Abstract
Background
Intrauterine growth restriction (IUGR) is a fetal condition characterized by growth-rate reduction. Afflicted fetuses tend to display abnormalities in heart rate.
Objective
To study the differences in the heart-rate variability of low-risk fetuses and IUGR fetuses during different behavioral states.
Methods
A total of 40 fetal magnetocardiograms were recorded from 20 low-risk and 20 IUGR fetuses using a 151-sensor SQUID-array system. The maternal cardiac signals were attenuated using signal-space projection. Fetal R waves were identified using an adaptive Hilbert transform approach and fetal heart rate calculated. In each three-minute window, the heart rate was classified into patterns reflective of quiet sleep (pattern A) and active sleep (pattern B) using the criteria of Nijhuis. Two adjacent 3-minute windows exhibiting the same pattern were selected for analysis from every dataset. Heart-rate variability in that 6-minute window was characterized using three measures, Standard Deviation of Normal to Normal (SDNN), Root Mean Square of Successive Differences (RMSSD) and Phase Plane Area (PPA).
Results
All three measures tended to be lower in the IUGR group compared to the low-risk group. However, when the measures were analyzed in patterns, only PPA showed significant difference between the risk groups in Pattern A, where as both PPA and SDNN showed highly significant risk-group differences in Pattern B. RMSSD did not show any significant risk-group difference.
Conclusion
The result signifies that the heart-rate variability of IUGR fetuses is different from that of low-risk fetuses, and only PPA was able to capture the HRV differences in both quiet and active states. The difference between these two groups of fetuses shows that the fetal-activity states are potential confounders when characterizing heart-rate variability.
Introduction
Intrauterine growth restriction (IUGR) is a condition characterized by reduction in fetal growth rate [1]. Fetuses with IUGRare identified by having estimated weights below the 10th percentile for gestational age (GA) by ultrasound measurement[2]. IUGR increases morbidity and mortality among premature neonates [3]. Maternal conditions such as chronic hypertension and preeclampsia often result in compromised placental function and, therefore, a decrease in fetal nutrition and inadequate transplacental delivery of oxygen (hypoxemia)[4]. When these conditions are severe, the fetus develops IUGR. During hypoxemia, the fetal cardiovascular-system function becomes impaired, resulting in abnormalities in heart-rate characteristics such as reduction in variability and amplitude of heart rate accelerations and movements [1,4,5].
The human fetus undergoes transitions through different characteristics of quiet and active sleep cycles with increasing gestational age; this is indicative of maturation of the autonomic nervous system [6]. In order to improve the assessment of fetal well-being, Nijhuis [7] proposed the concept of classifying fetal behavior states. To define these states he included the variability in the heart rate in combination with eye and body movements. In the process of characterizing the fetal state, Nijhuis defined and labeled these heart rate patterns as A, B, C and D. Each of these FHR patterns is based on the oscillation bandwidth and the absence or presence of acceleration. Patterns A and C are stable patterns with no or infrequent accelerations with C exhibiting wider oscillation bandwidth than A. Pattern B also has wider oscillation bandwidth but with frequent accelerations during movements whereas D is unstable with large and long-lasting accelerations [7,8].Sleep cycles and their dampening effect on heart-rate patterns are one of the major confounders in the study of fetal HRV. Since the heart rate’s characteristics are different for the quiet and active sleep states, it is important to analyze HRV based on fetal heart rate patterns. It has been shown that better characterization of HRV can be achieved if viewed in the light of fetal heart-rate patterns [6]. Thus HRV in conjunction with pattern classification may provide a better insight into fetal well-being.
Over the past few years, fetal magnetocardiographic (fMCG) recordings have been used to obtain the heart rate parameters [9–11]. It has been shown and is well documented that the fMCG is capable of accurately and reliably detecting cardiac rhythm [12–14]. In general, the peak of the QRS is detected to acquire the RR intervals and calculate the heart rate [13]. In our recent work we introduced a new HRV measure, Phase Plane Area (PPA) and applied it to fMCG recordings[15]. PPA is a non-linear metric based on the concept of Poincaré analysis that is used to characterize the recurrence property of trajectories in the phase space. We compared the ability of PPA and other standard HRV measures such as standard deviation of normal to normal interval (SDNN) and root mean square of successive differences (RMSSD) in distinguishing the different heart patterns. The results of the study showed that PPA performed markedly better in identifying the patterns A, B and D in fetuses that were defined as low-risk and exhibiting normal growth.
