Abstract
BACKGROUND
A pilot study was conducted to determine the safety and feasibility of intravenous administration of autologous umbilical cord blood (CB) in young children with acquired neurologic disorders. Most CB units (CBUs) were electively stored in private CB banks. Unlike public banks, which utilize specific criteria and thresholds for banking, private banks generally store all collected CBUs.
STUDY DESIGN AND METHODS
CBUs of eligible patients containing more than 1 × 107 cells/kg were shipped to Duke from the banks of origin after confirming identity by HLA typing. On the day of infusion, CBUs were thawed and washed in dextran-albumin and infused intravenously. Patients were medicated with acetaminophen, diphenhydramine, and methylprednisolone before transfusion. Data regarding patients, infusions, and CBUs were collected retrospectively. Characteristics of CBUs were compared to existing data from CBUs publicly banked at the Carolinas Cord Blood Bank.
RESULTS
From March 2004 to December 2009, 184 children received 198 CB infusions. Three patients had infusion reactions, all responsive to medical therapy and stopping the infusion. Median precryopreservation volume (60 mL vs. 89 mL, p < 0.0001), total nucleated cell count (4.7 × 108 vs. 10.8 × 108, p < 0.0001), and CD34 count (1.8 × 106 vs. 3.0 × 106, p < 0.0001) were significantly lower than publicly stored CBUs. Postthaw sterility cultures were positive in 7.6% of infused CBUs.
CONCLUSION
IV infusion of autologous CB is safe and feasible in young children with neurologic injuries. Quality parameters of privately banked CBUs are inferior to those stored in public banks. If efficacy of autologous CB is established clinically, the quality of autologous units should be held to the same standards as those stored in public banks.
Brain injuries sustained in utero or early in childhood are often the result of a stroke or perinatal hypoxic-ischemic event, which can cause life-long developmental and functional impairments that negatively impact quality of life for patients and their families. Existing therapies are aimed solely at managing sequelae of the injury, because there are no known treatments that can repair the damaged brain tissue. Cellular therapy is currently under investigation as a method of promoting neural repair and/or regeneration. Human umbilical cord blood (CB) is a unique source of progenitor cells for this therapy.
CB is rich in highly proliferative stem and progenitor cells mobilized by placental signals promoting homing of these cells to developing organs.1,2 In addition to their function in normal development, the cells and factors contained in CB may facilitate and improve endogenous repair and plasticity after injury to the brain or spinal cord.3–5 In animal models, CB has been shown to lessen the clinical and radiographic impact of hypoxic brain injury and stroke.6–10 In humans with inborn errors of metabolism undergoing allogeneic, unrelated donor CB transplantation, CB also engrafts and differentiates in the brain, facilitating neural cell repair.11,12
Based on these observations, we conducted a pilot study of intravenous (IV) autologous CB infusion in children with acquired neurologic diseases to determine safety and feasibility of the procedure. Most of the CB units (CBUs) had been electively stored in private CB banks at the time of the child’s birth.
In the past, CB was routinely discarded with the placenta as medical waste after a baby’s birth. Currently, at the time of delivery CB may be discarded, privately banked, donated to a public bank, or donated for research. Private CB banks store CBUs at the request and expense of the parents for possible future autologous or related allogeneic use. Conversely, public CB banks collect, process, and store donated CBUs intended for unrelated allogeneic transplantation at no expense to the donor or their family. Unlike public banks, which utilize specific criteria for banking (based on current clinical indications) to ensure optimum product safety and potency, private banks generally store any collected unit regardless of size or cell content, in part because they predict that conditions for potential future uses have yet to be defined.
To date, few privately banked CBUs have been used for infusion in the clinic. Most of those infused were used for a sibling with a disease that can be treated by hematopoietic stem cell transplantation. Therefore, this study provided a unique opportunity to analyze a large cohort of privately banked CBUs used for autologous infusion in the clinic. In this report, we examine the quality parameters of the CBUs used for autologous infusion and compare them to those units stored publicly at the Carolinas Cord Blood Bank, a public bank participating in the CW Bill Young Cell Transplantation’s National Cord Blood Inventory.
