Abstract
Objective
To identify clinical and demographic factors associated with a worse visual outcome in Acanthamoeba keratitis (AK).
Design
Retrospective, case control study.
Participants
A total of 72 eyes of 65 patients with AK who were diagnosed at the University of Illinois Eye and Ear Infirmary between May of 2003 and May of 2007 with treatment complete by October of 2007. The first affected eye was analyzed in bilateral disease.
Methods
Patient demographic, clinical characteristics, treatment methods, and final visual outcome data were collected through medical record reviews for all patients diagnosed with AK. Cases were defined as patients with AK with a visual outcome worse than 20/25 or those requiring penetrating keratoplasty (PKP). Controls were defined as patients with AK with a visual outcome of 20/25 or better. Logistic regression was used to estimate the odds ratio (OR) identifying prognostic factors associated with a worse visual outcome.
Main Outcome Measures
Final visual outcome worse than 20/25.
Results
AK was confirmed through microbiologic evidence in 48 of 65 eyes (73.8%) or with confocal microscopy in 62 of 65 eyes (95.4%). Final visual acuity data were available in 61 of 65 eyes (93.8%); of these 61 eyes, 40 (65.6%) achieved a final visual acuity of 20/25 or better. In multivariable analysis, deep stromal involvement or the presence of a ring infiltrate at presentation was independently associated with worse visual outcomes (OR, 10.27; 95% confidence interval [CI], 2.91–36.17). Symptom duration before diagnosis was statistically predictive of disease stage at presentation (OR, 4.43; 95% CI, 0.99–19.83; multivariable analysis) but not final visual outcome (OR, 2.55; 95% CI, 0.83–7.88; univariate analysis). PKP was performed in 11 of 12 eyes with active disease.
Conclusions
Corneal disease staging at presentation with slit-lamp examination was highly predictive of worse outcomes, allowing the identification of patients who might benefit from more aggressive medical or surgical intervention. Unlike in previous reports, patient-reported duration of symptoms before treatment was not reliable in predicting the final visual result in our series.
Acanthamoeba keratitis (AK) is perceived as a recalcitrant, painful, and devastating corneal infection that can lead to significant visual loss and ocular morbidity. Delayed recognition resulting from a nonspecific clinical presentation and poor microbiologic isolation rate, in addition to a cyst form highly insensitive to conventional antimicrobials, adversely affect clinical management. Although diagnostic and therapeutic advances have significantly improved overall prognosis, recalcitrant infections and poor outcomes persist in some cases.1,2 Previous reports suggest worse outcomes are associated primarily with later disease presentation,3–6 although the most appropriate measure of late disease and confounding variable effects, such as previous medication use, clinical disease characteristics, and diagnostic modalities, have not been identified. The purpose of this study is to identify prognostic factors for disease outcome and visual morbidity through analysis of a recent AK outbreak in the Chicago area.7,8
Materials and Methods
The University of Illinois at Chicago (UIC) Institutional Review Board reviewed and approved this research. Briefly, a retrospective case-control study including all University of Illinois Eye and Ear Infirmary patients with AK who were diagnosed between May of 2003 and May of 2007 with treatment complete by October of 2007 was conducted to determine the clinical and demographic factors associated with a worse outcome, defined as a visual acuity worse than 20/25. Because none of the visual outcome prognostic factors have been reported or analyzed, those patients involved in previous analyses of geographic case distribution and contact solution use were not excluded.7,8