In this study, we use the same three measures to study the differences in the heart-rate variability between IUGR and low-risk fetuses. Although the heart-rate variability of IUGR and normal fetuses has been investigated and compared in the past using SDNN and RMSSD, the results reported from these studies differed based on severity of IUGR and fetal state [16,17]. In this study we wanted to investigate if the PPA, with its shown ability to distinguish fetal heart rate patterns, would also prove to be a better method to study the differences in the HRV characteristics between the two groups. In order to accomplish this, we defined the status variable called Pattern, to indicate visually scored fetal heart rate pattern during a given 6-minute window. We then we analyzed the three HRV measures adjusting for Pattern and gestational age.
Methods
Subjects
We obtained 40 fetal magnetocardiogram (fMCG) datasets, 20 from low-risk fetuses and the other 20from IUGR fetuses. The gestational ages ranged from 30 weeks GA to 38 weeks GA in both groups. The duration of the recordings lasted between 10 and 30 minutes. The sampling rate was 312.5 Hz [18,19]. This study was approved by the University of Arkansas for Medical Sciences institutional review board, and all study subjects gave their informed written consent to participate. The exclusion criteria for study groups were fetal heart-related complications during pregnancy such as fetal arrhythmia or fetal cardiac-conduction defects and a history of drugs known to cause abnormal fetal heart-rate tracings. For the IUGR condition, fetuses having estimated weights below the 10th percentile for gestational age by ultrasound measurement were selected. The research protocol included the performance of the following ultrasound measurements at each visit: fetal biparietal diameter, head circumference (HC), and abdominal circumference (AC). The IUGR fetuses were recorded serially until delivery and a continued reduction in growth rate was observed in each case. For this study, only the recording of the last IUGR visit was chosen in order to minimize the imbalance in GA distributions between IUGR and low-risk fetuses.Table 1 shows the growth measurements of IUGR fetuses at first diagnosis, and HC and AC at the time of last fMCG measurement.
Table 1.
Growth measurements of IUGR fetuses at first diagnosis and HC and AC at the time of last FMCG measurement.
| IUGR PatID |
US GA and growth % when first diagnosed in clinic |
Actual (by LMP or early US) GA |
Head Circumference (cm) with corresponding US GA estimate |
Abdominal Circumference (cm) with corresponding US GA estimate |
|---|---|---|---|---|
| hr66 | 34 weeks | 38 weeks 5 days | 32.64 (36 weeks 3 days) | 30.68 (34 weeks 5 days) |
| 0 day b3% | ||||
| hr77 | 27 weeks | 32 weeks 5 days | 29.45 (32 weeks 1 day) | NM* |
| 0 day b3% | ||||
| hr78 | 32 weeks | 35 weeks 3 days | 30.80 (33 weeks 6 days) | 29.77 (33 weeks 6 days) |
| 2 days 8% | ||||
| hr79 | 31 weeks | 35 weeks 0 days | NM* | NM* |
| 1 day 3% | ||||
| hr82 | 33 weeks | 38 weeks 0 days | 32.32 (36 weeks 0 days) | 33.08 (37 weeks 1 day) |
| 6 days 9.3% | ||||
| hr110 | 31 weeks | 33 weeks 2 days | NM* | NM* |
| 1 day 9% | ||||