MATERIALS AND METHODS
Study design
This study is a retrospective data review of patients treated with IV autologous CB infusion for brain injury by the Duke Pediatric Blood and Marrow Transplant Program. A waiver of authorization to conduct this study was approved by the Duke University Medical Center Institutional Review Board (Durham, NC). Parents signed routine hospital consent for treatment as well as consent for data to be collected and shared with the National Marrow Donor Program and Center for International Blood and Marrow Transplant Research (NMDP/CIBMTR; Minneapolis, MN, and Milwaukee, WI) for entry into the Stem Cell Transplant Outcomes Database. Charts of children infused from March 2004 to December 2009 were reviewed.
Patients
Pediatric patients with acquired neurologic disorders as diagnosed by their local doctors were self-referred or referred by their treating physicians. Medical records were reviewed by the transplant team before the patient was accepted for treatment. Patients were eligible for autologous CB infusion if their parents had elected to bank their CB at birth. Patients with known genetic diseases, ineligible CBUs, a history of prior cellular therapy of any kind, or medical conditions that precluded safe travel, such as respiratory instability or intractable seizures, were excluded.
Treatment plan
On referral, information regarding the patient’s performance status, etiology of their brain injury, and location of their autologous CBU was acquired. With parental permission, data regarding the banked CBU were obtained to determine eligibility for infusion (see below). After the CBU was deemed eligible, identity and potency were confirmed. It was then shipped to the Duke Stem Cell Lab in a dry shipper maintaining temperatures of less than −150°C and stored under liquid nitrogen until the time of infusion. Upon receipt, an appointment was scheduled for treatment. Patients traveled to Duke for a 3-day visit including on Day 1, a baseline history, physical, and laboratory evaluation including donor screening labs; on Day 2, infusion of the autologous CB; and on Day 3 (since January 2009) a neurology consult for baseline evaluation in anticipation of a return visit 1 year later.
CBU criteria
Cryopreserved CBUs had to meet the following minimum criteria for infusion as documented by the bank of origin: precryopreservation total nucleated cell count (TNC) documented and more than 1 × 107 cells/kg calculated for the child’s current body weight, bacterial culture performed and negative, maternal infectious history screen and infectious disease markers (minimally human immunodeficiency virus Types 1 and 2, human T-lymphotropic virus Types I and II, hepatitis B and C, cytomegalovirus, West Nile virus, and syphilis) performed and negative, and identity confirmed via HLA typing of both a test sample of the CBU and peripheral blood of the patient. Viability and potency, measured by thawing a segment or reference vial and testing for TNC, colony forming units (CFUs), and CD34, were confirmed on a test sample when available. Once these criteria were met, the unit was shipped in a dry shipper to the Duke Stem Cell Lab from the bank of origin.
CBU thawing and infusion procedure
Cryopreserved CBUs were thawed and washed as described by Rubinstein and colleagues13 and re-suspended in dextran 40 plus 5% human serum albumin solution on the day of infusion. All thawed CBUs were filtered using a 170-µm blood set filter routinely used in red blood cell (RBC) transfusions. Thawed CBUs were tested for enumeration of TNC, CD34+ cells, CFUs, cell viability, ABO and Rh typing, and bacterial and fungal cultures. On the day of infusion, patients were admitted to the Duke Children’s Health Center Day Hospital and IV access was established via a peripheral vein. After medication with acetaminophen (10 mg/kg PO), diphenhy-dramine (1 mg/kg IV), and methylprednisolone (1 mg/kg IV), patients received either a portion of or their entire CBU via peripheral IV infusion over 15 to 30 minutes. IV fluids were administered at twice maintenance for 2 to 4 hours after the CB infusion. Vital signs and pulse oximetry were monitored every 5 minutes during the infusion and every 30 minutes for 2 to 4 hours post infusion.
