wall (Figure 2). We will briefly review the mechanism of action of each.
Figure 2.
Schematic depicting the inhibitory effects of rapamycin and paclitaxel on smooth muscle proliferation.
Rapamycin
Rapamycin (AY-22,989, USAN name Sirolimus; Rapamune) is a macrocyclic triene antibiotic produced by the filamentous bacterium (Streptomycetes hygroscopius). Rapamycin was isolated from a soil sample from Easter Island (Rapa Nui).51 At Ayerst Research Laboratories, rapamycin was found to have antifungal properties. However, the immunosuppressive activity of rapamycin precluded its development as an antibiotic/antifungal agent. Subsequently, rapamycin was shown to have potent antitumor activity as well.52 Because it is extremely lipophilic, it easily passes through cell membranes.
The receptor for rapamycin in all eukaryotes is a 12 000-Dalton cytosolic protein, known as FKBP12 (FK506 binding protein, 12 kDa), which is a member of the immunophilin family of proteins possessing peptidyl-prolyl cis-trans isomerase activity (PPIase). A rapamycin-FKBP12 “gain of function” complex interacts with the target of rapamycin (TOR) proteins to potently inhibit signaling to downstream targets (Figure 2). A mammalian homologue has been cloned from several species and has been termed mTOR, FRAP (FKBP12 and rapamycin associated protein), RAFT (rapamycin and FKBP12 target), SEP (sirolimus effector protein), or RAPT (rapamycin target).53–56
The mTOR complex 1 (mTORC1) regulates growth through effectors such as S6K1 and 4E-BP1. The mTOR complex 2 (mTORC2) regulates the prosurvival kinase Akt/PKB by phosphorylating it on Ser473, which is necessary for full activation of Akt, along with PDK1 phosphorylation of Thr308.57 Rapamycin binds to the intracellular protein FKBP12 to generate a drug-receptor complex that binds to and inhibits mTORC1. FKBP12-rapamycin does not bind to mTORC2, suggesting that mTORC2 is not directly inhibited by rapamycin. Rapamycin suppresses the assembly and function of mTORC2, which inhibits the phosphorylation of Akt Ser473.58 Thus, rapamycin and its analogues are universal inhibitors of mTORC1 and S6K1 and cell-type specific inhibitors of mTORC2 and Akt. Akt stands at the crossroads of growth factor and metabolic signaling and is strongly activated by insulin.59
Rapamycin inhibits phosphorylation of the ribosomal S6 kinases (S6K1 and S6K2). S6Ks regulate translation of mRNAs possessing a 5′ terminal oligopyrimidine tract (5′TOP), a stretch of 4 to 14 pyrimidines found at the extreme 5′ terminus of some protein translation machinery mRNAs (reviewed in Reference 60).60 Rapamycin has also been shown to inhibit phosphorylation of 4E-BPs (eukaryotic initiation factor 4E binding protein), which then can compete with eIF4G proteins for binding with eIF4E.61 In addition, rapamycin can also modulate the phosphorylation of eIF4B and the translation elongation factor eEF2. Additional targets involved in translational control may also be downstream of TOR. Depletion of amino acids or glucose leads to rapid dephosphorylation of 4E-BP1 (PHAS-I) and p70 S6 kinase (S6K)-1.61
Rapamycin also significantly reduces the kinase activity of the cyclin-dependent kinase (cdk) 4/cyclin D and cdk2/cyclinE complexes. This is achieved by increasing the cdk inhibitor p27Kip1 in various cell lines.62–64 The cdk inhibitor p27Kip1 inhibits cyclin E/cdk2 kinase activity by forming a complex (cyclinE/cdk2-p27Kip1), leading to inhibition of phosphorylation of the retinoblastoma protein (pRb), as well as inhibition of the dissociation of the pRb:E2F complex. Loss of the ability to upregulate p27Kip1 in BC3H1 myogenic cells is associated with rapamycin resistance and apoptosis on serum withdrawal.63
