Abstract
Background
Although interactive voice response (IVR) calls can be an effective tool for chronic disease management, many regions of the world lack the infrastructure to provide these services.
Objective
This study evaluated the feasibility and potential impact of an IVR program using a cloud-computing model to improve diabetes management in Honduras.
Methods
A single group, pre-post study was conducted between June and August 2010. The telecommunications infrastructure was maintained on a U.S. server, and calls were directed to patients’ cell phones using VoIP. Eighty-five diabetes patients in Honduras received weekly IVR disease management calls for six weeks, with automated follow-up emails to clinicians, and voicemail reports to family caregivers. Patients completed interviews at enrollment and a six week follow-up. Other measures included patients’ glycemic control (A1c) and data from the IVR calling system.
Results
55% of participants completed the majority of their IVR calls and 33% completed 80% or more. Higher baseline blood pressures, greater diabetes burden, greater distance from the clinic, and better adherence were related to higher call completion rates. Nearly all participants (98%) reported that because of the program, they improved in aspects of diabetes management such as glycemic control (56%) or foot care (89%). Mean A1c’s decreased from 10.0% at baseline to 8.9% at follow-up (p<.01). Most participants (92%) said that if the service were available in their clinic they would use it again.
Conclusions
Cloud computing is a feasible strategy for providing IVR services globally. IVR self-care support may improve self-care and glycemic control for patients in under-developed countries.
Introduction
Eighty percent of all deaths due to chronic diseases occur among patients in low- to middle income countries.1-3 Diabetes alone represents an international crisis, with an expected global prevalence in 2030 of 439 million people compared to 285 million people in 2009.4, 5 The current study was conducted in Honduras, the second poorest country in the Western Hemisphere. Limited healthcare resources, inadequate transportation systems, and populations struggling with growing social instability make Honduras protypical of the challenges to providing disease management in the most under-developed regions of the world.
Mobile phones are ubiquitous in developing countries,6 and in Latin America, nearly half a billion people have cell phones.7-9 In a survey of more than 600 chronically-ill adults in Honduras, 84% reported telephone access.10 Outgoing telehealth calls to patients’ cell phones are particularly accessible in Latin America because in many countries, a person can receive a mobile phone call without a monthly subscription to a calling plan or paying any “minute” charges.
As in more developed countries,11, 12 telephone disease management is feasible and beneficial in Latin America. Low-income diabetes patients in Santiago Chile receiving telephone nurse counseling have better glycemic control than similar patients treated in usual care,13 and a randomized trial in Argentina found that between-visit telephone support for heart failure patients significantly reduced long-term readmission rates.14, 15 Unfortunately, decision-leaders across Latin America feel that there is insufficient capacity to meet the growing need for self-management support within their countries and that innovation in this important area is lagging.16 Interactive voice response or IVR is the technology allowing patients to receive information and communicate with others asynchronously using their mobile- or landline telephones. Using IVR, patients interact with a structured series of voice-recorded message components and respond to queries for information either using their touch-tone keypad or voice-recognition technology. Based on patients’ responses, they receive recorded messages tailored to their individual needs, and others involved in their care can receive updates about how the patient is doing along with structured feedback about ways they can promote more effective disease management. Because the per-contact cost of IVR is low, patients can receive health monitoring and behavior change messages much more frequently than would be possible in almost any health care system relying on face-to-face encounters or “live” nurse calls.
Spanish-speakers and other language minority patients in the U.S. are willing to use IVR as part of their disease management,17-23 and report reliable and clinically-meaningful information via IVR calls.24-31 Studies have shown that IVR-supported disease management services can improve outcomes for patients with diabetes and other chronic health problems.32-38 In Honduras, 81% of chronically-ill adults reported that they were willing to participate in self-management education via automated calls, and respondents who had cancelled an appointment due to a transportation problem were particularly likely to report a willingness to receive support for their self-care via IVR.10 Unfortunately, very low-income areas in under-developed countries seldom have the resources to implement and maintain the informatics infrastructure necessary for a program of IVR-supported disease management.