Disease Definition and Data Collection
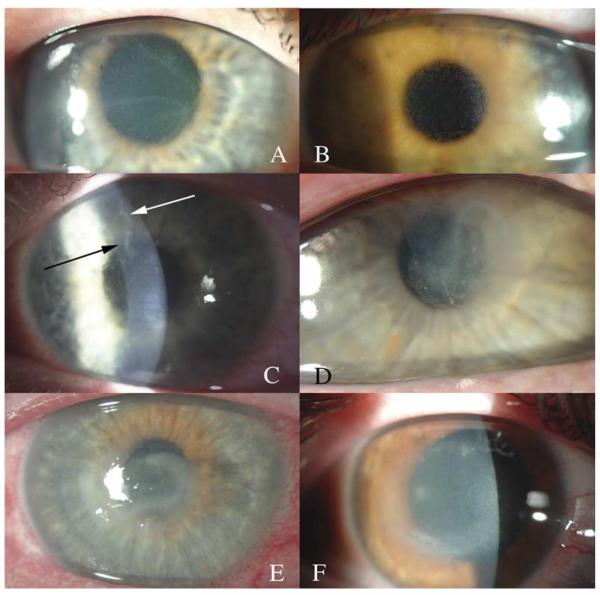
AK was diagnosed as previously described;7 in summary, diagnosis was based on clinical characteristics and identification of 1 or more of the following conditions: (1) identification of trophozoites or cysts on confocal microscopy; (2) identification of trophozoites or cysts through smears when specimens were stained with Diff-Quick stain; (3) positive Acanthamoeba cultures; or (4) pathologic identification of Acanthamoeba-like cysts on keratoplasty specimens. Corneal disease stage was evaluated by a single clinician (EYT) at presentation on the basis of clinically evident findings through slit-lamp biomicroscopy and categorized into 1 of 5 levels of progressive anatomic involvement and disease severity (epitheliitis, epitheliitis with radial neuritis, anterior stromal disease, deep stromal disease, or ring infiltrate) (Fig 1). Confocal microscopy was not used in disease staging because of its variability in detecting deeper stromal involvement, which is a function of the focal and often sparse nature of corneal stromal involvement in addition to the technical challenges with more opaque corneas.
Figure 1.
Slit-lamp photographs of AK. A and B, Isolated epitheliitis. Two patients with an isolated epithelial presentation of AK. Note the granular, cystic-appearing epithelium with little or no stromal inflammation. C, Epitheliitis with radial neuritis. A patient with irregular-appearing epithelium and an inflamed, nodular corneal nerve superiorly. D, Anterior stromal disease. A patient with anterior stromal involvement with clear surrounding cornea and without associated deep inflammation. E, Deep stromal keratitis. Note the diffuse central infiltrate with generalized central haze without a clear lateral or deep border. F, Ring infiltrate. An early ring infiltrate demonstrating an area of central corneal haze with a relative paracentral demarcation ring characteristic of an immune Acanthamoeba ring.
Anterior stromal disease was defined as the presence of sub-epithelial infiltrates, shallow non-necrotic ulcers, or other isolated involvement of the anterior cornea. Extracorneal manifestations of inflammation, such as limbal or scleral inflammation, were categorized as the most advanced level of involvement, the ring infiltrate group.
Treatment was initiated at the University of Illinois Eye and Ear Infirmary for nearly all patients, most commonly with propamidine isethionate (Brolene) and either chlorhexidine gluconate 0.02% or polyhexamethylene biguanide 0.02% hourly, which was tapered according to clinical response. On the basis of previous reports9–11 and our experience, wide mechanical epithelial debridement was performed regardless of the stage at presentation in an attempt to reduce organism load through mechanical extirpation and to facilitate initial topical drug penetration when it is presumed that the number of the less resistant trophozoite forms is at its peak.
Subject data were collected through a retrospective review of the records of patients with AK; all patients with AK were tracked through an Excel-based spreadsheet (Microsoft Corp, Redmond, WA).
Statistical Analysis
Statistical analyses were performed using SAS (v. 9.1.3; SAS Institutes, Cary, NC). Treatment completion was defined as quiescent disease for a period of 3 months after discontinuation of all anti-acanthamoebal medications. Descriptive statistics were calculated on all diagnosed cases, whereas analysis of factors associated with final visual outcome was restricted to eyes with final visual acuity data. In patients with bilateral AK, analysis was restricted to the first afflicted eye.