| hr128 | 30 weeks | 36 weeks 3 days | 27.75 (30 weeks 3 days) | 29.21 (33 weeks 1 day) |
| 1 day b3% | ||||
| hr130 | 23 weeks | 32 weeks 4 days | 30.12 (33 weeks 3 days) | 28.54 (32 weeks 4 days) |
| 2 days 7% | ||||
| hr137 | 33 weeks | 35 weeks 1 day | 29.68 (32 weeks 6 days) | 31.24 (35 weeks 1 day) |
| 5 days <2% | ||||
| hr139 | 28 weeks | 32 weeks 0 days | 28.61 (31 weeks 4 days) | 22.80 (27 weeks 1 day) |
| 6 days b3% | ||||
| hr141 | 30 weeks | 33 weeks 4 days | 28.62 (31 weeks 2 days) | 28.41 (32 weeks 2 days) |
| 1 day 9% | ||||
| hr143 | 30 weeks | 37 weeks 1day | 31.59 (35 weeks 3 days) | 28.60 (32 weeks 4 days) |
| 6 days b3% | ||||
| hr155 | 30 weeks | 32 weeks 6 days | 28.30 (31 weeks 0 days) | 26.00(30 weeks 1 day) |
| 5 days b3% | ||||
| hr158 | 27 weeks | 32 weeks 5 days | 27.10 (29 weeks 4 days) | 22.79 (27 weeks 1 day) |
| 3 days 3% | ||||
| hr164 | 28 weeks | 33 weeks 4 days | 29.11 (32 weeks 1 day) | 21.82 (26 weeks 2 days) |
| 6 days <10% | ||||
| hr173 | 33 weeks | 36 weeks 6 days | 30.4 0(33 weeks 6 days) | 28.20 (32 weeks 2 days) |
| 4 days 4% | ||||
| hr178 | 32 weeks | 32 weeks 4 days | 28.28 (31 weeks 0 days) | 26.83 (31 weeks 0 days) |
| 1 day 9% | ||||
| hr179 | 32 weeks | 35 weeks 2 days | 29.99 (33 weeks 2 days) | 29.07 (33 weeks 1 day) |
| 4 days 10% | ||||
| hr181 | 31 weeks | 36 weeks 1 day | 28.30 (31 weeks 0 days) | 29.50 (33 weeks 3 days) |
| 2 days <3% | ||||
| hr190 | 30 weeks | 34 weeks 2 days | 28.17 (30 weeks 6 days) | 29.95 (33 weeks 6 days) |
| 1 day <2% |
NM – Not measured during the last recording only.
Data Analysis
The data were band-pass filtered between 0.5 Hz and 50 Hz. Interfering maternal cardiac signals were attenuated using signal-space projection [20]. Using the adaptive Hilbert-transform approach [21], the fetal R waves were identified and the heart rate in beats per minute (bpm) was calculated. We analyzed the heart rate using three different measures, namely, SDNN, RMSSD and PPA. Using Nijhuis’ definition of patterns, the heart rate was scored visually in each disjoint three-minute window by experts. We applied the following criteria for scoring - Pattern A: Steady heart rate with infrequent accelerations and an oscillation bandwidth of less than 5 beats per minute (bpm); Pattern B: Varying heart rate with frequent acceleration and wider oscillation bandwidth greater than 5 bpm; Pattern C: Stable heart rate with no accelerations and an oscillation bandwidth slightly greater than 5 bpm; Pattern D: Long-lasting and large acceleration from the baseline with a wider oscillation bandwidth of greater than 10 bpm. Patterns A and B correspond to quiet and active sleep states, respectively, whereas patterns C and D correspond to quiet and active awake states, respectively.
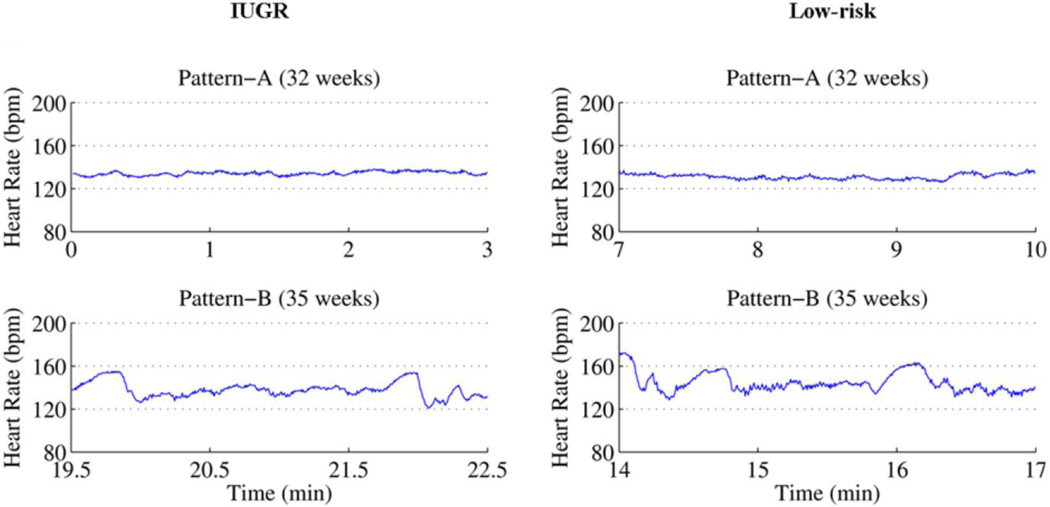
Although all four patterns were scored, in this work we focused only on patterns A and B. We excluded windows scored as pattern C and D from the analysis because they were found in less than 1% of the data. Further, we included only the windows in which both experts mutually agreed on a pattern and excluded all windows with spurious beats.Figure 1 shows 3-minute windows of pattern A and pattern B of an IUGR fetus and a low-risk fetus.