Data collection
Autologous CBUs
Data regarding patients (diagnosis, age, birth history, and symptoms), infusions, and autologous CBU characteristics (collection volume, precryopreservation and post-thaw TNC, CD34 count, viability, sterility cultures, and post thaw CFUs) were obtained from both a prospectively maintained clinical database and a retrospective review of routine medical records.
Publicly banked CBUs
Precryopreservation parameters (collection volume, TNC, and CD34 count) of publicly banked CBUs were obtained from records at the Carolinas Cord Blood Bank, a public CB bank located at Duke University.
Statistical analysis
Medians and ranges were calculated for each precryopreservation variable. The Wilcoxon ranked sum test was used to determine differences in medians of precryopreservation variables between autologous CBUs and CBUs publicly banked at the Carolinas Cord Blood Bank. All analyses were performed in computer software (SAS 9.2 for Windows, SAS Institute, Inc., Cary, NC).
RESULTS
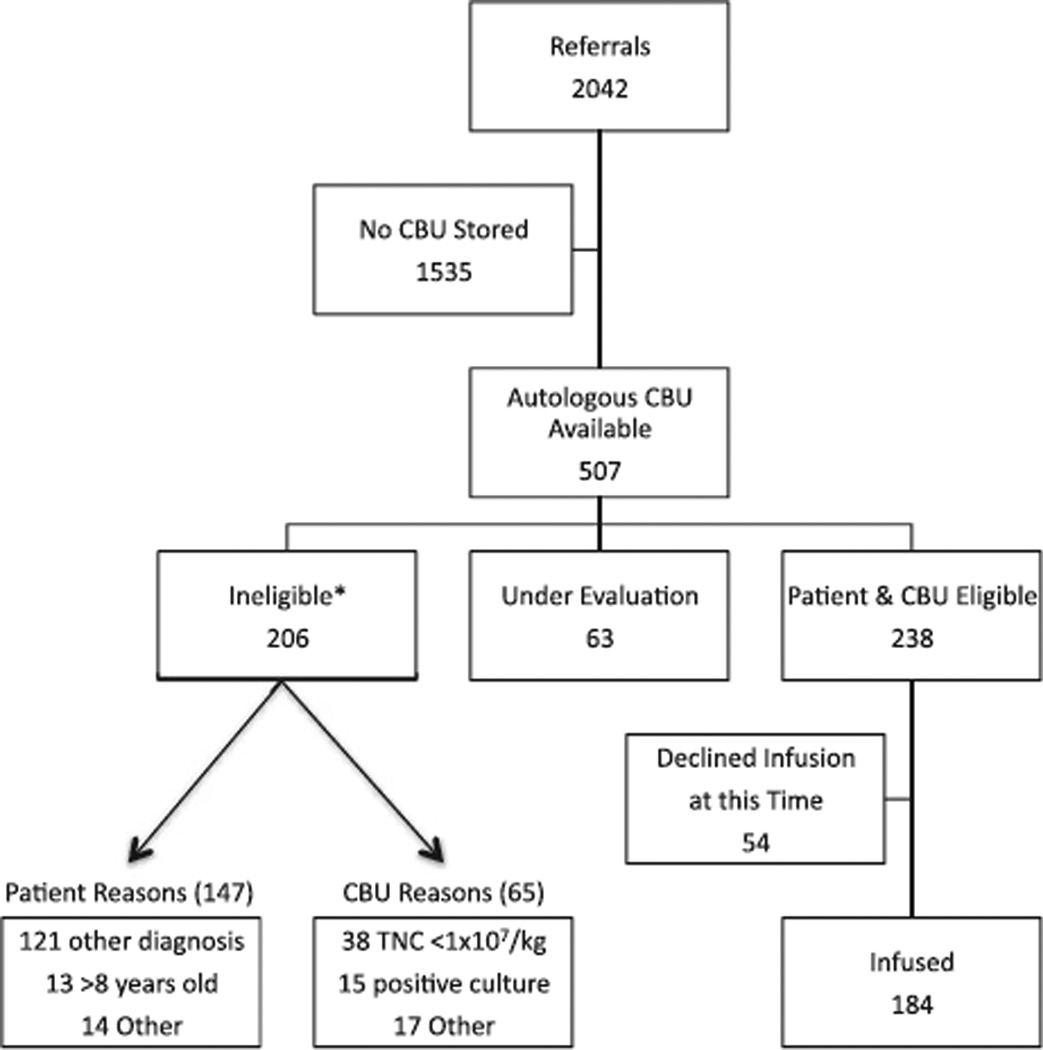
Over the study period of March 2004 to December 2009, the Pediatric Blood and Marrow Transplantation Program at Duke received 2042 referrals for autologous CB infusions. Of those, 75% (n = 1535) did not have a banked CBU. Of the remaining 507 patients with banked autologous CBUs, 41% (n = 206) did not meet the criteria for infusion, for either patient (n = 147) or CBU (n = 65) reasons. While the major reason for patient exclusion was an ineligible diagnosis, the major reason for CBU exclusion was inadequate cell dose (see Fig. 1).