Final visual outcome was stratified into 4 categories according to data distribution and other important visual acuity measures (i.e., requirements for a restricted or unrestricted driver’s license). The distribution of visual acuity and disease stage at presentation was evaluated in a matrix, and both variables were collapsed into dichotomous classifications that were plausible and consistent with known disease biology based on variable distribution. Specifically, classifications were chosen to minimize the likelihood of artifactual findings resulting from inadequately sized comparisons. Dichotomous classifications were defined as a final best spectacle corrected visual outcome of 20/25 or better versus an outcome worse than 20/25; similarly, disease stage was defined as presentation with either epitheliitis with or without radial neuritis or anterior stromal disease versus presentation with deep stromal disease or a ring infiltrate. Patients requiring penetrating keratoplasty (PKP) for therapeutic or optical reasons were assigned to the worse visual outcome category, because advanced disease without further intervention would most likely result in vision less than 20/25 (according to current disease knowledge). Patients with AK who had a final visual outcome of worse than 20/25 or requiring PKP were considered “cases” for analysis, and patients with AK who had a final visual outcome of 20/25 or better were considered “controls.”
Univariate analysis was performed using logistic regression to estimate the odds ratio (OR) and 95% confidence intervals (CIs) to determine factors associated with a worse outcome. Multivariable logistic regression was performed using forward stepwise addition to determine factors associated with worse visual outcome while controlling for all confounding variables.
To confirm the robustness of the dichotomous visual outcome and disease classification results, univariate and multivariable logistic regression modeling was performed in a similar fashion using an additional dichotomous visual outcome defined as a visual acuity of 20/40 or worse, as well as both a collapsed trichotomous disease stage (epitheliitis with or without radial neuritis; anterior stromal disease; and deep stromal or ring infiltrate) and the expanded 5-category disease stage classification.
In a separate univariate analysis, factors associated with a worse disease stage at presentation, as defined by presentation with deep stromal disease or a ring infiltrate, were analyzed using logistic regression to estimate the OR and 95% CI. Multivariable logistic regression performed with forward stepwise addition was used to determine factors associated with a worse disease stage at presentation while controlling for all confounding variables.
Results
Seventy-two AK cases in 65 patients were diagnosed at UIC between May of 2003 and May of 2007 with treatment complete by October of 2007. Of these, 38 of 72 patients (52.8%) were male, the mean age was 28.69 ± 14.94 years, and 64 of 72 patients (90.1%) used soft contact lenses. Seven patients (10.8%) had bilateral, usually asymmetric disease.
Microbiologic evidence of disease, as defined by a positive result on superficial corneal cultures, smear, or pathology specimens in patients who underwent PKP, was present in 48 of 65 (73.8%) of eyes, and yield exceeded 66.7% for all groups without discernable effect of depth of involvement. Nearly all patients had either evidence of disease with microbiologic testing or confocal microscopy imaging in 62 of 65 eyes (95.4%).
Final visual acuity data after completing treatment were available in 61 of 65 eyes (93.8%), of which 40 (65.6%) achieved a final outcome of 20/25 or better. The dichotomous final visual outcome classification defined as 20/25 or better versus an outcome of worse than 20/25 was selected on the basis of these 65.6% of eyes achieving a 20/25 or better outcome. Final visual outcome was nearly identical between patients with epitheliitis with or without radial neuritis: Nine of 11 eyes (81.8%) with epitheliitis achieved 20/25 or better compared with 13 of 16 eyes (81.3%) with epitheliitis and radial neuritis. Outcomes in patients presenting with anterior stromal disease were not significantly different statistically from patients with epithelial disease, with 11 of 15 eyes (68.8%) achieving 20/25 (anterior stromal disease vs epitheliitis with or without radial neuritis OR, 1.60; 95% CI, 0.36–7.18; P = 0.25). In contrast, patients with deep stromal disease and ring infiltrates performed significantly worse, with only 1 of 5 eyes (20.0%) and 6 of 14 eyes (37.5%), respectively, achieving a final outcome of 20/25 or better (deep stromal or ring infiltrate vs epitheliitis with or without radial neuritis OR, 12.32; 95% CI, 3.01–50.42). Because final visual outcome was not statistically different between patients presenting with epitheliitis with or without radial neuritis and anterior stromal disease, subsequent analyses used a collapsed, dichotomous disease categorization comparing anterior disease (epitheliitis with or without radial neuritis and anterior stromal disease) versus deeper disease (deep stromal disease and ring infiltrate).