Rapamycin inhibits intimal hyperplasia after both alloimmune and mechanical injury in rats.65,66 The mechanism of this inhibition was demonstrated to involve a direct effect of rapamycin on BC3H1 (myogenic cells)67 and smooth muscle cell proliferation and migration.68,69 The inhibition of cellular proliferation is associated with a reduction in the activity of CDKs and in retinoblastoma protein phosphorylation (pRb), leading to G1- to S-phase transition arrest.68 FK506 (tacrolimus) and FK520 (an analog of FK506) failed to inhibit either vascular smooth muscle cell proliferation or migration and in fact antagonize rapamycin's antiproliferative and antimigratory properties through competition with FKBP12.68,69
Mitogen-induced downregulation of p27Kip1 is blocked by rapamycin. In p27Kip1 null mice, we demonstrated relative rapamycin resistance in mixed embryonic fibroblasts (MEF) and splenic T-lymphocytes.63 In rapamycin-resistant myogenic cells (RR), constitutively low levels of p27Kip1 were observed, which did not increase with serum withdrawal and rapamycin. The lack of p27Kip1 reduces rapamycin-mediated inhibition of smooth muscle cell migration, which suggests an important role for p27Kip1 in the signaling pathway(s) regulating smooth muscle cell migration.70,71 Silencing of p27Kip1 also blocked the inhibitory effects of rapamycin on migration of endothelial cells.72 Decreased levels of p27Kip1 in the vessel wall have been associated with markedly increased neointimal response after wire-injury.26 This study by the Nabel group demonstrated that the increased neointimal formation was due to lack of p27Kip1 in cells within the vessel wall as well as bone marrow–derived cells. Thus, regulation of p27Kip1 in nonvascular cells by rapamycin may be important in modulating the arterial response to injury. There is some controversy regarding the requirement for p27Kip1 in mediating rapamycin's inhibition of neointimal formation in vivo, with 1 study reporting that neointimal formation in p27Kip1 null mice was not enhanced after wire injury and that rapamycin effectively reduced neointimal formation in p27Kip1 null mice.73
Systemic (intramuscular) administration of rapamycin (0.5 mg/kg×3 days before injury and 0.25 mg/kg for 14 days) was shown to significantly inhibit intimal proliferation in a porcine model of coronary artery balloon injury.74 Coronary arteries were analyzed at 4 weeks after injury revealing that rapamycin inhibited coronary intimal proliferation (control, 63±3.4% versus rapamycin, 36±4.5%, P<0.001).74 Rapamycin-coated stents were shown to inhibit restenosis in a porcine model of balloon injury/stent implantation.75 These preclinical data led to studies in humans demonstrating marked reduction of restenosis after rapamycin-coated stent implantation.76–78 Stents eluting analogs of rapamycin, including zotarolimus and everolimus, have also been shown to be efficacious in reducing restenosis after stent implantation.79,80
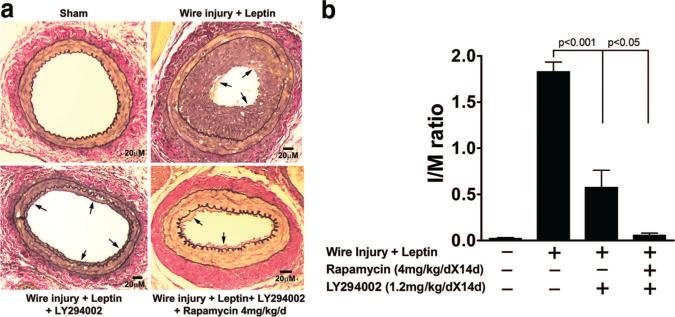
Diabetic patients may remain at increased risk of developing in-stent restenosis, despite the use of rapamycin-coated stents.81,82 Hyperleptinemia, which is seen in diabetes and metabolic syndrome, is a risk factor for stroke and progression of coronary artery disease.83–85 Leptin can promote platelet aggregation, inflammation, endothelial dysfunction, and vascular smooth muscle proliferation and migration, which are involved in atherogenesis.86 Leptin activates the mTOR-signaling pathway in primary murine vascular smooth muscle cells, stimulating proliferation in vitro.87 Exogenous leptin, administered at levels comparable to those found in obese humans, promoted neointimal hyperplasia and significantly increased the dose of rapamycin required for effective inhibition of neointimal formation (Figure 3). Combination therapy with a PI3K inhibitor, LY294002, and rapamycin effectively inhibited the leptin-enhanced neointimal hyperplasia.87 These data suggest that in the setting of hyperleptinemia, higher doses of an mTOR inhibitor or combination therapy with mTOR and PI3K inhibitors may be more efficacious in limiting neointimal formation.