Cloud computing is an approach to the design of technologic resources in which end-users (e.g., a clinic or health system) access the computing infrastructure remotely over the internet. As a result, health care provider organizations do not need to maintain the hardware, software or expertise that comprise the infrastructure in the “cloud” supporting them. For IVR-supported disease management programs, a cloud computing model means that clinics could potentially access the program from almost anywhere in the world. Patient information can be entered via the system’s website, and IVR calls can be generated from the central server to patients’ cell phones using the low-cost technology for Voice over IP (VoIP) that makes possible services such as SkypeTM and VonageTM. Because the fixed costs of service delivery can be spread across potentially large numbers of users, a cloud computing model could be financially sustainable by health care organizations with limited budgets.
The purpose of the current study was to determine whether a cloud computing approach could be used to deliver IVR-supported chronic illness care within a very low income community located in an under-developed area of Latin America. Specifically, we evaluated: the technical feasibility of delivering IVR-supported diabetes management internationally, whether patients were willing to enroll in such a program, and the frequency with which patients would complete IVR disease management calls. We also evaluated patients’ satisfaction with the service and changes in their health behaviors and glycemic control over time.
Methods
Site Description and Recruitment
Adult patients with diabetes were identified at the time of outpatient primary care visits to clinics in a semi-rural region of Honduras. Prior surveys of patients receiving care in the study site found an average of five years of formal education, 27% of patients reporting illiteracy, and 44% reporting that they had missed one or more outpatient visits in the prior year due to cost concerns.10, 39, 40 To be eligible for the current study, patients had to have access to a cellular phone, a life expectancy of at least one year, and no diagnosed psychiatric disorders. All patients completed a written informed consent, and the study was approved by the Human Subjects Committees at the University of Michigan and the local health center.
Intervention
A translational science approach was used for program development.41 During the planning phase, investigators traveled to Honduras to established collaborative relationships supporting the intervention design, implementation, and research plan. IVR health status monitoring and behavior change calls focused on medication adherence, general health status, hyperglycemia, hypoglycemia, glucose self-monitoring, and foot care. Calls were professionally translated into Spanish, recorded by a native Spanish-speaker, and tested by clinicians and patients in Honduras prior to initiating the study.
The intervention was designed to have three mechanisms of action: First, patients received recorded information during weekly automated calls about how to better manage their diabetes based on their symptoms and self-care challenges. Second, patients’ clinical team received updates via structured emails based on concerning patient reports such as symptoms of hyperglycemia or low self-monitored glucose levels. Third, patients had the option of enrolling in the program with an informal caregiver – usually an adult child or other family member. For such patients, that family member received structured IVR calls to their cell phone after each patient assessment, that included feedback and suggestions that the family member could use to support the patient in his/her disease self-care.
Research staff in Honduras used a web interface to enter participants’ telephone numbers and preferred calling times into the calling database maintained on a server at the University of Michigan. Using VoIP, the system generated multiple calling attempts to each patient’s cell phone per calling week. When patients were reached, IVR interactions took roughly 15 minutes to complete.
Measurement
Participants were asked to complete in-person interviews at baseline and follow-up. Surveys were constructed in English and professionally translated into Spanish. Depressive symptoms were measured using the Spanish version of the 10-item CES-D screener (α reliability in this dataset = .81), with the recommended cutoff indicating significant depression.42 Diabetes-related distress was measured with the Problem Areas in Diabetes Scale (PAID, α=.87).43 Medication adherence was measured with the Morisky Index44 (α=.71). Diabetes self-efficacy was measured using a scale developed by Heisler et al. (α=.64).45 Three items asked about the priority of diabetes relative to other issues (e.g., “I have other health problems more important than diabetes”). Diabetes-specific social support was measured using a subscale from the Diabetes Care Profile (α=.91).46 As a measure of treatment access, patients were asked how long they had to travel to reach their source of primary care. Illiteracy was self-reported. Self-reported monthly income was annualized and converted to dollars.
Questions about perceived intervention impact were developed for the study. The first set used the item stem: “How much would you say that you’ve improved in each of the following areas as a result of the CarePartner Program?” In the second set, respondents were asked “Due to the information you received during the automated calls since you have been in the CarePartner Program, have you ever done any of the following?” Items included talking with their doctor about diabetes, making changes in their diet, and doing diabetic foot care. As part of their follow-up survey, participants also were asked to respond to open ended questions regarding their impressions of the program.
