Abstract
In this review, we discuss the possible pathophysiological mechanisms and the role of arterial stiffness as a biomarker, a blood pressure–independent predictor of cardiovascular morbidity and mortality. The effects of different antihypertensive drug classes on noninvasively assessed markers of arterial stiffness are also discussed. Current evidence will be reviewed regarding the effect of drugs on arterial stiffness, including the peripheral and central effects of angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, dihydropyridine calcium channel blockers, beta blockers (including vasodilating beta blockers), diuretics, and mineralocorticoid antagonists.
Keywords: pulse wave analysis, pulse contour analysis, pulse wave velocity, pulse wave amplification, augmentation index, hypertension
In most industrialized countries, cardiovascular disease is the leading cause of morbidity and mortality,1 and elevated brachial artery blood pressure (BP) is a classic major risk factor and powerful predictor of cardiovascular organ damage, morbidity, and mortality2 (Fig. 1). Abundant clinical data have shown that treatment of elevated BP is associated with reduced target organ damage, cardiovascular morbidity, and mortality.3,4
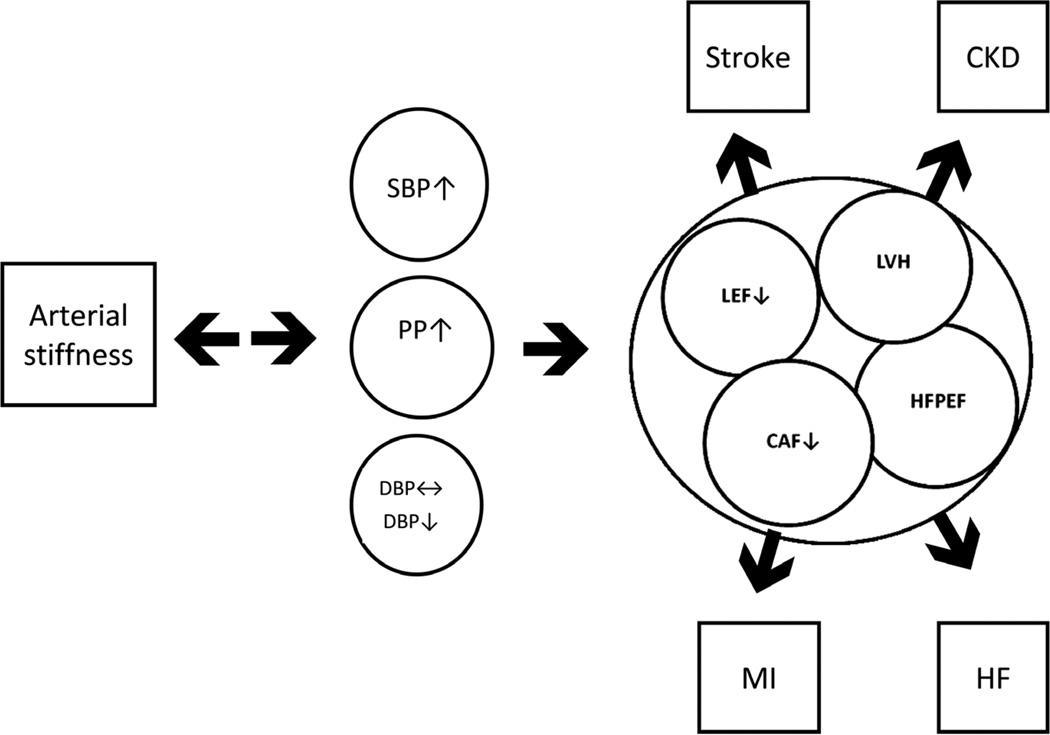
Figure 1.
The diagram describes the effects of arterial stiffness on the hophysiology of cardiovascular disease. CAF indicates coronary artery flow; CKD, chronic kidney disease; DBP, diastolic blood pressure; HF, heart failure; HFPEF, heart failure with preserved ejection fraction; LEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; MI, myocardial infarction; PP, pulse pressure; SBP, systolic blood pressure.