Figure 3.
Combination of PI3K inhibitor (LY294002) and mTOR inhibitor (rapamycin) inhibits leptin-induced neointimal hyperplasia in mice. a, Femoral artery injury. Micrographs of leptin-induced neointimal formation (arrows) in injured femoral arteries after treatment with LY294002 and/or rapamycin. Elastic–van Gieson staining to highlight elastic laminae (black rings) and collagen (red). b, Intima:media (I/M) ratios. Tukey probability values are over brackets. Adapted from Shan J, Nguyen TB, Totary-Jain H, Dansky H, Marx SO, Marks AR. Leptin-enhanced neointimal hyperplasia is reduced by mTOR and PI3K inhibitors. Proc Natl Acad Sci USA. 2008;105:19006 –19011.87
As discussed above, reendothelialization is an important component of the healing process after vascular injury. Rapamycin has been shown to prevent vascular endothelial growth factor–mediated and serum-stimulated proliferation of endothelial cells in vitro.88,89 Additionally, rapamycin inhibits endothelial cell migration in a p27Kip1-dependent manner.72 The first-generation drug-eluting stents are highly effective at blocking neointimal hyperplasia but also exhibit reduced stent endothelialization. These observations may be due to rapamycin's direct effects on endothelial proliferation and migration.
Paclitaxel
Paclitaxel was isolated from the bark of the Western yew tree in 1971.90 Paclitaxel induces cell cycle arrest in vascular smooth muscle and inhibits neointimal formation in animal models.91 Paclitaxel is extremely lipophilic, which promotes the rapid cellular uptake of the drug. Paclitaxel triggers the assembly of microtubules into extraordinarily stable yet disorganized and dysfunctional polymers.92,93 Unlike colchicine, which inhibits microtubule assembly, paclitaxel shifts the microtubule equilibrium toward assembly and polymerization. Several important biological processes are inhibited by paclitaxel, including mitotic spindle formation during cell division, intracellular transport, maintenance of cellular shape, and motility.94 Paclitaxel inhibits smooth muscle cell proliferation and migration in vitro and in vivo.95,96 Stents eluting paclitaxel reduced neointimal formation in a porcine coronary artery injury model. The arteries demonstrated incomplete healing with the late persistence of macrophages and fibrin deposition. These findings indicated the need for tightly controlled drug release and a narrow toxic-therapeutic window.8 Clinical trials with the paclitaxel-eluting stent have shown efficacy in preventing in-stent restenosis.97–100
Concluding Remarks
Smooth muscle proliferation and migration after percutaneous intervention represent the end result of natural healing processes triggered by vascular injury. Vascular smooth muscle cell proliferation, especially after stent implantation, plays a critical role in neointimal hyperplasia through cellular expansion and extracellular matrix deposition. Elucidating the molecular mechanisms responsible for smooth muscle cell proliferation has led to the development of novel therapeutic approaches, including rapamycin- and paclitaxel-eluting stents that have significantly improved the care of patients with coronary artery disease. To address the concerns about the potentially increased incidence of stent thrombosis in patients treated with drug-eluting stents, newer stents and coronary devices have been developed such as drug-eluting stents with biodegradable polymers, drug-eluting stents that are polymer-free, stents with novel coatings, completely biodegradable stents, bifurcation stents, and drug-eluting balloons. Many of these are currently undergoing preclinical and clinical trials. Newer, potentially more efficacious, more specific, and safer approaches may be on the horizon. Although targeting miRNAs represents a potential therapeutic strategy to specifically inhibit vascular smooth muscle proliferation and migration, to inhibit vascular inflammation, or to enhance vascular reendothelialization, additional work is required to identify precise targets and improve delivery.