Univariate analysis to compare factors associated with worse visual outcomes identified deeper disease presentation (OR, 10.27; 95% CI, 2.91–36.17) and continued postdiagnosis steroid use (OR, 17.00; 95% CI, 3.19–90.66) as statistically significant; no additional demographic or clinical factors, including prediagnosis medication use, were significant (Table 1). No anti-acanthamoebal medications were significantly associated with a worse visual outcome. In multivariable analysis, only disease stage at presentation remained statistically significant in the association with a final visual outcome worse than 20/25 (OR, 10.27; 95% CI, 2.91–36.17). When controlling for disease stage at presentation, no demographic or clinical factors including medication use pre- and postdiagnosis were statistically significant in predicting a worse visual outcome. Multivariable analysis performed with the different visual outcome and disease classifications resulted in consistent findings, confirming the robustness of the analyses.
Table 1.
Comparison of Demographic and Clinical Characteristics in 61 Acanthamoeba Keratitis Cases with Final Visual Acuity of <20/25* Versus ≥20/25
|
Visual Acuity
<20/25 |
Visual Acuity
≥20/25 |
Univariate Analysis
|
||||||
|---|---|---|---|---|---|---|---|---|
| ntotal = 23 | % | ntotal = 38 | % | OR | 95% CI | P Value | ||
| Clinical factors | ||||||||
| Duration between symptom onset and UIC presentation |
||||||||
| 3+ wk | 17 | 73.9 | 20 | 52.6 | 2.55 | 0.83 | 7.88 | 0.10 |
| <3 wk | 6 | 26.1 | 18 | 47.4 | ||||
| Disease presentation | ||||||||
| Deep stromal disease or ring infiltrate | 14 | 60.9 | 5 | 13.2 | 10.27 | 2.91 | 36.17 | <0.001 |
| Epitheliitis with or without radial neuritis or anterior stromal disease |
9 | 39.1 | 33 | 86.8 | ||||
| Medication use prediagnosis | ||||||||
| Steroid use prediagnosis | ||||||||
| Yes | 21 | 91.3 | 29 | 76.3 | 3.26 | 0.64 | 16.66 | 0.16 |
| No | 2 | 8.7 | 9 | 23.7 | ||||
| Duration of prediagnosis steroid use | ||||||||
| 2+ wk | 15 | 71.4 | 14 | 46.7 | 2.86 | 0.87 | 9.37 | 0.08 |
| <2 wk | 6 | 28.6 | 16 | 53.3 | ||||
| HSV treatment prediagnosis | ||||||||
| Any HSV treatment | ||||||||
| Yes | 7 | 31.8 | 19 | 50.0 | 0.47 | 0.16 | 1.40 | 0.17 |
| No | 15 | 68.2 | 19 | 50.0 | ||||
| Antibiotic treatment prediagnosis | ||||||||
| Fourth-generation fluoroquinolones | ||||||||
| Yes | 13 | 61.9 | 16 | 47.1 | 1.83 | 0.60 | 5.54 | 0.29 |
| No | 8 | 38.1 | 18 | 52.9 | ||||
| Third-generation fluoroquinolones | ||||||||
| Yes | 7 | 33.3 | 5 | 14.7 | 2.90 | 0.78 | 10.78 | 0.11 |
| No | 14 | 66.7 | 29 | 85.3 | ||||
| Medication use postdiagnosis | ||||||||
| Steroid use postdiagnosis | 0.003*† | |||||||
| Added after diagnosis (vs immediate taper or discontinue) |
2 | 9.1 | 2 | 5.3 | 3.40 | 0.42 | 27.30 | 0.86 |
| Continued after diagnosis (vs immediate taper or discontinue) |
10 | 45.5 | 2 | 5.3 | 17.00 | 3.19 | 90.66 | 0.02† |
| Immediate taper or discontinued | 10 | 45.5 | 34 | 89.5 | ||||
OR = odds ratio; CI = confidence interval; UIC = University of Illinois at Chicago; HSV = herpes simplex virus.