Fig. 1.
Patient referrals. *Some patients or CBUs have more than one exclusion criterion.
Patient characteristics
Of those referred who met both patient and CBU criteria (n = 238), 184 children received 198 autologous CB infusions (14 patients received two infusions based on availability of larger cell doses). Median age at diagnosis was 3 months (range, prenatally-6 years), and median age at infusion was 27 months (range, 6 days–9.5 years). Median weight at the time of infusion was 12.3 kg (range, 1.7–34.9 kg). Fifty-four percent of patients were male, and 46% were female. The majority of patients (91%) were Caucasian. Diagnoses are listed in Table 1. Of patients who received autologous CB infusions, approximately 10% were later found to have an underlying genetic disorder (Angelman’s syndrome, mitochondrial diseases, chromosomal deletions) causing their symptoms.
TABLE 1.
Patient characteristics
| Characteristic | Number | Percent |
|---|---|---|
| Age at infusion | ||
| ≤6 months | 30 | 15 |
| 7 months-3 years | 120 | 61 |
| ≥4 years | 48 | 24 |
| Sex | ||
| Male | 99 | 54 |
| Female | 85 | 46 |
| Diagnosis | ||
| Cerebral palsy | 140 | 76 |
| Congenital hydrocephalus | 23 | 12 |
| Other injuries | 23 | 12 |
CBU characteristics
Autologous CBUs were obtained from 24 different CB banks: 149 (81%) CBUs came from 11 private US banks (113 from two of the larger private US banks), 13 (7%) CBUs from 11 international banks, and 22 (12%) CBUs from two public banks. Units stored at public banks were collected as directed donations and therefore did not have to meet volume or content criteria typically required of units stored for public use. Forty-six percent (n = 84) of CBUs were stored in vials, and 54% (n = 100) in bags. The majority of CBUs stored in vials had been subjected to density gradient separations in Ficoll before cryopreservation, while those stored in bags underwent more traditional RBC depletion and volume reduction. Only 18% (32/179) of CBUs were stored submerged under liquid nitrogen, the storage method commonly used in public banks, whereas 82% (147/179) were stored in the vapor phase of liquid nitrogen. Storage practices of the most commonly used CB banks are described in Table 2.
TABLE 2.