Footnotes
Disclosures
None.
References
- 1.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury, I: smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 2.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- 3.Ip JH, Fuster V, Israel D, Badimon L, Badimon J, Chesebro JH. The role of platelets, thrombin and hyperplasia in restenosis after coronary angioplasty. J Am Coll Cardiol. 1991;17:77B–88B. doi: 10.1016/0735-1097(91)90942-3. [DOI] [PubMed] [Google Scholar]
- 4.Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998;98:82–89. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 5.Kim MS, Dean LS. In-Stent Restenosis. Cardiovasc Ther. 2010 Apr 9; doi: 10.1111/j.1755-5922.2010.00155.x. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 6.Forrester JS, Fishbein M, Helfant R, Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991;17:758–769. doi: 10.1016/s0735-1097(10)80196-2. [DOI] [PubMed] [Google Scholar]
- 7.Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 8.Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257–2273. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ Res. 1986;58:427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- 10.Orlandi A, Bennett M. Progenitor cell-derived smooth muscle cells in vascular disease. Biochem Pharmacol. 2010;79:1706–1713. doi: 10.1016/j.bcp.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox JN, Cipolla GD, Martin FH, Simonet L, Dunn B, Ross CE, Scott NA. Contribution of adventitial myofibroblasts to vascular remodeling and lesion formation after experimental angioplasty in pig coronary arteries. Ann N Y Acad Sci. 1997;811:437–447. doi: 10.1111/j.1749-6632.1997.tb52025.x. [DOI] [PubMed] [Google Scholar]
- 13.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 14.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 15.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 16.Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci U S A. 2003;100:4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 18.Sherr CJ. The ins and outs of RB: coupling gene expression to the cell cycle clock. Trends Cell Biol. 1994;4:15–18. doi: 10.1016/0962-8924(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 19.Sherr CJ. Growth factor-regulated G1 cyclins. Stem Cells. 1994;12(Suppl 1):47–55. [PubMed] [Google Scholar]
- 20.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 21.DeGregori J, Kowalik T, Nevins JR. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 23.Tanner FC, Boehm M, Akyurek LM, San H, Yang ZY, Tashiro J, Nabel GJ, Nabel EG. Differential effects of the cyclin-dependent kinase inhibitors p27(Kip1), p21(Cip1), and p16(Ink4) on vascular smooth muscle cell proliferation. Circulation. 2000;101:2022–2025. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- 24.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 25.Chang MW, Barr E, Lu MM, Barton K, Leiden JM. Adenovirus-mediated over-expression of the cyclin/cyclin-dependent kinase inhibitor, p21 inhibits vascular smooth muscle cell proliferation and neointima formation in the rat carotid artery model of balloon angioplasty. J Clin Invest. 1995;96:2260–2268. doi: 10.1172/JCI118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehm M, Olive M, True AL, Crook MF, San H, Qu X, Nabel EG. Bone marrow-derived immune cells regulate vascular disease through a p27(Kip1)-dependent mechanism. J Clin Invest. 2004;114:419–426. doi: 10.1172/JCI20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007;100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 28.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–C668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graf K, Xi XP, Yang D, Fleck E, Hsueh WA, Law RE. Mitogen-activated protein kinase activation is involved in platelet-derived growth factor-directed migration by vascular smooth muscle cells. Hypertension. 1997;29:334–339. doi: 10.1161/01.hyp.29.1.334. [DOI] [PubMed] [Google Scholar]