BP varies in different arterial systems, and invasive and noninvasive studies have shown that brachial arterial BP does not necessarily reflect central aortic BP.5 Although young individuals have a higher brachial than central aortic BP, older individuals develop higher central BP mediated by increasing arterial stiffness. An increased central BP may lead to target organ damage through a variety of mechanisms. Clinical data indicate that lowering brachial arterial BP does not necessarily correlate with equal lowering of central BP or arterial stiffness. Increased arterial stiffness independent of brachial arterial BP appears to be a novel and independent risk factor for cardiovascular disease and mortality6 in well-functioning older adults7 and hypertensive patients.8 In this article, the effects of various antihypertensive drugs on arterial stiffness are discussed.
PATHOPHYSIOLOGY OF ARTERIAL STIFFNESS IN HYPERTENSION
The mechanism of elevated BP and arterial stiffness is not fully understood. Vascular change in the composition of tissue components within the vascular wall through mechanical factors, that is, mainly uncontrolled BP and the combination of metabolic factors,9 may lead to increased arterial stiffness. Available data indicate that uncontrolled BP and aging leads to a synergistic increase in arterial stiffness. Recent data suggest that arterial stiffness may even precede hypertension,10 challenging the classic paradigm that hypertension results in altered vascular structure and function and resultant increased arterial stiffness. Finally, accumulating data suggest that despite similar brachial arterial BP-lowering effects, the impact on central aortic BP and arterial stiffness differs with various antihypertensive drug classes (Table 1).11,12
TABLE 1.
Summary of the Effect of Selected Antihypertensive Drug Classes
| Drug Class | Wave Reflection | PWV |
|---|---|---|
| ACE inhibitor | ||
| Ramipril | ↓ | ↓ |
| Tranolapril | ↓ | ↓/↔ |
| Quinapril | ↓ | ↓ |
| Lisinopril | ↔ | ↔ |
| Perindopril | ↓ | ↓ |
| Enalapril | ↓ | ↓ |
| Beta blocker | ||
| Atenolol | ↓ | ↔/↓/↑ |
| Metoprolol | ↔ | |
| Bisoprolol | ↓ | ↑ |
| Nebivolol | ↓ | ↔/↓ |
| Propanolol | ↓ | ↑ |
| Diuretics | ||
| Hydrochlorothiazide | ↔ | ↔ |
| Indapamide | ↔ | ↔ |
| CCB | ||
| Amlodipine | ↔ | ↓ |
| Nitrendipine | ↓ | ↓ |
| Nifedipine | ↓ | |
| Felodipine | ↓ | ↓ |
| Verapamil | ↓ | |
| Direct renin inhibitors | ||
| Aliskiren | ↓ | ↓ |
| MCRA | ||
| Spironolactone | ↔/↓ | ↓ |
| Eplerenone | ↓ | ↓ |
Adapted from Drugs.12
ACE indicates angiotensin-converting enzyme; CCB, calcium channel blocker; MCRA, mineralocorticoid receptor antagonist; PWV, pulse wave velocity.
EFFECTS OF ANTIHYPERTENSIVE DRUGS ON ARTERIAL STIFFNESS
A meta-analysis by the BP Lowering Treatment Trialists’ Collaboration from randomized, controlled trials demonstrated significant differences in cause-specific outcomes due to beneficial effects of individual antihypertensive agents.13 The Losartan Intervention For Endpoint Reduction in Hypertension (LIFE)14 study and the Anglo-Scandinavian Cardiovascular Outcomes Trial (ASCOT)15 compared antihypertensive regimens with an angiotensin receptor blocker (ARB) (losartan with or without a diuretic) or a calcium channel blocker (CCB) (amlodipine with or without perindopril) with beta blocker (atenolol) treatment. In the LIFE study, cardiovascular death, stroke, and myocardial infarction were reduced by losartan vs atenolol in hypertensive patients with left ventricular hypertrophy. In the highest vs lowest quartile of pulse pressure (PP), there was a significantly increased risk for stroke and total mortality with atenolol-based treatment. Williams et al16 describe findings of the Conduit Artery Function Evaluation (CAFE) study, a substudy of ASCOT,15 which compared the effects of atenolol-based and amlodipine-based regimens on central aortic pressure and hemodynamics in 2199 hypertensive patients. Data from this study suggest a significant reduction in arterial systolic BP (SBP) and PP in hypertensive patients treated with CCBs compared with those taking beta blockers, whereas peripheral BP did not show a difference between groups. Central BP and cardiohemodynamic parameters were assessed using applanation tonometry to evaluate arterial stiffness. One interesting finding of the CAFE study16 was that beta blockers did not lower central SBP as much as CCBs, supporting the idea that therapy with antihypertensive agents may have different effects on arterial stiffness, and thus, central hemodynamic parameters despite a similar effect on brachial artery BP. Selecting antihypertensive agents that not only lower brachial artery BP but have a favorable impact on central BP and arterial stiffness may be an important consideration in selecting the optimal cardiovascular drug therapy.