Figure 1.
Represents fetal heart rate patterns A and B of IUGR and low-risk fetuses.
After pattern classification, the difference in the HRV of the low-risk and IUGR fetuses were studied. Since the standard minimum time window to analyze HRV is 5 minutes[22], we combined two adjunct 3-minute windows having the same pattern to form a 6-minute window. Only the first 6-minute window was picked from each dataset and the three measures were calculated. The SDNN was calculated as the standard deviation of the heart rate, RMSSD was calculated as the root-mean-square of the successive difference of the heart rate and PPA was calculated as the area of the trajectory obtained by plotting heart rate versus derivative of the heart rate, which is called the phase plane plot. The heart rate was calculated as hri = 60/ti- ti-1, where ti was the time marker of the ith R wave and t the time in seconds. The derivative of heart rate (hr’) is defined as
The area was calculated by a Monte Carlo approach, in which the phase plane was populated with uniformly distributed random numbers and the probability (P) of the random number that fell on the trajectories of the heart rate and its time derivative was calculated. The probability P multiplied by the range of the hri and the range of hr’i will provide the area occupied by the trajectories.
Statistical Analysis
Gestational ages and heart-rate patterns were compared for imbalance between risk groups by Wilcoxon rank-sum and Fisher exact tests, respectively. Data for the 3 HRV measures were transformed to their base-2 logarithms to stabilize variance and facilitate inference on fold changes, then analyzed via Analysis of Covariance (ANCOVA), with Risk Group, Pattern, and their interaction as class variables and Gestational Age (GA) as the continuous covariate. The ANCOVA post-hoc comparisons focused on the risk-group differences within each heart-rate pattern, and the resulting estimated differences were reverse-transformed by base-2 exponentiation to yield GA-adjusted IUGR/LR ratios in the original units of the HRV measure. Statistical significance was defined as P<0.05 despite the multiple comparisons, in order not to inflate Type II error.
Results
The two groups of 20 each were well-balanced with respect to GA at the time of the SARA recording, with means (standard deviations) of 34.3 (1.9) weeks in the IUGR group versus 35.1 (2.1) weeks in the low-risk group (P=0.20). The two groups were also well-balanced with respect to heart-rate pattern during the recording, with the 6-minute windows classified as pattern A and pattern B in 13 (65%) and 7 (35%) recordings, respectively, from IUGR fetuses, compared to12 (60%) and 8 (40%) recordings, respectively, from low-risk fetuses (P=1.00).