Practices at the most frequently used CB banks
| Bank | Number | Liquid nitrogen or vapor phase | Bags or vials | ABO performed (%) | CD34 performed (%) |
|---|---|---|---|---|---|
| 1 | 70 | Vapor | Both | 33 | 4 |
| 2 | 43 | Vapor | Bags | Yes | Yes |
| 3 | 21 | Liquid | Bags | Yes | Yes |
| 4 | 17 | Vapor | Both | 59 | Yes |
Thawing and postthaw graft characteristics
The autologous CBUs were packaged in various ways—some in vials, some in single bags, and some in compartmentalized bags. When a large collection was obtained and when the CBU was stored in multiple vessels, a portion of a CBU was used for a single infusion. The amount of the CBU thawed for infusion depended on the TNC dose/kg delivered to a patient. Based on experience in allogeneic CB transplantation, doses between 3 × 107 and 5 × 107 cells/kg were targeted for a single infusion, but doses of 10 × 107 cells/kg or more were allowed. There were no criteria for maximum cell dose. When possible, some of the unit was maintained frozen for possible future use. When there were sufficient cells, a small number of patients (n = 14) received a second infusion 6 to 12months after the first. A median precryopreservation TNC of 3.1 × 108 cells (2.6 × 107 cells/kg) were thawed for infusion. Median postthaw recovery of TNC was 82% (range, 13%–200%), and patients were dosed with a median of 2.0 × 107 TNC/kg (range, 0.1 × 107–13.3 × 107), 0.7 × 105 CD34+ cells/kg (range 0.04 × 105–6.4 × 105), and 6.5 × 104 CFU/kg (range, 0 × 104–315 × 104). Postthaw sterility cultures were positive in 7.6% (n = 14) of CBUs infused, versus 0.5% of public units used for transplantation. Organisms cultured are shown in Table 3. Upon thawing, a white particulate matter was visible in the bag of eight autologous CBUs, all of which had been processed using the AXP system before cryopreservation.
TABLE 3.
Positive postthaw sterility cultures
| Organism | Number |
|---|---|
| Coagulase-negative Staphylococcus | 5 |
| Streptococcus viridans | 2 |
| Diphtheroids | 2 |
| Ralstonia pickettii | 2 |
| Gram-negative rods | 2 |
| Lactobacillus | 1 |
Infusions
Infusions were generally well tolerated. Three patients (1.5%) experienced anaphylactic reactions during their CB infusion characterized by wheezing with or without urticaria 2 to 10 minutes after the IV infusion was initiated. The reactions resolved after discontinuation of the infusion and treatment with additional IV diphenhydramine and bronchodilators. The remainder of the CB cells were discarded for two of the infusions stopped before completion; one patient was able to restart and complete the CB infusion. One patient’s mother experienced an allergic reaction consisting of urticaria, presumably due to contact with dimethyl sulfoxide exhaled onto her face and neck by her child receiving a CB infusion. The reaction resolved with oral diphenhydramine. Despite 14 positive cultures, there were no clinically apparent infections, and no children were treated with antibiotics.
Follow-up