In the following discussion, we will review individual antihypertensive agents and their effects on noninvasively assessed markers of arterial stiffness, that is, augmentation index (AIx), pulse contour analysis, and pulse wave velocity (PWV) in patients with arterial hypertension. The assessment of AIx and central BP from radial pulse-derived waveforms using applanation tonometry17,18 and the assessment of carotid-femoral aortic PWV are considered by some to be the most reliable measurements to evaluate arterial stiffness.6 Data suggest that increased PWV predicts a greater risk of cardiovascular morbidity and mortality in patients with comorbidities such as hypertension,9 diabetes mellitus,19 and end-stage renal disease20 compared to those without these conditions.5,21
Beta-adrenergic Blockers
Beta blockers have been well-studied in regard to their effect on brachial arterial and central BP. Recent data suggest that they may be inferior to other antihypertensive drugs and in preventing stroke,22,23 an observation that may be partially explained by different effects of beta blockers on peripheral vs central BP. For example, the reduced effectiveness of atenolol on central BP and arterial stiffness parameters has been shown in several studies. In the Preterax in REgression of Arterial Stiffness in a contrOlled double-bliNd (REASON) study,24 a subgroup of 124 patients treated with either 50 or 100 mg atenolol daily were analyzed at baseline and again after 1 year of treatment. The effect on brachial arterial BP was more pronounced than on central aortic SBP (16.2 vs 8 mm Hg decrease). In this study, central PP showed a nonsignificant increase of 2.3 mm Hg after treatment with atenolol, suggesting an increase of PW reflection measured by an increased AIx of 2.5%. Two small randomized, double-blind, crossover studies conducted by Dhakam et al25 and Morgan et al26 came to similar conclusions. Dhakam et al25 compared atenolol 50 mg with nebivolol 5 mg in 16 patients with isolated systolic hypertension. The brachial BP-lowering effect of both agents was similar, as was the PWV, while there was an increase in AIx and a higher heart rate in the nebivolol vs the atenolol group. However, aortic PP was significantly lower in the nebivolol group (50 vs 54 mm Hg). In a randomized study by Mahmud et al,27 the effect of atenolol and nebivolol on brachial BP and PWV was similar in 40 patients with untreated hypertension. However, while atenolol reduced PP, nebivolol’s effect on PP was more pronounced, as was its effect on AIx. The possible mechanism for nebivolol’s superior effect may include increased levels of nitric oxide associated with the vasodilating effects of the drug.