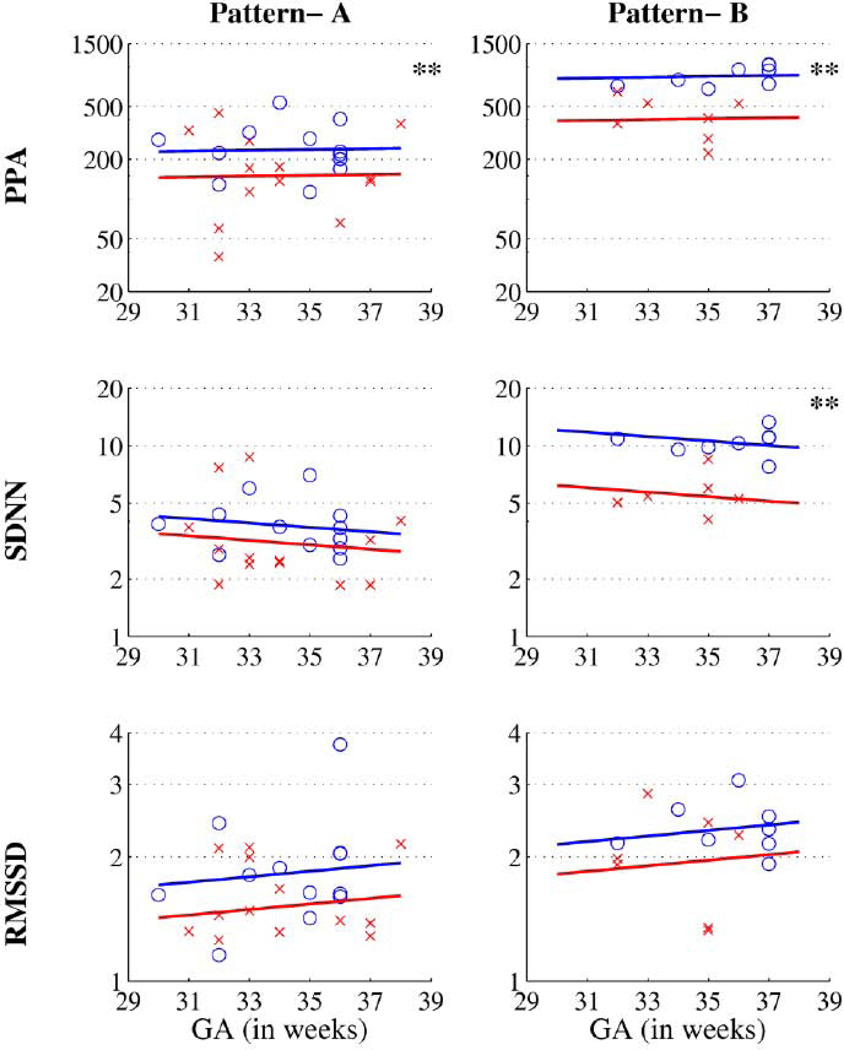
In total, 40 six-minute segments were analyzed, 20 from each risk-group. Table 2 presents the results of the ANCOVA post-hoc comparisons between risk groups. In every case, after adjusting for GA, the IUGR group had lower average HRV than the low-risk group. For PPA, the decrease in the IUGR group attained statistical significance in both patterns, being 36% lower in the pattern-A recordings (IUGR/LR ratio = 0.637; P=0.042) and 52% lower in the pattern-B recordings (IUGR/LR ratio = 0.479; P=0.013). For SDNN, the decrease in the IUGR group was 49% lower in pattern-B recordings (IUGR/LR ratio = 0.511; P=0.0010), but only 19% lower in pattern-A recordings (IUGR/LR ratio = 0.810; P=0.15). For RMSSD, however, the decreases with IUGR were not statistically significant in either pattern, being only 17% in the pattern-A recordings (P=0.096) and only 14% in the pattern-B recordings (P=0.25).Figure 2 shows the scatter plot of the three measures and the two patterns in both the risk groups in each gestational age. The Y-axes are the values of the three measures in log scale. The lines are constructed from the predicted mean of low-risk and IUGR fetuses at different GAs, and illustrate the GA-adjusted risk-group differences quantified in Table 2.
Table 2.
Gestational Age-Adjusted IUGR–LR Difference in HRV measures
| When Heart-Rate Pattern=A | When Heart-Rate Pattern=B | |||||||
|---|---|---|---|---|---|---|---|---|
| HRV measure |
Estimated Difference1 |
Standard Error2 |
P3 | IUGR/LR Ratio4 |
Estimated Difference1 |
Standard Error2 |
P3 | IUGR/LR Ratio4 |
| PPA | −0.6504 | 0.3073 | 0.0415 | 0.637 | −1.0622 | 0.4059 | 0.013 | 0.479 |
| RMSSD | −0.2639 | 0.1541 | 0.0956 | 0.833 | −0.2401 | 0.2035 | 0.2461 | 0.847 |
| SDNN | −0.3039 | 0.205 | 0.1472 | 0.81 | −0.9682 | 0.2708 | 0.001 | 0.511 |
Estimated IUGR–LR difference in log2-transformed HRV measure, adjusted for gestational age (GA).
Standard error of the estimated difference.
Two-tailed P value testing the estimated difference, based on Student’s t-distribution with 35 degrees of freedom.
GA-adjusted IUGR/LR ratio of HRV measure in original units, obtained by reverse-transformation (i.e. base-2 exponentiation) of the estimated difference.
Figure 2.
Scatter plots of the three metrics in log2 value. The X indicates IUGR and O indicates low-risk fetuses. The Y-axis is shown in log log scale. The lines represent the predicted means of the measures.