Median length of follow-up is 12 months. No adverse events have been reported with short-term follow-up. Specifically, no infections, autoimmune diseases, tumors, or other adverse events have been observed.
Public versus private CBUs
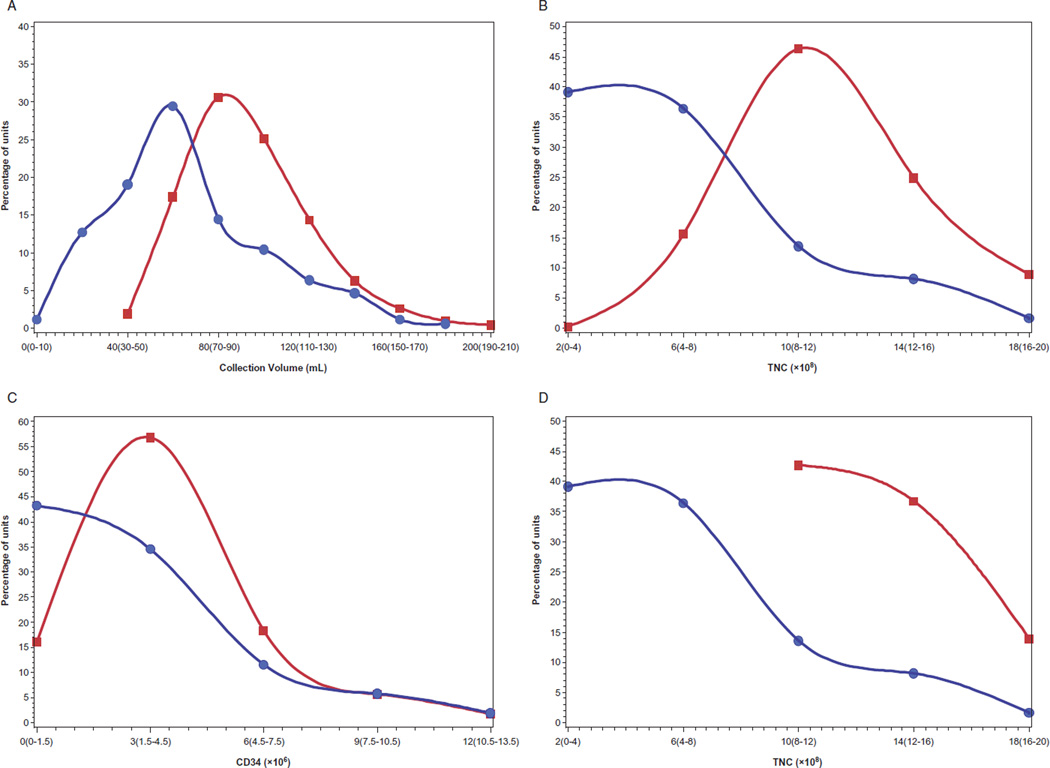
We compared quality parameters of the CBUs infused in this study to the inventory of the Carolinas Cord Blood Bank (n = 22,624), a public US CB bank. Median precryopreservation volume, TNC, and CD34 counts of autologous CBUs used for infusion were significantly lower than publicly stored CBUs. The median collection volumes of autologous CBUs was 60 mL (range, 5–180 mL) versus 89 mL (range, 40–304 mL) for publicly banked CBUs (p < 0.0001). The median precyropreservation TNC of autologous CBUs was 4.7 × 108 (range, 0.3 × 108–33.8 × 108) versus 10.8 × 108 (range, 2.1 × 108–58.4 × 108) for publicly banked CBUs (p < 0.0001). Precryopreservation CD34 counts were only available for 104 of 184 autologous CBUs, and the median, 1.8 × 106 (range, 0 × 106–19.1 × 106), was significantly below that of units stored publicly, 3.0 × 106 (range, 0.01 × 106–112.2 × 106; p< 0.0001). Distribution curves are shown in Fig. 2.
Fig. 2.
Distributions of quality variables (A–C, collection volume, TNC, and CD34 of autologous CBUs [●] and entire Carolinas Cord Blood Bank inventory [■]; D, TNC of autologous CBUs [●] and NCBI-eligible Carolinas Cord Blood Bank CBUs [■]).
Some groups have instituted acceptance criteria for banking CBUs in public banks. The Stem Cell Therapeutic and Research Act of 2005 established the National Cord Blood Inventory (NCBI) to increase the number of quality CBUs available for allogeneic transplantation. Among other criteria, this program requires banked CBUs to have a TNC of 9 × 108 or greater, viability of 85% or greater, and attached segments for confirmatory testing.14 Only 14% (25/184) of autologous CBUs used for infusion in this study met these NCBI eligibility criteria. In October 2009, the FDA issued final guidance for licensure of CBUs intended for allogeneic use including safety and potency criteria.15 Specifically, a precryopreservation TNC of 5 × 108 or greater and viable CD34 count of 1.25 × 106 or greater were recommended. Of the autologous units used for this study with available CD34 counts, only 47% (49/104) would meet these criteria for licensure. Currently, many private banks do not even perform CD34 counts before storage.
DISCUSSION
In this report, we describe our experience with IV infusion of autologous CB in young children with acquired neurologic disorders. This study also represents the largest experience to date of privately banked CBUs utilized for autologous purposes. This study primarily evaluated the safety and feasibility of the procedure in the outpatient setting. Clinical adverse events were limited to three infusion reactions in patients and one contact reaction in a child’s mother, all successfully treated with medical management with no lasting sequelae, demonstrating the procedure to be both safe and feasible. An upcoming randomized placebo-controlled clinical trial will formally assess the effectiveness of this approach.