To further investigate the effects of nebivolol on central pressure and left ventricular wall thickness, Kampus et al28 conducted a randomized, double-blind study in 80 patients comparing nebivolol with metoprolol succinate. Patients were randomized to either nebivolol 5 mg or metoprolol succinate 50 or 100 mg daily for 12 months. Both beta blockers similarly reduced heart rate, brachial BP, and mean arterial pressure. AIx and PWV remained the same in both treatment groups. However, central aortic BP, PP, and left ventricular septal wall thickness were significantly reduced after 1 year of treatment in the nebivolol treatment group. The change in left ventricular septal wall thickness was correlated with central aortic BP (r = 0.41; P = 0.001) and PP (r = 0.32; P = 0.01). The investigators postulated that the possible beneficial effects of nebivolol may be attributed to the enhanced release of endothelium-derived nitric oxide, and therefore improved endothelial function and reduced arterial stiffness. Although there are differential effects of beta blockers on arterial stiffness, overall, they have generally been shown to be inferior to ARBs, angiotensin-converting enzyme (ACE) inhibitors and CCBs.29,30
Diuretics
Diuretics have been shown to lower BP both as monotherapy and as an add-on agent.29,31 The most commonly used diuretic agent in the United States is hydrochlorothiazide,32 despite the fact that chlorthalidone has been shown to be more potent, has a longer duration of action, and has been better validated in clinical outcome trials.33 The effects of diuretics on arterial stiffness measures have not been as well studied as other drug classes. In a small randomized crossover study conducted by Morgan et al26 with previously untreated essential hypertensive patients, the effect of 25 and 50 mg hydrochlorothiazide on arterial stiffness was assessed after a 4-week treatment phase. Brachial artery SBP was significantly reduced (by 15.2 mm Hg) compared to placebo, whereas changes in AIx were not significant. In a double-blind randomized study of 471 patients with essential hypertension, Asmar et al34 evaluated low-dose combination treatment with indapamide (0.625 mg) and perindopril (2 mg) compared with atenolol (50 mg). Patients were followed for 12 months, and although both drug regimens resulted in the same diastolic BP (DBP) reduction, the combination of indapamide and perindopril reduced SBP and PP significantly more than atenolol. These studies indicate that diuretics have a rather neutral effect on central BP without any favorable effect on arterial wall composition and arterial stiffness beyond brachial artery BP reduction. Although chlorthalidone is considered the better thiazide-like diuretic compared to hydrochlorothiazide, to our knowledge there are no clinical trials evaluating the effects of chlorthalidone on arterial stiffness.
Calcium Channel Blockers
Long-acting CCBs are safe and established antihypertensive agents. Dihydropyridine-type CCBs like amlodipine not only antagonize the L-type calcium channel, but in animal models also have been shown to have antioxidant effects.35,38 A number of CCBs have been evaluated regarding their effect on central BP and arterial stiffness. London et al36 investigated the effect of nitrendipine 20 or 40 mg once daily in 10 patients with end-stage renal disease using direct carotid tonometry. After 1 year of therapy, brachial artery BP and central BP were significantly reduced, with a more pronounced effect on central PP. The investigators also observed a significant decrease in aortic stiffness assessed by carotid-femoral PWV and a decrease in AIx. Deary et al37 investigated the effect of amlodipine 5 mg once daily on brachial artery BP and central BP in 30 patients after 6 weeks of treatment. Both parameters were significantly reduced. In a randomized, crossover study of the effects of felodipine (n = 16) or amlodipine (n = 28) on arterial stiffness, Morgan et al26 evaluated 44 elderly untreated patients with essential hypertension. Neither treatment demonstrated any difference on central BP at the lower dosage. However, with increasing dosage (10 vs 5 mg) the effect on central BP and brachial artery BP was more pronounced. In comparison with placebo, the CCB-treated groups showed a more pronounced effect on central than brachial artery pressure (−20.0 and −17.7 mm Hg) and on PPs (−12.0 and −11.2 mm Hg). In addition, a significant reduction of AIx was observed (−10%) in the treatment groups vs placebo.
ACE Inhibitors
In most of the conducted randomized studies, ACE inhibitors lower central aortic BP more than brachial artery BP.29 Possible mechanisms of this beneficial effect on arterial compliance and central BP have been postulated, including a reduction of oxidative stress and inflammation and vasodilation through angiotensin II inhibition,38 causing smooth muscle relaxation and recomposition of the vessel wall. For example, in a randomized, crossover, placebo-controlled study,26 the effect of enalapril 20 and 40 mg once daily was compared to perindopril 4 and 8 mg on peripheral and central BP after 4 weeks of treatment. Both treatment arms were similarly independent of the dosage regarding their effect on central BP, while demonstrating a greater reduction of central compared with brachial artery BP (−13.0 vs −8.3 mm Hg) and PP (−9.0 vs −3.9 mm Hg). Both agents also significantly reduced the AIx. In another randomized, double-blind, placebo-controlled, crossover study, Hirata et al38 investigated the acute change in BP and arterial stiffness in 30 patients with high cardiovascular risk. The investigators observed reduced AIx and arterial stiffness, along with reduced central and brachial artery BP, 5 hours after administration of 10 mg ramipril.