** indicates P value <0.05.
Discussion
Fetal heart-rate assessment is a cornerstone of modern obstetrical practice and a primary means of ensuring fetal well-being. Assessment of fetal HRV is critical to appropriate interpretation of a heart-rate tracing, and a key component of the standardized categorization currently used for clinical decision-making in the United States [23]. Pathologically decreased HRV has been attributed to fetal injury-induced metabolic acidosis that depresses the fetal brainstem [24]. Because metabolic acidosis and the resultant changes in HRV is more likely in the setting of the IUGR fetus, this population was an ideal target for determining the optimal HRV metric for detection of IUGR-related heart-rate changes.
As mentioned earlier, the heart-rate variability of IUGR and normal fetuses has been investigated and compared in the past. Lange et al, compared SDNN and RMSSD values of low-risk and IUGR fetuses but did not show a clear difference: a finding that is quite contrary to the anticipation that distress in IUGR fetuses may display different HRV properties compared to low-risk fetuses. They attributed this discrepancy to low degree of severity of IUGRs included in their study [16]. In a later study Schneider et al, reported a difference in SDNN and RMSSD where one-third of the IUGR study population included compromised fetuses with only segments from the quiet sleep state analyzed [17]. Further, Detrended Fluctuation Analysis (DFA) of fetal heart-rate tracings has shown that, even though the normal fetuses’ short-term (α1) scaling exponent is not significantly different from that of IUGR fetuses or those deemed small for gestational age (SGA), the long-term (α2) scaling exponent of IUGR/SGA were significantly higher than that of normal fetus, indicating that heart-rate dynamics of IUGR fetuses are different from those of normal healthy fetus [25]. This implies that DFA shows the difference between low-risk and IUGR/SGA fetuses only in low-frequency components but not in high-frequency components. A phase-rectified signal-averaging technique showed that acceleration-related fluctuations are important when studying the heart rate of an IUGR fetus, since it tends to show decreased variations [26]. Further, complexity analysis of heart rate showed that the heart rate of IUGR fetuses exhibits decreased complexity when compared to low-risk fetuses [27].
Conflicting findings are not surprising considering that the variation in the severity of IUGRs included in these studies, and that the metabolic acidosis associated with decreased HRV is usually associated with severe fetal compromise and precedes fetal death.
A physiologic decrease in HRV during quiet sleep confounds the standard visual interpretation of fetal heart rate that is used in clinical practice, and can cause a misleading, non-reassuring reading. Thus far, the PPA measure is unique in its ability to differentiate the low-risk and IUGR fetus based on HRV during the quiet-sleep state, which predominates throughout pregnancy, but especially during earlier gestational ages. In the pattern-A recordings, which are dominated by high-frequency components that indicate parasympathetic activity [8], PPA, clearly distinguished the two groups of fetuses in this study while the other two measures failed. Also, in the pattern-B recordings, which is characterized predominantly by low-frequency components (accelerations) that indicate both sympathetic and parasympathetic activity [8], PPA, along with SDNN, showed differences that reached two-fold between risk groups in this study. One possible reason for the differences in performance may be because SDNN and RMSSD quantify the low- and high-frequency components, respectively, whereas in PPA these two frequency components and their interactions are kept intact. In PPA, the interaction between low-frequency and high-frequency components is quantified in terms of the fill factor which is a fraction of the phase plane area occupied by the trajectories. A first step towards the characterization of a time series using the tools of nonlinear dynamics is to represent the one-dimensional signal in a multidimensional phase space using Taken’s embedding theorem [28]. This is usually accomplished using the delayed coordinates with delay representing the decorrelation time (corresponds to time at which autocorrelation falls to 30%). In this work, instead of a delay coordinate, the derivative of heart rate is used as a second coordinate. Heart rate can be thought of as a complex interaction between sympathetic and parasympathetic arms of the autonomic nervous system (ANS) to maintain homeostasis. Thus, the very presentation of the heart rate and its derivative in phase plane provides opportunity to quantify the dynamics, and interactions between the two branches of the ANS. This property makes PPA superior to SDNN and RMSSD. The latter two capture only one arm of the ANS, and not the dynamical interaction occurring between the arms of ANS. Furthermore, our results also suggest that the interaction between the low- and high-frequency components plays a significant role in characterizing fetal heart rate, and that PPA captures this interaction reliably regardless of the fetal state.