Body Mass Index was calculated based on participants’ height and weight wearing light clothing. Blood pressure was measured in both arms with a repeat measurement in the arm with the highest pressure after several minutes of rest. For analyses presented here, we identified patients over the recommended blood pressure level for persons with diabetes (i.e., 120/80 mmHg).47 Patients’ glycemic control was measured at baseline and follow-up using point-of-care analyzers for HbA1c48 and fasting blood glucose (FBG). Due to a malfunction of the A1c analyzer, 33% of patients at baseline were unable to have that test. FBG values are highly correlated with A1c’s, and both are used for diagnosing diabetes.40, 49 In the current study, the correlation between baseline A1cs and FBG was .803 (p<.0001); for patients missing baseline A1c data, we imputed those values based on their FBG test results. Where relevant, results below are presented for the overall sample with imputed A1c data and for the subset with measured baseline A1c values. The IVR calling system recorded the date of each attempted patient contact, the outcome of each attempt (completed assessment, patient not reached, etc.), and patients’ IVR-reported responses.
Analyses
Engagement with the IVR calls was measured using summary variables representing the proportion of participation weeks in which an attempted assessment was completed. These summary data were merged with patient surveys and physiologic measures collected at baseline and follow-up. Variation in assessment completion rates was examined across groups defined by patients’ baseline sociodemographic and clinical characteristics. Stepwise logistic regression with backwards elimination and a p-value > .20 for exclusion was used to identify baseline characteristics identifying patients who completed their IVR assessment calls more frequently. In analyses presented here, two logistic models were constructed using as outcomes completion rates of > 50% and > 80%. Poisson regression was used in auxiliary analyses using the number of completed calls as the outcome; those analyses are available on request and had similar findings. Finally, changes between baseline and the six-week follow-up in patients’ glycemic control were examined, as well as patients’ impressions at follow-up regarding intervention satisfaction and perceived intervention-related changes in their diabetes care. Qualitative data were reviewed for key themes.
Results
Baseline Characteristics of Enrollees
Of the 94 patients screened for eligibility, one had no telephone, three were lost to follow-up before completing the enrollment process, five were excluded due to problems completing their initial IVR calls, and 85 participated in the study. Enrollees were mainly women (70%) and on average 55.7 years of age (Table 1). More than half had five or fewer years of formal education and on average patients’ had annual household incomes of $2,591. Eighty-nine percent reported taking diabetes related medication, and 85% of those participants reported at least one episode of cost-related medication non-adherence in the prior year. Baseline A1c levels based on the imputed sample were on average 10.2% (SD=3.0), and 21% had blood pressures greater than the recommended level of 120/80 mmHg.
Table 1.
Baseline Characteristics of the Sample
| All Patients % or mean(SD) |
With CarePartner % or mean(SD) |
Without CarePartner % or mean(SD) |
|
|---|---|---|---|
| N | 89 | 63 | 26 |
| Female (a) | 70.1 | 68.9 | 76.9 |
| Age | 55.7(11.0) | 52.2(11.6) | 59.5(11.2) |
| Years of Education | 4.7(4.1) | 4.9(4.0) | 4.1(4.2) |
| Annual Household Income | $2591(2521) | $2639(2467) | $2469(2705) |
| Number of Family in Household | 4.0(2.5) | 4.0(2.5) | 4.0(2.6) |
| Insulin Use | 12.6 | 11.5 | 15.4 |
| Body Mass Index | 29.3(6.7) | 29.8(7.1) | 28.1(6.7) |
| Depressed(b) | 53.3 | 55.6 | 48.2 |
| A1c | 10.2(3.0) | 10.3(2.9) | 9.9(3.3) |
| BP > 120/80 | 21.1 | 19.1 | 25.9 |
P=.04. No other differences between patients with and without a CarePartner were observed.
CESD-10 scores > 4.
Call Completion Rates
On average, 4.5 call attempts were made per patient per week, yielding 250 successful contacts. Overall 16 (19%) of participants completed none of their IVR monitoring and behavior-change calls, 23 (27%) completed 1-2, 26 (30%) completed 3-4, and 20 (23%) completed 5+.