Notably, despite a higher prevalence of cerebral palsy among African American children,16 the vast majority of participants in this study were Caucasian. This may indicate a racial disparity in access to private autologous CB banking and to this procedure that could extend to any future cellular therapies developed using autologous CB. The cause of such a disparity is likely multifactorial, and awareness of the study or ability to travel for the procedure may play a role. However, it also likely reflects unequal access (possibly due to socioeconomic factors) to private CB banking, an issue that will be amplified as use of autologous CB increases.17
Also of note, approximately 10% of children diagnosed with cerebral palsy in this study were subsequently found to have an underlying genetic diagnosis responsible for their symptoms. This reflects the lack of specificity of a diagnosis of cerebral palsy and the need to conduct more thorough diagnostic evaluations in these patients. In the case of genetic diseases, autologous CB infused in this fashion would not be expected to have benefit. In future trials of children with cerebral palsy, strict diagnostic and inclusion criteria will be necessary to ensure that only patients with potential for benefit are included.
This series highlights substantial variability in processing and storage methods among CB banks that may affect CBU quality. Almost half of autologous CBUs were stored in vials. Smaller than bags, cryovials are less costly to store as more can be housed in a given freezer unit. To be thawed and infused, however, the CB must be transferred to another container, thereby increasing the risk of bacterial contamination and decreasing cell recovery. Additionally, most autologous units were stored in the vapor phase of liquid nitrogen, a less expensive and space-saving alternative to submersion under liquid nitrogen as utilized by many public banks. Vapor-phase storage increases exposure to temperature fluctuations, a factor to which hematopoietic stem cells are exquisitely sensitive and that carries a potential risk of decreasing the potency of the CBU.
We examined quality parameters of these autologous CBUs including precryopreservation collection volume, TNC, and CD34 count of the autologous units and found that they were all substantially inferior to units stored in public banks.18 The medians obtained in this study over-estimate the true parameters of all privately stored CBUs, because the CBUs of many referred patients were deemed ineligible for infusion due to low cell counts. There was also a significantly higher incidence of bacterial contamination upon thawing among privately banked CBUs compared to public CBUs. Only a small proportion of the infused CBUs from private banks met FDA (47%) or NCBI (14%) banking criteria.
Collection practices may account for some of the quality differences between public and private CBUs, particularly collection volume. Currently, public CB donation is only available at predefined collection sites which utilize trained personnel for the collection of CB. In our bank, a trained CB collector does not reach proficiency until they have collected 80 CBUs. On the other hand, privately stored units are collected by the mother’s obstetrician or midwife, who is generally not formally trained in CB collection. In addition, because the obstetrician’s primary responsibility at the time of delivery is to the mother and child, private CB collections do not always receive the undivided attention afforded to public donations.
If autologous CB infusion is determined to be efficacious in the treatment of neurologic or other diseases, then the paradigm around CB banking might shift and CB collection might become the standard of care in all hospitals in the future. Therefore, we suggest that banked autologous CBUs be held to the same standards as publicly banked units. In an effort to enhance the quality of autologous CBUs, collection practices could be expanded. Experienced collection staff could be employed as routine hospital staff at busy centers, and training in CB collection could be incorporated into obstetric and gynecology residency programs. Processing and storage methods could be optimized to maximize recovery of cells collected, and efforts should be made to improve access to CB banking.
Many children with cerebral palsy and other brain injuries of infancy are not diagnosed at birth but develop symptoms in the first few months of life. Therefore, if clinical benefit is shown in this population, public banks could allow parents to store their child’s CBU regardless of cell dose but delay listing it on a donor registry for up to 1 year. At that time, the parents could elect to continue to store the CBU privately at a cost or donate it for public use. This option would require public banks to increase their inventory to accommodate some units that do not meet current criteria for public donation. It also does not take into account potential future uses of autologous CB in older children and adults.