IUGR fetuses demonstrate movement to a lesser extent [29]and do not respond as well to external stimuli, and their heart rates do not accelerate like those of normal fetuses [30]. This explains the decreased average HRV obtained for IUGR fetuses compared to low-risk fetus.
In summary, our fetal MCG results show that the heart-rate variability of IUGR fetuses is different from that of low-risk fetuses in both patterns using PPA analysis. The difference between these two groups of fetuses shows that the fetal-activity states are potential confounders when characterizing heart-rate variability.
Acknowledgements
This work was supported by grants from National Institutes of Health (NIH) R01EB007826-01A1 and 1R01NS368277-08A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
None of the authors have any conflict of interest.
References
- 1.Odendall H. Fetal heart rate patterns in patients with intrauterine growth retardation. Obstet Gynecol. 1976;48:187–190. [PubMed] [Google Scholar]
- 2.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–133. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 3.Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol. 2004 Aug;191:481–487. doi: 10.1016/j.ajog.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 4.Baschat AA, Hecher K. Fetal growth restriction due to placental disease. Semin Perinatol. 2004 Feb;28:67–80. doi: 10.1053/j.semperi.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Henson G, Dawes GS, Redman CW. Characterization of the reduced heart rate variation in growth-retarded fetuses. Br J Obstet Gynaecol. 1984 Aug;91:751–755. doi: 10.1111/j.1471-0528.1984.tb04844.x. [DOI] [PubMed] [Google Scholar]
- 6.Schneider U, Frank B, Fiedler A, Kaehler C, Hoyer D, Liehr M, Haueisen J, Schleussner E. Human fetal heart rate variability-characteristics of autonomic regulation in the third trimester of gestation. J Perinat Med. 2008;36:433–441. doi: 10.1515/JPM.2008.059. [DOI] [PubMed] [Google Scholar]
- 7.Nijhuis JG. The third trimester. In: Nijhuis JG, editor. Fetal behaviour: Development and perinatal aspects. NY: Oxford University Press; 1992. pp. 26–40. [Google Scholar]
- 8.Van Laar JO, Peters CH, Vullings R, Houterman S, Oei SG. Power spectrum analysis of fetal heart rate variability at near term and post term gestation during active sleep and quiet sleep. Early Hum Dev. 2009 Dec;85:795–798. doi: 10.1016/j.earlhumdev.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhuravlev YE, Rassi D, Mishin AA, Emery SJ. Dynamic analysis of beat-to-beat fetal heart rate variability recorded by SQUID magnetometer: quantification of sympathovagal balance. Early Hum Dev. 2002;66:1–10. doi: 10.1016/s0378-3782(01)00225-0. [DOI] [PubMed] [Google Scholar]
- 10.Van Leeuwen P, Lange S, Bettermann H, Grönemeyer D, Hatzmann W. Fetal heart rate variability and complexity in the course of pregnancy. Early Hum Dev. 1999;54:259–269. doi: 10.1016/s0378-3782(98)00102-9. [DOI] [PubMed] [Google Scholar]
- 11.Wakai RT, Wang M, Pedron SL, Reid DL, Martin CB., Jr Spectral analysis of antepartum fetal heart rate variability from fetal magnetocardiogram recordings. Early Hum Dev. 1993;35:15–24. doi: 10.1016/0378-3782(93)90134-g. [DOI] [PubMed] [Google Scholar]
- 12.Leuthold A, Wakai RT, Martin CB. Noninvasive in utero assessment of PR and QRS intervals from the fetal magnetocardiogram. Early Hum Dev. 1999;54:235–243. doi: 10.1016/s0378-3782(98)00100-5. [DOI] [PubMed] [Google Scholar]
- 13.Lowery CL, Campbell JQ, Wilson JD, Murphy P, Preissl H, Malak SF, Ewaran H. Noninvasive antepartum recording of fetal S-T segment with a newly developed 151- channel magnetic sensor system. Am J Obstet Gynecol. 2003;188:1491–1497. doi: 10.1067/mob.2003.367. [DOI] [PubMed] [Google Scholar]