In bivariate analyses, patients reporting that diabetes was their most significant health problem were significantly more likely than other participants to complete the majority of their calls (66% versus 42%, p=.027). In multivariate analyses, call completion rates were not statistically different across groups defined by patients’ age, gender, educational attainment, depressive symptoms, or baseline glycemic control (Table 2). Several factors associated with a greater need for support were positively associated with call completion rates. Specifically, patients traveling more than an hour to reach their clinic had nearly twice the odds of completing the majority of their IVR calls, and patients reporting at baseline that diabetes was their most important health problem had nearly 12 times the odds of completing half or more of their IVR assessments. Patients reporting more diabetes-related distress also had a greater likelihood of completing the majority of their assessments. Patients with high blood pressures at baseline were more likely to complete 50% or more of their assessments, and these patients were nine times as likely as other patients to complete 80% or more calls. In contrast, patients with poorer medication adherence at baseline were less likely to complete IVR assessments, and both poor perceived health plus illiteracy were marginally associated with lower completion rates.
Table 2.
Adjusted Odds Ratios for Patient Characteristics Associated with Completing a Greater Percentage of Automated Diabetes Assessment and Behavior Change Calls
| Completing > 50% of Calls | Completing > 80% of Calls | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| AOR | 95% CI | P-Value | AOR | 95% CI | P-Value | |
| Traveling 1+ Hours to Clinic | 1.9 | 1.0, 3.4 | .04 | --- | ||
| DM is most important health problem | 11.9 | 2.6, 49.0 | .001 | 8.4 | 1.7, 42.2 | .01 |
| Poor perceived health | 0.2 | .05, 1.1 | .06 | 0.2 | 0.3, 1.1 | .07 |
| Illiteracy | 0.2 | .05, 1.1 | .07 | — | ||
| Greater Rx Adherence Problems | 0.6 | 0.3, 0.9 | .03 | 0.6 | 0.3, 1.1 | 0.7 |
| Greater Diabetes Distress | 1.2 | 1.1, 1.4 | .005 | 1.2 | 1.1, 1.4 | .01 |
| Blood pressure > 120/80 mmHg | 1.2 | 1.1, 1.4 | .005 | 9.4 | 1.9, 46.9 | .006 |
| Greater diabetes-related support | — | 1.3 | 0.9, 42.2 | .13 | ||
Notes: DM=diabetes mellitus. AOR = Adjusted Odds Ratios. Results were derived from a stepwise logistic regression with backwards selection and a p>.20 criteria for exclusion. Factors found not to be associated with call completion rates included: age, gender, educational attainment, baseline A1c, diabetes-specific social support, depression, diabetes self-efficacy, and participation with an informal caregiver (“CarePartner”).
Satisfaction with the System
Sixty four participants (75% of enrollees) completed endpoint surveys. There were no differences in the baseline glycemic control, medication adherence, diabetes distress, depressive symptoms, or other characteristics of those who did and did not return for the endpoint assessment. However, only 29% of patients lost to follow-up completed the majority of their IVR calls compared to 68% of those with follow-up surveys (p=.001).
Overall, 83% of follow-up survey respondents agreed that the IVR system was easy to use, 84% agreed that the language used was consistent with the language with which they were familiar, and 89% agreed that the system provided useful suggestions for managing their diabetes. Seventy percent of participants reported that either all or most of their needs had been satisfied by the program, and 80% reported that if a friend had a similar need, they would recommend the CarePartner program to them. Ninety-two percent said that if the program were available in the clinic, they would use it again, and 100% said that they were “very satisfied” (88%) or “generally satisfied” (12%) with the program.