- 30.Scherberich A, Campos-Toimil M, Ronde P, Takeda K, Beretz A. Migration of human vascular smooth muscle cells involves serum-dependent repeated cytosolic calcium transients. J Cell Sci. 2000;113(Pt 4):653–662. doi: 10.1242/jcs.113.4.653. [DOI] [PubMed] [Google Scholar]
- 31.Fukui R, Amakawa M, Hoshiga M, Shibata N, Kohbayashi E, Seto M, Sasaki Y, Ueno T, Negoro N, Nakakoji T, Ii M, Nishiguchi F, Ishihara T, Ohsawa N. Increased migration in late G(1) phase in cultured smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C999–C1007. doi: 10.1152/ajpcell.2000.279.4.C999. [DOI] [PubMed] [Google Scholar]
- 32.Goukassian D, Diez-Juan A, Asahara T, Schratzberger P, Silver M, Murayama T, Isner JM, Andres V. Overexpression of p27(Kip1) by doxycycline-regulated adenoviral vectors inhibits endothelial cell proliferation and migration and impairs angiogenesis. FASEB J. 2001;15:1877–1885. doi: 10.1096/fj.01-0065com. [DOI] [PubMed] [Google Scholar]
- 33.Fukui R, Shibata N, Kohbayashi E, Amakawa M, Furutama D, Hoshiga M, Negoro N, Nakakouji T, Ii M, Ishihara T, Ohsawa N. Inhibition of smooth muscle cell migration by the p21 cyclin-dependent kinase inhibitor (Cip1). Atherosclerosis. 1997;132:53–59. doi: 10.1016/s0021-9150(97)00086-5. [DOI] [PubMed] [Google Scholar]
- 34.Diez-Juan A, Andres V. Coordinate control of proliferation and migration by the p27Kip1/cyclin-dependent kinase/retinoblastoma pathway in vascular smooth muscle cells and fibroblasts. Circ Res. 2003;92:402–410. doi: 10.1161/01.RES.0000059306.71961.ED. [DOI] [PubMed] [Google Scholar]
- 35.Parmacek MS. MicroRNA-modulated targeting of vascular smooth muscle cells. J Clin Invest. 2009;119:2526–2528. doi: 10.1172/JCI40503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 37.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 40.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 41.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 48.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic, II: fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28:727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 52.Sehgal SN, Bansbach CC. Rapamycin: in vitro profile of a new immunosuppressive macrolide. Ann N Y Acad Sci. 1993;685:58–67. doi: 10.1111/j.1749-6632.1993.tb35852.x. [DOI] [PubMed] [Google Scholar]
- 53.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 54.Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci USA. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 56.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 57.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 58.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 59.Kohn AD, Kovacina KS, Roth RA. Insulin stimulates the kinase activity of RAC-PK, a pleckstrin homology domain containing ser/thr kinase. EMBO J. 1995;14:4288–4295. doi: 10.1002/j.1460-2075.1995.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas G. The S6 kinase signaling pathway in the control of development and growth. Biol Res. 2002;35:305–313. doi: 10.4067/s0716-97602002000200022. [DOI] [PubMed] [Google Scholar]
- 61.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 62.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 63.Luo Y, Marx SO, Kiyokawa H, Koff A, Massague J, Marks AR. Rapamycin resistance tied to defective regulation of p27Kip1. Mol Cell Biol. 1996;16:6744–6751. doi: 10.1128/mcb.16.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawamata S, Sakaida H, Hori T, Maeda M, Uchiyama T. The upregulation of p27Kip1 by rapamycin results in G1 arrest in exponentially growing T-cell lines. Blood. 1998;91:561–569. [PubMed] [Google Scholar]
- 65.Morris RE, Cao W, Huang X, Gregory CR, Billingham ME, Rowan R, Shorthouse RA. Rapamycin (Sirolimus) inhibits vascular smooth muscle DNA synthesis in vitro and suppresses narrowing in arterial allografts and in balloon-injured carotid arteries: evidence that rapamycin antagonizes growth factor action on immune and nonimmune cells. Transplant Proc. 1995;27:430–431. [PubMed] [Google Scholar]