- 14.Stinstra J, Golbach E, van Leeuwen P, Lange S, Menendez T, Moshage W, Schleussner E, Kaehler C, Horigome H, Shigemitsu S, Peters MJ. Multicentre study on the fetal cardiac time intervals using magnetocardiography. British J of Ob and Gyn. 2002;109:1235–1243. doi: 10.1046/j.1471-0528.2002.01057.x. [DOI] [PubMed] [Google Scholar]
- 15.Govindan RB, Vairavan S, Sriram B, Wilson JD, Preissl H, Eswaran H. Phase plane based identification of fetal heart rate patterns. Conf Proc IEEE Eng Med Biol Soc. 2011;1:1455–1458. doi: 10.1109/IEMBS.2011.6090337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange S, Van Leeuwen P, Leven A, Klein A, Hatzmann W, Grönemeyer D. Heart rate variability in growth retarded fetuses as determined by fetal magnetocardiography. In: Nowak H, Haueisen J, Giessler F, Huonker R, editors. Biomag 2002. Proceedings of the 13th International Conference on Biomagnetism; 2002 Aug 10–14; Jena, Germany. Berlin, Offenbach: VDE Verlag; 2002. pp. 633–635. [Google Scholar]
- 17.Schneider U, Fiedler A, Liehr M, Kähler C, Schleussner E. Fetal heart rate variability in growth restricted fetuses. Biomed Tech (Berl) 2006 Oct;51:248–250. doi: 10.1515/BMT.2006.048. [DOI] [PubMed] [Google Scholar]
- 18.Gustafson KM, May LE, Yeh HW, Million SK, Allen JJ. Fetal cardiac autonomic control during breathing and non-breathing epochs: the effect of maternal exercise. Early Hum Dev. 2012 Jul;88:539–546. doi: 10.1016/j.earlhumdev.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moraes ER, Murta LO, Baffa O, Wakai RT, Comani S. Linear and nonlinear measures of fetal heart rate patterns evaluated on very short fetal magnetocardiograms. Physiol Meas. 2012 Oct;33:1563–1583. doi: 10.1088/0967-3334/33/10/1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrba J, Robinson SE, McCubbin J, Lowery CL, Eswaran H, Wilson JD, Murphy P, Preissl H. Fetal MEG redistribution by projection operators. IEEE Trans. on Biomed. Eng. 2004;51:1207–1218. doi: 10.1109/TBME.2004.827265. [DOI] [PubMed] [Google Scholar]
- 21.Ulusar UD, Govindan RB, Wilson JD, Lowery CL, Preissl H, Eswaran H. Adaptive rule based fetal QRS complex detection using hilbert transform. Conf Proc IEEE Eng Med Biol Soc, 2009;1:4666–4669. doi: 10.1109/IEMBS.2009.5334180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 23.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114(1):192–202. doi: 10.1097/AOG.0b013e3181aef106. [DOI] [PubMed] [Google Scholar]
- 24.Signore C, Freeman RK, Spong CY. Antenatal testing-are evaluation: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;113(3):687–701. doi: 10.1097/AOG.0b013e318197bd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikuchi A, Unno N, Kozuma S, Taketani Y. Detrended fluctuation analysis of heart rate variability in normal and growth-restricted fetuses. Gynecol. Obstet. Invest. 2008;65:116–122. doi: 10.1159/000109266. [DOI] [PubMed] [Google Scholar]
- 26.Huhn EA, Lobmaier S, Fischer T, Schneider R, Bauer A, Schneider KT, Schmidt G. New computerized fetal heart rate analysis for surveillance of intrauterine growth restriction. Prenat Diagn. 2011;31:509–514. doi: 10.1002/pd.2728. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi A, Shimizu T, Hayashi A, Horikoshi T, Unno N, Kozuma S, Taketani Y. Nonlinear analyses of heart rate variability in normal and growth-restricted fetuses. Early Hum Dev. 2006;82:217–226. doi: 10.1016/j.earlhumdev.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan D, Glass L. Understanding Nonlinear Dynamics. N.Y: Springer-Verlag; 1995. [Google Scholar]
- 29.Mor-Yosef S, Sadovsky E, Brzezinski A, Levinsky R, Ohel G. Fetal movements and intrauterine growth retardation. Int J Gynaecol Obstet. 1983;21:315–318. doi: 10.1016/0020-7292(83)90022-x. [DOI] [PubMed] [Google Scholar]
- 30.Timor-Tritsch IE, Dierker LJ, Zador I, Hertz RH, Rosen MG. Fetal movements associated with fetal heart rate accelerations and decelerations. Am J Obstet Gynecol. 1978;131:276–280. doi: 10.1016/0002-9378(78)90600-2. [DOI] [PubMed] [Google Scholar]