Potential Impacts on Patients’ Self-Care and Glycemic Control
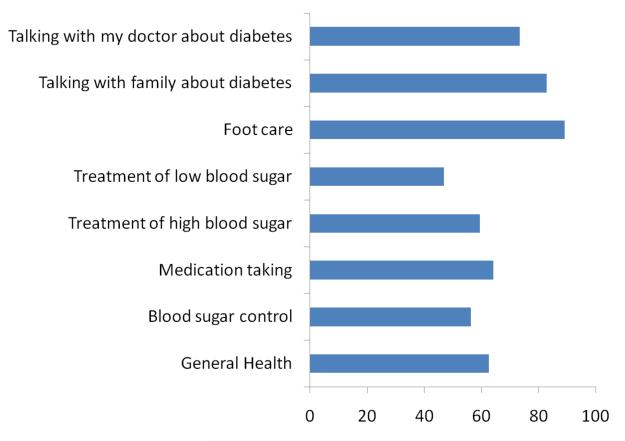
All but one respondent (98%) completing the follow-up survey reported that they had improved in some aspect of their health and self-care as a result of participating in the program (figure). More than half (56%) reported that they improved in their blood sugar control as a result of being in the program, 66% reported that their diet improved, and 89% reported that due to the information they received during the automated calls their foot care improved. In the subgroup of patients with A1c values at baseline and follow-up (N=40), levels improved significant from an average of 10.0% to 8.9%, or a difference of 1.1% (95% CI: 0.5% – 1.5%). Using imputation for the patients with fasting glucose data at baseline but no baseline A1c, A1c values decreased 1.3% (95% CI: 0.8% – 1.9%) between baseline and follow-up. Participants who completed more than half of their weekly IVR assessments had significantly larger average decreases in their A1c levels than patients who completed fewer calls (mean change of 1.1% versus .34%; p=.04). Patients who participated with a CarePartner also had larger decreases in A1c than those who participated alone, although the result did not reach statistical significance (1.1% versus 0.41%; p=.23).
Figure.
Percent of patients reporting an improvement as a result of being in the CarePartner Program
Qualitative Data
In response to the open-ended question about participants’ favorite part of the program, many patients spoke in general terms about appreciating the “advice” and “attention” they received. Other respondents mentioned that they appreciated program features such as “explanations of low and high blood sugar,” “advice on how to treat high and low blood pressure,” and “advice about how to take care of [my] feet.” When asked about their least favorite aspect of the program, participants mainly focused on the sound quality and problems with calls cutting-off prematurely. When asked what they would change about the program, the majority responded that there was nothing they would change.
Discussion
To our knowledge, this is the first study to demonstrate the technical feasibility, acceptability, and potential impact of an mHealth tool for chronic disease management that can be provided to resource-poor areas of under-developed countries using a cloud-computing model and standard mobile phones. Call completion rates were acceptable, and in follow-up interviews, patients reported that the service was easy to use and provided information useful for their diabetes self-care. More than 90% of patients at follow-up reported that they would use such a service if it were available in their clinic. Most also reported important changes in their self-management, and pre-post changes in patients’ A1c were clinically significant. These changes in glycemic control are particularly notable because A1c is an objective measure of patients’ diabetes-related risk, and because changes measured after only six weeks would tend to under-estimate the long-term impact on patients’ health status.
Because this disease management tool uses cloud computing and only requires cell phones, it could be used to expand access to health information in low income communities around the world. To be sustainable, such service models will require a feasible financial plan for system adoption and maintenance, either through health ministries, regional private sector providers, or large insurers. A more robust and portable software platform also will be required, ideally allowing the system to be maintained within multiple regional service points that receive technical support and system updates from a centralized source. Models that blend centralization and decentralization of resources may address the technical issues contributing to the sound quality problems reported by some patients in the current study.
It was encouraging to see that call completion rates were consistent across groups defined by patients’ age, gender, and educational attainment, and that the significant number of patients with depression did not have increased difficulty participating. Several factors associated with a greater need for self-management support were associated with higher call completion rates. Specifically, patients with longer travel times to the clinic, greater diabetes distress, and higher blood pressures all completed more of their calls controlling for covariates. In contrast, poorer perceived health status, illiteracy and medication adherence problems may be associated with worse call completion rates. Taken together, these findings suggest that interventions such as this hold promise for reaching some of the most vulnerable patients, but more intensive targeting and training may be required to ensure that patients who need additional motivation and ongoing support are appropriately served.
This model is feasible in areas with little infrastructure for developing and maintaining an IVR program. Costs for VoIP calls between the United States and Latin America are often less than $0.03 per minute although there is substantial variation across countries and providers. Nevertheless, the quality and durability of the telecommunication network can affect the success of these programs. In the current study, several weeks of nearly hurricane-level storms interrupted VoIP calls to participants’ telephones. While this can be circumvented by increasing the number of call attempts (something that was done in this study), ultimately, services such as this must depend on the local telephone service.