Eyes requiring PKP for therapeutic or optical reasons assigned to the dichotomous category of <20/25.
OR, CIs, and P values presented per stratum; the Cochran-Mantel-Haenszel P value, a stratum-adjusted P value, is presented for the composite variable significance value.
Factors significantly associated statistically with worse disease stage at presentation in univariate analysis were the duration between symptom onset and UIC presentation (OR, 5.33; 95% CI, 1.35–21.05) and the duration of prediagnosis steroid use of 2 or more weeks (OR, 5.15; 95% CI, 1.24–21.30). Only 1 variable remained statistically significant or nearly significant in multivariable analysis: duration between symptom onset and UIC presentation (OR, 4.43; 95% CI, 0.99–19.83). Duration of prediagnosis steroid use was no longer statistically significant after controlling for confounding variables (OR, 2.65; 95% CI, 0.45–15.60).
Discussion
Corneal disease stage at presentation was independently and strongly associated with final visual outcome in multivariable analysis: Patients with deep stromal disease or a ring infiltrate had more than a 10-fold increase in the odds of a visual outcome worse than 20/25 (OR, 10.27; 95% CI, 2.91–36.17). Although it is unclear whether corneal disease stage as graded by slit-lamp examination represents the level of Acanthamoeba involvement or the reactive host immune response, the technique is easily accessible and familiar to clinicians. More precise characterization of cor-neal depth and area of involvement might further refine results, but no widely available technology is currently validated for this purpose.
Previous studies have suggested that an earlier time to diagnosis results in more favorable outcomes,4–6,12,13 yet our results suggest symptom duration is not a strong predictor of final visual outcome when compared with corneal disease stage at presentation. Patients presenting with a symptom duration of 3 or more weeks were more likely to have a final visual acuity less than 20/25, but it was not statistically significant in univariate analysis (OR, 2.55; 95% CI, 0.83–7.88). This is not unexpected, because identification of the anatomic layer of corneal involvement is more objective than patient recall of symptom onset, particularly given the variable, nonspecific symptoms characteristic of early AK that make identification of disease onset unreliable.
Nonetheless, a statistically significant association between symptom duration of 3 or more weeks and presentation with deep corneal disease remained in both univariate and multivariable analyses (OR, 4.43; 95% CI, 0.99–19.83), which may explain the perceived relationship between symptom duration and final visual outcome. However, it is unlikely that the rate of clinical and anatomic progression is constant among individuals because it likely depends on such factors as organism strain, the magnitude of initial exposure or “load,” the presence of sustaining amoebic prey, and host factors in the form of immune function and corneal integrity. This may explain the considerable variability of symptom duration within stages and the overlap among categories, as well as the lack of association with final visual outcome.