- 66.Gregory CR, Huie P, Billingham ME, Morris RE. Rapamycin inhibits arterial intimal thickening caused by both alloimmune and mechanical injury. Its effect on cellular, growth factor, and cytokine response in injured vessels. Transplantation. 1993;55:1409–1418. doi: 10.1097/00007890-199306000-00037. [DOI] [PubMed] [Google Scholar]
- 67.Jayaraman T, Marks AR. Rapamycin-FKBP12 blocks proliferation, induces differentiation, and inhibits cdc2 kinase activity in a myogenic cell line. J Biol Chem. 1993;268:25385–25388. [PubMed] [Google Scholar]
- 68.Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- 69.Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB, Marks AR. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98:2277–2283. doi: 10.1172/JCI119038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun J, Marx SO, Chen HJ, Poon M, Marks AR, Rabbani LE. Role for p27(Kip1) in vascular smooth muscle cell migration. Circulation. 2001;103:2967–2972. doi: 10.1161/01.cir.103.24.2967. [DOI] [PubMed] [Google Scholar]
- 71.Marx SO, Marks AR. Bench to bedside: the development of rapamycin and its application to stent restenosis. Circulation. 2001;104:852–855. doi: 10.1161/01.cir.104.8.852. [DOI] [PubMed] [Google Scholar]
- 72.Moss SC, Lightell DJ, Jr, Marx SO, Marks AR, Woods TC. Rapamycin regulates endothelial cell migration through regulation of the cyclin-dependent kinase inhibitor p27Kip1. J Biol Chem. 2010;285:11991–11997. doi: 10.1074/jbc.M109.066621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roque M, Reis ED, Cordon-Cardo C, Taubman MB, Fallon JT, Fuster V, Badimon JJ. Effect of p27 deficiency and rapamycin on intimal hyperplasia: in vivo and in vitro studies using a p27 knockout mouse model. Lab Invest. 2001;81:895–903. doi: 10.1038/labinvest.3780298. [DOI] [PubMed] [Google Scholar]
- 74.Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, Chesebro J, Fallon J, Fuster V, Marks A, Badimon JJ. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99:2164–2170. doi: 10.1161/01.cir.99.16.2164. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki T, Kopia G, Hayashi S, Bailey LR, Llanos G, Wilensky R, Klugherz BD, Papandreou G, Narayan P, Leon MB, Yeung AC, Tio F, Tsao PS, Falotico R, Carter AJ. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001;104:1188–1193. doi: 10.1161/hc3601.093987. [DOI] [PubMed] [Google Scholar]
- 76.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 77.Sousa JE, Costa MA, Abizaid A, Abizaid AS, Feres F, Pinto IM, Seixas AC, Staico R, Mattos LA, Sousa AG, Falotico R, Jaeger J, Popma JJ, Serruys PW. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103:192–195. doi: 10.1161/01.cir.103.2.192. [DOI] [PubMed] [Google Scholar]
- 78.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 79.Ruygrok PN, Desaga M, Van Den Branden F, Rasmussen K, Suryapranata H, Dorange C, Veldhof S, Serruys PW. One year clinical follow-up of the XIENCE V Everolimus-eluting stent system in the treatment of patients with de novo native coronary artery lesions: the SPIRIT II study. EuroIntervention. 2007;3:315–320. doi: 10.4244/eijv3i3a58. [DOI] [PubMed] [Google Scholar]
- 80.Leon MB, Kandzari DE, Eisenstein EL, Anstrom KJ, Mauri L, Cutlip DE, Nikolsky E, O'Shaughnessy C, Overlie PA, Kirtane AJ, McLaurin BT, Solomon SL, Douglas JS, Jr, Popma JJ. Late safety, efficacy, and cost-effectiveness of a zotarolimus-eluting stent compared with a paclitaxel-eluting stent in patients with de novo coronary lesions: 2-year follow-up from the ENDEAVOR IV trial (Randomized, Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Taxus Paclitaxel-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). J Am Coll Cardiol Cardiovasc Interv. 2009;2:1208–1218. doi: 10.1016/j.jcin.