In a randomized, double-blind, controlled study by Jiang et al,39 101 patients with mild essential hypertension were randomized to either enalapril 10 mg or indapamide 2.5 mg a day. Both agents reduced brachial artery SBP and DBPs, mean arterial pressure, and PP, while the effect on central BP and PP was more pronounced with enalapril. There was also a 5.4% reduction in the enalapril-treated group in pulse wave reflection measured by AIx. The aggregate evidence supports the beneficial effect of ACE inhibitors on central BP and arterial stiffness.
Angiotensin Receptor Blockers
The newer ARBs and their effects on central BP and arterial stiffness have been investigated in only a few studies. Mahmud and Feely40 investigated the effect of valsartan 80 mg as add-on treatment after 2 weeks in 18 patients with uncontrolled hypertension. The investigators found that valsartan 80 mg significantly reduced brachial and central BP. There was an even more pronounced reduction of central SBP than brachial artery SBP, resulting in a significant increase of the PP amplification (from 8 ± 3 at baseline vs 12 ± 7 at 2 hours to 14 ± 5 mm Hg at 2 weeks, P < 0.01). The augmentation pressure decreased following valsartan. AIx was also significantly reduced from 21 ± 8% at baseline to 11 ± 7% at 2 hours and 10 ± 5% at 2 weeks (P < 0.01). Similar effects were found by Dhakam et al25 in a double-blind, randomized, crossover study comparing treatment with atenolol 50 mg with eprosartan 600 mg daily in 21 subjects with newly discovered essential hypertension. The investigators found no difference in the reduction of brachial artery BP, mean arterial pressure, and PWV between the 2 regimens. Central BP lowering was significantly more pronounced with eprosartan, and AIx was increased in the atenolol group and decreased in the eprosartan group. The investigators postulated that atenolol’s effects on arterial stiffness (in contrast to ARBs) could have been a possible explanation for the failure of atenolol to improve cardiovascular outcomes.
Direct Renin Inhibitors
Increased activity of the renin-angiotensin-aldosterone system has been associated with increased cardiovascular morbidity and mortality in hypertensive patients. It has been shown that antagonizing the renin-angiotensin-aldosterone system improves arterial stiffness and endothelial function.41 Recently, aliskiren, a direct renin inhibitor, has become available for the treatment of essential hypertension. In animal studies, aliskiren improves vascular function and prevents atherosclerosis.42 In a pilot study on the effect of aliskiren 300 mg daily in 10 patients with uncomplicated type 1 diabetes mellitus,43 arterial stiffness was measured by AIx and PWV. Patients were evaluated at baseline and after 30 days of treatment. All measures of arterial stiffness showed improvement, with changes in central SBP of 104 at baseline vs 97 mm Hg, in AIx of 21.7 vs 16.6 mm Hg, and in PWV of 7.4 vs 6.7 m/s. However, there is one note of caution with the use of the direct renin inhibitors separate from the effects on arterial stiffness. The Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (ALTITUDE) was a randomized cardiovascular outcomes trial comparing aliskiren vs placebo added to an ACE inhibitor or an ARB in patients with high-risk type 2 diabetes mellitus. The ALTITUDE data safety and monitoring board stopped the trial due to “… increased risk for nonfatal stroke, renal complications, hyperkalemia, and hypotension in patients taking aliskiren after 18–24 months” and the futility for seeing cardiovascular or renal benefits.44 This follows the results of the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET),45 which compared the ARB telmisartan to the ACE inhibitor ramipril, or the combination of the 2 in a large cardiovascular outcomes trial of adults at high risk for cardiovascular events [like the Heart Outcomes Prevention Evaluation (HOPE)46 trial entry criteria]: although ONTARGET showed no difference between the ACE inhibitor and ARB, the combination showed no further cardiovascular benefit but more serious adverse outcomes, especially renal. Therefore, we would discourage using this combination for further BP reduction or for presumed cardiovascular prevention.