Because progressively deeper disease presents an escalating treatment challenge in addition to increasing the likelihood of visual impairment from stromal scarring, we initially classified patients into 5 categories according to progressive depth and severity of corneal involvement by slit-lamp examination (Fig 1). Although radial perineuritis represents mid-to-deep stromal involvement both clinically and histopathologically, a separate category was created for patients with primarily epithelial disease and perineuritis because of the unique localized nature of perineuritis and the previous reports suggesting it is more responsive to therapy.14 The presence of radial perineuritis at any stage did not predict final visual outcome, and visual results for epitheliitis with radial neuritis were statistically indistinguishable from purely epithelial disease. Anterior stromal disease was also categorized separately to distinguish it from the deep inflammatory state seen in deep stromal keratitis and immune ring infiltrates, and similarly, there was no statistical difference between anterior stromal disease and epithelial disease with or without perineuritis. Because these anterior disease stages commonly result in negligible corneal scarring and irregularity because of a muted immune response and minimal stromal alteration, it is consistent that prognosis in these patients would be favorable.
Medication use before diagnosis in general and specifically to either the third and fourth-generation fluoroquinolone antibiotics or antiviral topical and systemic medications was not statistically associated with poorer visual outcome in multivariable analysis. Similarly, 50 of 61 eyes (82%) received steroids before diagnosis, statistically limiting our ability to examine the effect of steroids on disease outcome.
The targeted time frame for visual acuity data abstraction was at visits 3 to 6 months after discontinuation of anti-acanthamoebal medications; data were not specifically attributable to this interval for only 17 subjects. Of these, 11 subjects had 20/25 vision or better (which presumably did not worsen because disease did not recur), and 6 subjects had 20/30 or worse. Of these 6 subjects, the visual acuity data used in analyses were taken from visits at least 1 or more years after disease resolution indicating stability, and for the remaining 2 subjects, visual acuity was provided through the referring doctor at a time point at least 3 months after treatment, but an exact time point was not specified. Similarly, all other data were collected at least 3 months after drug cessation. As such, the likelihood that patient visual acuity data were changing with time, which could cause visual acuity misclassification and bias results, is minimal.15
Unique to this series is the ability to identify a significant proportion of the patients in earlier stages of disease, thereby providing sufficient power to statistically evaluate and validate prognostic factors associated with visual outcome. Because the microbiologic identification of Acanthamoeba has historically approximated 50%,1,16 inclusion of false-positive cases could skew results making comparisons among previous studies less informative. The percentage of cases with microbiologic confirmation of disease in our cases, however, exceeded most reported series, and definitive identification through routine surface scrapings was possible regardless of disease stage at presentation. Previous analyses of the diagnostic accuracy within our series suggest the likelihood of false-positive cases is low; in fact, 48 of 65 eyes (73.8%) had microbiologic confirmation of disease, which, with the addition of confocal microscopy, increased to 62 of 65 eyes (95.4%) with supportive, objective evidence of disease suggesting these are true cases. It is possible that a simultaneous confocal microscopy and corneal smear diagnostic protocol as performed at the University of Illinois Eye and Ear Infirmary, which supports treatment initiation at the first office visit, may have reduced diagnostic delay and skewed the population to an earlier disease stage.
Conclusions
Slit-lamp grading of corneal disease stage or depth of involvement at presentation was strongly associated with final visual outcome and provides a practical method for clinicians to identify high-risk patients in whom to consider more aggressive medical and surgical therapy. This advanced understanding of prognostic factors and outcomes of AK should help direct treatment in future cases such that visual outcomes improve to levels comparable to more common forms of infectious keratitis.
Acknowledgments
This study was supported by grants from National Institutes of Health (EY15689 and EY09073), Bethesda, Maryland; Prevent Blindness America, Chicago, Illinois; Midwest Eye-Banks, Ann Arbor, Michigan; University of Illinois at Chicago Campus Research Board, Chicago, Illinois; AOF AAO William C. Ezell Fellowship, Rockville, Maryland; and Karl Cless Foundation, Northbrook, Illinois.
Footnotes
No authors have a financial interest in the study.