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 81.Moussa I, Costa RA, Leon MB, Lansky AJ, Lasic Z, Cristea E, Trubelja N, Carlier SG, Mehran R, Dangas GD, Weisz G, Kreps EM, Collins M, Stone GW, Moses JW. A prospective registry to evaluate sirolimuseluting stents implanted at coronary bifurcation lesions using the “crush technique.”. Am J Cardiol. 2006;97:1317–1321. doi: 10.1016/j.amjcard.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 82.Scheen AJ, Warzee F, Legrand VM. Drug-eluting stents: meta-analysis in diabetic patients. Eur Heart J. 2004;25:2167–2169. doi: 10.1016/j.ehj.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 83.Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, Gami AS, Sert Kuniyoshi FH, Wolk R, Somers VK. Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am J Cardiol. 2007;100:234–239. doi: 10.1016/j.amjcard.2007.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, Sattar N. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 85.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819–1824. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 86.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Shan J, Nguyen TB, Totary-Jain H, Dansky H, Marx SO, Marks AR. Leptin-enhanced neointimal hyperplasia is reduced by mTOR and PI3K inhibitors. Proc Natl Acad Sci U S A. 2008;105:19006–19011. doi: 10.1073/pnas.0809743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu Y, Sato JD. MAP kinases, phosphatidylinositol 3-kinase, and p70 S6 kinase mediate the mitogenic response of human endothelial cells to vascular endothelial growth factor. J Cell Physiol. 1999;178:235–246. doi: 10.1002/(SICI)1097-4652(199902)178:2<235::AID-JCP13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 89.Vinals F, Chambard JC, Pouyssegur J. p70 S6 kinase-mediated protein synthesis is a critical step for vascular endothelial cell proliferation. J Biol Chem. 1999;274:26776–26782. doi: 10.1074/jbc.274.38.26776. [DOI] [PubMed] [Google Scholar]
- 90.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents, VI: the isolation and structure of taxol, a novel anti-leukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 91.Herdeg C, Oberhoff M, Baumbach A, Blattner A, Axel DI, Schroder S, Heinle H, Karsch KR. Local paclitaxel delivery for the prevention of restenosis: biological effects and efficacy in vivo. J Am Coll Cardiol. 2000;35:1969–1976. doi: 10.1016/s0735-1097(00)00614-8. [DOI] [PubMed] [Google Scholar]
- 92.Arnal I, Wade RH. How does taxol stabilize microtubules? Curr Biol. 1995;5:900–908. doi: 10.1016/s0960-9822(95)00180-1. [DOI] [PubMed] [Google Scholar]
- 93.Caplow M, Shanks J, Ruhlen R. How taxol modulates microtubule disassembly. J Biol Chem. 1994;269:23399–23402. [PubMed] [Google Scholar]
- 94.Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 95.Sollott SJ, Cheng L, Pauly RR, Jenkins GM, Monticone RE, Kuzuya M, Froehlich JP, Crow MT, Lakatta EG, Rowinsky EK. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest. 1995;95:1869–1876. doi: 10.1172/JCI117867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Axel DI, Kunert W, Goggelmann C, Oberhoff M, Herdeg C, Kuttner A, Wild DH, Brehm BR, Riessen R, Koveker G, Karsch KR. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–645. doi: 10.1161/01.cir.96.2.636. [DOI] [PubMed] [Google Scholar]
- 97.Grube E, Silber S, Hauptmann KE, Mueller R, Buellesfeld L, Gerckens U, Russell ME. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107:38–42. doi: 10.1161/01.cir.0000047700.58683.a1. [DOI] [PubMed] [Google Scholar]
- 98.Colombo A, Drzewiecki J, Banning A, Grube E, Hauptmann K, Silber S, Dudek D, Fort S, Schiele F, Zmudka K, Guagliumi G, Russell ME. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788–794. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]
- 99.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004;109:1942–1947. doi: 10.1161/01.CIR.0000127110.49192.72. [DOI] [PubMed] [Google Scholar]
- 100.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]