Provision of information-based services within resource-poor environments will require careful considerations of how to make such services relevant given the sometimes daunting barriers to access. In the current study, much of the information provided during IVR calls focuses on behaviors such as blood glucose self-monitoring, medication adherence, and regular follow-up with patients’ clinical team. Each of these activities assumes the availability of resources that may not always be accessible to low-income patients in developing countries. In a prior study within this same clinic,10 43% of survey respondents reported that they had to miss an appointment in the prior year due to transportation problems, 44% reported missing an appointment due to cost problems, and 63% reported cost-related medication non-adherence. That latter number was even higher in the current study (85%). Mobile technologies may provide information to patients that circumvents the need for in-person visits and may be able to alert them to the importance of their medications or low-cost options for receiving care. Between-visit IVR assessments also could allow health systems to more effectively target scarce medical resources. Nevertheless, advice to use medications or other resources will have little effect if patients or their health systems are unable to access these necessities.
Moreover, many low-income patients have low health literacy, including poor understanding of their self-management goals or preventive medicine and little familiarity with physiologic markers of their disease status such as A1c.50 Although interventions such as this one can play a crucial role in improving that understanding, the effectiveness of such programs may be constrained by patients’ ability to process the information provided. To be truly effective, new services such as this one must be culturally sensitive to those beliefs, while using effective behavior change approaches to move patients to a more active role in their disease management.
A limitation of the current study is that the sample was too small to detect potentially important differences in system usability or impact across patient subgroups identifying those who would or would not be especially likely to benefit from this type of service. Also, patients participated in the study for only six weeks, and this limited our ability to examine temporal trends in participants’ service use. It may be that system usage wanes over time or that it increases as participants become more accustomed to the program and initial technical challenges are addressed. The limited time frame of this study is particularly important because one of the principle outcomes, i.e., participants’ A1c-measured glycemic control, represents a running average of blood sugar values over the prior 10-12 weeks. Because of that, it is possible that the pre-post changes we report here under-estimate the true impacts of this intervention on patients’ physiologic health. On the other hand, this study did not include a control group, so we cannot definitively attribute changes in patients’ health or self-care to the intervention. Regression to the mean, Hawthorne effects, and other artifacts of participation in a research study may have contributed to the differences we observed between patients’ baseline and follow-up assessments. Finally, 25% of patients were lost to follow-up. Although patients who did and did not complete follow-up surveys were similar on a wide variety of baseline measures, those who failed to complete the follow-up assessment also completed fewer of their IVR calls and may have been less satisfied with their experience.
Taking these caveats into account, we conclude that a cloud computing model for IVR-supported diabetes management can be accessed by clinics and patients in remote areas of under-developed countries and used to provide low-cost self-care monitoring and behavior change support. Despite very little formal education or prior experience with IVR services, diabetes patients in Honduras were able to use such a system, and reported that it was acceptable and helpful for their diabetes self-management. Pre-post changes in patients’ A1c levels suggest that such a service may improve patients’ disease outcomes. Future controlled studies with larger samples, longer follow-up, and patients from other geographic areas are warranted to determine whether this service model is sustainable and has clear impacts on patients’ health.
Acknowledgements
Tim Woolley, Steve Christiansen, Pradip Patel, and Debra Haslam played essential roles in the development and implementation of the IVR system. Evan Milton assisted with project management and Armando Matiz Reyes assisted in identifying and supervising student researchers from the UM School of Public Health.
Funding This research was funded by the University of Michigan School of Public Health Global Health Summer Internship Program, the Michigan Diabetes Research and Training Center (NIH # DK020572), and the Michigan Institute for Clinical and Health Research (NIH #UL1RR024986). Dr. Piette is a VA Senior Research Career Scientist.
Biography
Dr. Piette is a Department of Veterans Affairs Senior Research Career Scientist, VA Ann Arbor Healthcare System, Ann Arbor, Michigan, United States. He is the Director of the program on Quality Improvement for Complex Chronic Conditions (QUICCC), the Associate Director for Global Health Technologies for the UM Center for Global Health, and a Professor of Internal Medicine at the University of Michigan.