Mineralocorticoid Receptor Antagonists
Historically, the nonselective mineralocorticoid receptor antagonist (MCRA) spironolactone has been primarily used as a potassium-sparing diuretic and antiandrogen. Within the last 2 decades, there has been a paradigm shift based on data, suggesting that MCRA is effective in the treatment of hypertension47 and that it also improves endothelial function and arterial stiffness.41 Furthermore, these improvements have been shown to be independent of its BP-lowering effect with the postulation that the effects are the result of antagonism of inflammatory and profibrotic pathways. This aforementioned paradigm shift is based on the findings that (1) aldosterone release is driven by additional factors other than its effects on angiotensin alone; (2) the MCR is also activated by cortisol in patients with heart failure and essential hypertension; and (3) aldosterone acts primarily on the vasculature and central nervous system with resultant hypertension.48 Incidentally, beneficial effects of MCRA blockade in patients with increased arterial stiffness have been found even in patients with low serum aldosterone levels, supporting the hypothesis that aldosterone is only one of the ligands on the MCRA receptor.48,49
In the past, the use of MCRA blockade was limited due to side effects. Recently, the selective MCRA eplerenone was developed as an alternative for patients experiencing antiandrogen effects with spironolactone (eg, breast tenderness, gynecomastia, and erectile dysfunction). Mahmud and Feely48 conducted a randomized single-blind crossover study of untreated essential hypertension in which patients received either 50 mg of spironolactone or 2.5 mg of bendroflumethiazide for 4 weeks. After 1-month crossover washout period, patients received the alternative antihypertensive agent. Brachial SBPs and DBPs were lowered significantly in both groups, but only treatment with spironolactone reduced PWV and AIx, even after correcting for bendroflumethiazide’s greater BP reduction. De Souza et al50 conducted an open-label prospective trial to assess the efficacy of spironolactone on BP reduction and arterial stiffness measured by aortic PWV in 175 resistant hypertensive patients as determined by ambulatory BP monitoring. As to the BP-lowering effect, mean BP reductions of 16/9 mm Hg in ambulatory BP monitoring and 14/7 mm Hg in office BP were observed, with predictors of better BP response being larger waist circumference, lower PWV, and lower serum potassium. A significant change in PWV could be observed in so-called good responders (defined as >10% SBP reduction). The beneficial effects of MCRA on arterial stiffness were also shown with eplerenone in normotensive and mild-to-moderate hypertensive patients by Savoia et al.51 In a double-blind study, 16 normotensive patients were randomized to either 50 mg of eplerenone or atenolol daily. After 1 year of treatment, BP in both groups was controlled. Interestingly, SBPs and DBPs were lower in the atenolol-treated vs eplerenone-treated group (121.4/79.2 and 129.1/84.5 mm Hg, respectively). In both groups, arterial media/lumen ratio and cross-sectional area were unchanged, whereas with eplerenone a reduction in stiffness was observed compared to increased stiffness with atenolol. These observations were independent of BP.
CONCLUSIONS
In conclusion, there is evidence that antihypertensive drug classes may have different effects on arterial stiffness. While ACE inhibitors, CCBs, and MCRAs are beneficial in reducing arterial stiffness and central BP, some beta blockers may have the opposite effects while lowering peripheral BP. However, the majority of studies on beta blockers have investigated the effects of atenolol and there are insufficient data available regarding the effects of vasodilating beta blockers. ARBs seemingly have a beneficial effect on arterial stiffness, with the caveat that results are conflicting and larger studies are needed. Diuretics seem to be neutral, in that they do not seem to affect arterial stiffness and central BP beyond their effects on brachial artery pressure reduction.
Noninvasive measurement of arterial stiffness is a potentially valuable tool in the detection of early vascular changes that precede hypertension and could serve to reinforce early life-style changes and to prevent the development of cardiovascular events, and help to direct the most appropriate antihypertensive therapy. However, most of the studies to date are rather small, and larger studies are needed before the more routine application of these techniques can be recommended.
Footnotes
Disclosure: The authors have no conflict of interest to report.