In summary, umbilical CB is a unique source of stem and progenitor cells with multiple potential applications in the fields of regenerative medicine and cellular therapies. Potential applications of autologous cells have not yet been elucidated. Currently, CB legislation primarily focused on informing parents about CB banking options has been enacted in 17 states, but the nuances and differences in CB banking quality have not been addressed. If CBUs are to be effectively utilized in the allogeneic and/or autologous setting, their safety and potency is of utmost importance. Tomaximize the likelihood that an individual CBU is suitable for use, a quality product must be collected, produced, and stored regardless of its intended recipient.
ACKNOWLEDGMENTS
The authors thank the entire staff of the Pediatric Blood and Marrow Transplant Program for responding to all patient referrals and caring for these patients, the Stem Cell Laboratory for processing the umbilical CBUs, and the families of these patients for allowing us to treat their children and study and report our experience with this therapy. We also thank the CB banks for providing the patient’s autologous CBU and test sample for this study without further charge to their family.
Supported by Training Grant 5-T32-HL007057-34.
ABBREVIATIONS
- CB
cord blood
- CBU(s)
cord blood unit(s)
- NCBI
National Cord Blood Inventory
- TNC
total nucleated cell count.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
REFERENCES
- 1.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci USA. 2003;100:645–650. doi: 10.1073/pnas.0237086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11:275–281. [PubMed] [Google Scholar]
- 4.Nishio Y, Koda M, Kamada T, Someya Y, Yoshinaga K, Okada S, Harada H, Okawa A, Moriya H, Yamazaki M. The use of hemopoietic stem cells derived from human umbilical cord blood to promote restoration of spinal cord tissue and recovery of hindlimb function in adult rats. J Neurosurg Spine. 2006;5:424–433. doi: 10.3171/spi.2006.5.5.424. [DOI] [PubMed] [Google Scholar]
- 5.Nan Z, Grande A, Sanberg CD, Sanberg PR, Low WC. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann N Y Acad Sci. 2005;1049:84–96. doi: 10.1196/annals.1334.009. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 7.Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 8.Vendrame M, Cassady J, Newcomb J, Butler T, Penny-packer KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- 9.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier C, Middelanis J, Wasielewski B, Neuhoff S, Roth-Haerer A, Gantert M, Dinse HR, Dermietzel R, Jensen A. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr Res. 2006;59:244–249. doi: 10.1203/01.pdr.0000197309.08852.f5. [DOI] [PubMed] [Google Scholar]
- 11.Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 12.Kurtzberg J, Kosaras B, Stephens C, Snyder EY. Umbilical cord blood cells engraft and differentiate in neural tissues after human transplantation. Biol Blood Marrow Transplant. 2003;9:128–129. [Google Scholar]
- 13.Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, Taylor PE, Stevens CE. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contract between the Health Resources and Services Administration and the Carolinas Cord Blood Bank at Duke University Medical Center, contract #HHSH23420073/002C. 2006 Nov [Google Scholar]
- 15.Office of Communication, Outreach and Development (OCOD) Rockville (MD): U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research; 2009. Guidance for industry: minimally manipulated, unrelated allogeneic placental/umbilical cord blood intended for hematopoietic reconstitution for specified indications. [Google Scholar]
- 16.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 17.Faden RR, Dawson L, Bateman-House AS, Agnew DM, Bok H, Brock DW, Chakravarti A, Gao XJ, Greene M, Hansen JA, King PA, O’Brien SJ, Sachs DH, Schill KE, Siegel A, Solter D, Suter SM, Verfaillie CM, Walters LB, Gearhart JD. Public stem cell banks: considerations of justice in stem cell research and therapy. Hastings Cent Rep. 2003;33:13–27. [PubMed] [Google Scholar]
- 18.Kurtzberg J, Cairo MS, Fraser JK, Baxter-Lowe L, Cohen G, Carter SL, Kernan NA. Results of the cord blood transplantation (COBLT) study unrelated donor banking program. Transfusion. 2005;45:842–855. doi: 10.1111/j.1537-2995.2005.04428.x. [DOI] [PubMed] [Google Scholar]