References
- 1.Radford CF, Lehmann OJ, Dart JK, National Acanthamoeba Keratitis Study Group Acanthamoeba keratitis: multicentre survey in England 1992– 6. Br J Ophthalmol. 1998;82:1387–92. doi: 10.1136/bjo.82.12.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathers WD. Acanthamoeba: a difficult pathogen to evaluate and treat. Cornea. 2004;23:325. doi: 10.1097/00003226-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Mathers WD, Sutphin JE, Folberg R, et al. Outbreak of keratitis presumed to be caused by Acanthamoeba. Am J Ophthalmol. 1996;121:129–42. doi: 10.1016/s0002-9394(14)70577-x. [DOI] [PubMed] [Google Scholar]
- 4.Duguid IG, Dart JK, Morlet N, et al. Outcome of Acanthamoeba keratitis treated with polyhexamethyl biguanide and propamidine. Ophthalmology. 1997;104:1587–92. doi: 10.1016/s0161-6420(97)30092-x. [DOI] [PubMed] [Google Scholar]
- 5.Claerhout I, Goegebuer A, Van Den Broecke C, Kestelyn P. Delay in diagnosis and outcome of Acanthamoeba keratitis. Graefes Arch Clin Exp Ophthalmol. 2004;242:648–53. doi: 10.1007/s00417-003-0805-7. [DOI] [PubMed] [Google Scholar]
- 6.Bacon AS, Dart JK, Ficker LA, et al. Acanthamoeba keratitis: the value of early diagnosis. Ophthalmology. 1993;100:1238–43. doi: 10.1016/s0161-6420(93)31499-5. [DOI] [PubMed] [Google Scholar]
- 7.Joslin CE, Tu EY, Shoff ME, et al. The association of contact lens solution use and Acanthamoeba keratitis. Am J Ophthalmol. 2007;144:169–80. doi: 10.1016/j.ajo.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joslin CE, Tu EY, McMahon TT, et al. Epidemiological characteristics of a Chicago-area Acanthamoeba keratitis outbreak. Am J Ophthalmol. 2006;142:212–7. doi: 10.1016/j.ajo.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 9.Holland GN, Donzis PB. Rapid resolution of early Acanthamoeba keratitis after epithelial debridement. Am J Ophthalmol. 1987;104:87–9. doi: 10.1016/0002-9394(87)90302-3. [DOI] [PubMed] [Google Scholar]
- 10.Newman W, Hay J, Browne B, Seal DV. Acanthamoeba as a ‘transient’ in the corneal scrape of a poorly compliant soft contact lens wearer with peripheral keratitis [letter] Eye. 1997;11:937–9. doi: 10.1038/eye.1997.233. [DOI] [PubMed] [Google Scholar]
- 11.Brooks JG, Jr, Coster DJ, Badenoch PR. Acanthamoeba keratitis: resolution after epithelial debridement. Cornea. 1994;13:186–9. [PubMed] [Google Scholar]
- 12.Park DH, Palay DA, Daya SM, et al. The role of topical corticosteroids in the management of Acanthamoeba keratitis. Cornea. 1997;16:277–83. [PubMed] [Google Scholar]
- 13.Niederkorn JY, Alizadeh H, Leher H, McCulley JP. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1999;1:437–43. doi: 10.1016/s1286-4579(99)80047-1. [DOI] [PubMed] [Google Scholar]
- 14.Thebpatiphat N, Hammersmith KM, Rocha FN, et al. Acanthamoeba keratitis: a parasite on the rise. Cornea. 2007;26:701–6. doi: 10.1097/ICO.0b013e31805b7e63. [DOI] [PubMed] [Google Scholar]
- 15.DiLoreto DA, Jr, Bressler NM, Bressler SB, Schachat AP. Use of best and final visual acuity outcomes in ophthalmological research. Arch Ophthalmol. 2003;121:1586–90. doi: 10.1001/archopht.121.11.1586. [DOI] [PubMed] [Google Scholar]
- 16.Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002;86:536–42. doi: 10.1136/bjo.86.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]