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, De Backer G, Dominiczak A, et al. Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 4.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037–2114. doi: 10.1016/j.jacc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glasser SP, Dudenbostel T. The global burden of cardiovascular disease: The role of endothelial function and arterial elasticity in cardiovascular disease as novel and emerging biomarkers. Curr Cardiovasc Risk Rep. 2011;5:187–195. doi: 10.1007/s12170-010-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 9.Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn JN, Finkelstein S, McVeigh G, et al. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26:503–508. doi: 10.1161/01.hyp.26.3.503. [DOI] [PubMed] [Google Scholar]
- 11.Blacher J, Protogerou AD, Safar ME. Large artery stiffness and antihypertensive agents. Curr Pharm Des. 2005;11:3317–3326. doi: 10.2174/138161205774424654. [DOI] [PubMed] [Google Scholar]
- 12.Boutouyrie P, Lacolley P, Briet M, et al. Pharmacological modulation of arterial stiffness. Drugs. 2011;71:1689–1701. doi: 10.2165/11593790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Czernichow S, Zanchetti A, Turnbull F, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration. The effects of blood pressure reduction and of different blood pressure-lowering regimens on major cardiovascular events according to baseline blood pressure: meta-analysis of randomized trials. J Hypertens. 2011;29:4–16. doi: 10.1097/HJH.0b013e32834000be. [DOI] [PubMed] [Google Scholar]
- 14.Fyhrquist F, Dahlöf B, Devereux RB, et al. LIFE Study Group. Pulse pressure and effects of losartan or atenolol in patients with hypertension and left ventricular hypertrophy. Hypertension. 2005;45:580–585. doi: 10.1161/01.HYP.0000161186.55933.6b. [DOI] [PubMed] [Google Scholar]
- 15.Dahlöf B, Sever PS, Poulter NR, et al. ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 16.Williams B, Lacy PS, Thom SM, et al. CAFE InvestigatorsAnglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 17.Avolio AP, Van Bortel LM, Boutouyrie P, et al. Role of pulse pressure amplification in arterial hypertension: experts’ opinion and review of the data. Hypertension. 2009;54:375–383. doi: 10.1161/HYPERTENSIONAHA.109.134379. [DOI] [PubMed] [Google Scholar]
- 18.Laurent S, Cockcroft J, Van Bortel L, et al. European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 19.Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 20.Blacher J, Guerin AP, Pannier B, et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 21.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 22.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 23.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366:1545–1553. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 24.Protogerou A, Blacher J, Stergiou GS, et al. Blood pressure response under chronic antihypertensive drug therapy: the role of aortic stiffness in the REASON (Preterax in Regression of Arterial Stiffness in a Controlled Double-Blind) study. J Am Coll Cardiol. 2009;53:445–451. doi: 10.1016/j.jacc.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 25.Dhakam Z, McEniery CM, Yasmin, et al. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens. 2006;19:214–219. doi: 10.1016/j.amjhyper.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Morgan T, Lauri J, Bertram D, et al. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17:118–123. doi: 10.1016/j.amjhyper.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Mahmud A. Reducing arterial stiffness and wave reflection-Quest of the holy grail? Artery Res. 2007;1:13–19. [Google Scholar]
- 28.Kampus P, Serg M, Kals J, et al. Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension. 2011;57:1122–1128. doi: 10.1161/HYPERTENSIONAHA.110.155507. [DOI] [PubMed] [Google Scholar]
- 29.Protogerou AD, Stergiou GS, Vlachopoulos C, et al. The effect of antihypertensive drugs on central blood pressure beyond peripheral blood pressure. Part II: evidence for specific class-effects of antihypertensive drugs on pressure amplification. Curr Pharm Des. 2009;15:272–289. doi: 10.2174/138161209787354186. [DOI] [PubMed] [Google Scholar]
- 30.Safar M, Levy B. The response of large arteries to antihypertensive treatment. In: O’Rourke M, Safar M, Dzau V, editors. Pharmacological Aspects in Arterial Vasodilation. Vol. 2. Philadelphia, PA: Lea & Febiger; 1993. pp. 157–166. [Google Scholar]
- 31.Glasser SP, Arnett DK, McVeigh GE, et al. The importance of arterial compliance in cardiovascular drug therapy. J Clin Pharmacol. 1998;38:202–212. doi: 10.1002/j.1552-4604.1998.tb04417.x. [DOI] [PubMed] [Google Scholar]
- 32.Hanselin MR, Saseen JJ, Allen RR, et al. Description of antihypertensive use in patients with resistant hypertension prescribed four or more agents. Hypertension. 2011;58:1008–1013. doi: 10.1161/HYPERTENSIONAHA.111.180497. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan NM. Chlorthalidone versus hydrochlorothiazide: a tale of tortoises and a hare. Hypertension. 2011;58:994–995. doi: 10.1161/HYPERTENSIONAHA.111.183525. [DOI] [PubMed] [Google Scholar]
- 34.Asmar RG, London GM, O’Rourke ME, et al. REASON Project Coordinators and Investigators. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38:922–926. doi: 10.1161/hy1001.095774. [DOI] [PubMed] [Google Scholar]
- 35.Mason RP, Walter MF, Trumbore MW, et al. Membrane antioxidant effects of the charged dihydropyridine calcium antagonist amlodipine. J Mol Cell Cardiol. 1999;31:275–281. doi: 10.1006/jmcc.1998.0867. [DOI] [PubMed] [Google Scholar]
- 36.London GM, Pannier B, Guerin AP, et al. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation. 1994;90:2786–2796. doi: 10.1161/01.cir.90.6.2786. [DOI] [PubMed] [Google Scholar]
- 37.Deary AJ, Schumann AL, Murfet H, et al. Double-blind, placebo-controlled crossover comparison of five classes of antihypertensive drugs. J Hypertens. 2002;20:771–777. doi: 10.1097/00004872-200204000-00037. [DOI] [PubMed] [Google Scholar]
- 38.Hirata K, Vlachopoulos C, Adji A, et al. Benefits from angiotensin-converting enzyme inhibitor ‘beyond blood pressure lowering’: beyond blood pressure or beyond the brachial artery? J Hypertens. 2005;23:551–556. doi: 10.1097/01.hjh.0000160211.56103.48. [DOI] [PubMed] [Google Scholar]
- 39.Jiang XJ, O’Rourke MF, Zhang YQ, et al. Superior effect of an angiotensin-converting enzyme inhibitor over a diuretic for reducing aortic systolic pressure. J Hypertens. 2007;25:1095–1099. doi: 10.1097/HJH.0b013e3280ac1533. [DOI] [PubMed] [Google Scholar]
- 40.Mahmud A, Feely J. Favourable effects on arterial wave reflection and pulse pressure amplification of adding angiotensin II receptor blockade in resistant hypertension. J Hum Hypertens. 2000;14:541–546. doi: 10.1038/sj.jhh.1001053. [DOI] [PubMed] [Google Scholar]
- 41.Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004;17(12 pt 1):1192–1200. doi: 10.1016/j.amjhyper.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 42.Lu H, Rateri DL, Feldman DL, et al. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest. 2008;118:984–993. doi: 10.1172/JCI32970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherney DZ, Lai V, Scholey JW, et al. Effect of direct renin inhibition on renal hemodynamic function, arterial stiffness, and endothelial function in humans with uncomplicated type 1 diabetes: a pilot study. Diabetes Care. 2010;33:361–365. doi: 10.2337/dc09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMurray JJ, Abraham WT, Dickstein K, et al. Aliskiren, ALTITUDE, and the implications for ATMOSPHERE. Eur J Heart Fail. 2012;14:341–343. doi: 10.1093/eurjhf/hfs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 46.Verma S, Gupta M, Holmes DT, et al. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur Heart J. 2011;32:2135–2142. doi: 10.1093/eurheartj/ehr066. [DOI] [PubMed] [Google Scholar]
- 47.Krum H, Nolly H, Workman D, et al. Efficacy of eplerenone added to renin-angiotensin blockade in hypertensive patients. Hypertension. 2002;40:117–123. doi: 10.1161/01.hyp.0000025146.19104.fe. [DOI] [PubMed] [Google Scholar]
- 48.Mahmud A, Feely J. Aldosterone-to-renin ratio, arterial stiffness, and the response to aldosterone antagonism in essential hypertension. Am J Hypertens. 2005;18:50–55. doi: 10.1016/j.amjhyper.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 49.Funder JW. Reconsidering the roles of the mineralocorticoid receptor. Hypertension. 2009;53:286–290. doi: 10.1161/HYPERTENSIONAHA.108.119966. [DOI] [PubMed] [Google Scholar]
- 50.de Souza F, Muxfeldt E, Fiszman R, et al. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–152. doi: 10.1161/HYPERTENSIONAHA.109.140988. [DOI] [PubMed] [Google Scholar]
- 51.Savoia C, Touyz RM, Amiri F, et al. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension. 2008;51:432–439. doi: 10.1161/HYPERTENSIONAHA.107.103267. [DOI] [PubMed] [Google Scholar]