Footnotes
conflicts of interest None of the authors has any conflict of interest.
References
- 1.Daar AS, Singer PA, Persad DL, et al. [Site accessed October 22, 2010];Grand challenges in chronic non-communicable diseases. Nature. 2007 450:494–496. doi: 10.1038/450494a. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization [Site accessed October 22, 2010];Cardiovascular Disease Prevention and Control. http://www.who.int/dietphysicalactivity/publications/facts/cvd/en/2010.
- 3.World Health Organization . WHO Facing the Facts: The Impact of Chronic Disease in the Americas. WHO; Geneva: [Site accessed October 22, 2010]. 2005. http://www.who.int/chp/chronic_disease_report/media/AMRO.pdf. [Google Scholar]
- 4.International Diabetes Federation [Site accessed October 22, 2010];The Diabetes Atlas. 2009 http://www.idf.org/Facts_and_Figures.
- 5.Pan American Health Organization [Site accessed October 22, 2010];Health in the Americas 2007. http://www.paho.org/hia/archivosvol2/paisesing/honduras%20english.pdf.
- 6.Castells M, Linchuan Qui J, Sey A. Mobile Communication and Society: A Global Perspective. Massachusetts Institute of Technology; Boston, MA: 2007. [Google Scholar]
- 7.International Telecommunications Union [Site accessed October 22, 2010];World Information Society 2007 Report: Beyond WSIS. http://www.itu.int/osg/spu/publications/worldinformationsociety/2007/report.html.
- 8.Asociación de la Industria Celular en Colombia [Site accessed October 22, 2010];Densidad celular en Colombia. http://www.asocel.org.co/pdf/densidad_celular_en_%20colombia_90.pdf.
- 9.Piette JD, Lange I, Issel M, et al. Use of telephone care in a cardiovascular disease management programme for type 2 diabetes patients in Santiago, Chiile. Chronic Illn. 2006;2:87–96. doi: 10.1177/17423953060020020401. [DOI] [PubMed] [Google Scholar]
- 10.Piette JD, Mendoza-Avelares M, Milton EC, et al. Access to mobile communication technology and willingness to participate in automated telemedicine calls among chronically-ill patients in Honduras. Telemed J E Health. doi: 10.1089/tmj.2010.0074. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark RA, Inglis SC, McAlister FA, Cleland JGF, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. British Medical Journal. 2007;334:942–950. doi: 10.1136/bmj.39156.536968.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L, Forbes A, While A, Griffiths P. Telephone follow-up to improve glycaemic control in patients with type 2 diabetes: a systeamtic review and meta-analysis of controlled trials. Cochrane Database of Systematic Reviews. doi: 10.1111/j.1464-5491.2010.03113.x. in press. [DOI] [PubMed] [Google Scholar]
- 13.Lange I, Campos S, Urrutia J, Bastamante C, Alcayaga C, Piette JD. Efecto de un modelo de apoyo telefonico en el auto-manejo y control metabolico de la diabetes tipo 2, en un centro de atencion primaria, Santiago, Chile. Revista de Medicina de Chile. doi: 10.4067/s0034-98872010000600010. in press. [DOI] [PubMed] [Google Scholar]
- 14.GESICA Investigators Randomised trial of telephone intervention in chronic heart failure: DIAL trial. British Medical Journal. 2007;331:425–435. doi: 10.1136/bmj.38516.398067.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GESICA Investigators Long-term results after a telephone intervention in chronic heart failure: DIAL (Randomized Trial of Phone Intervention in Chronic Heart Failure) follow-up. Journal of the American College of Cardiology. 2010;56(5):372–378. doi: 10.1016/j.jacc.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 16.Sapag JC, Lange I, Campos S, Piette JD. Innovaciones para el cuidado y autocuidado de personas con enfermedades cronicas en America Latina: oportunidades y desafios para el trabajo en red. [Innovation for the care and self-care of persons with chronic diseases in Latin America: Opportunities and challenges for collaborative work.] The Panamerican Journal of Public Health. 2010;27(1):1–9. doi: 10.1590/s1020-49892010000100001. [DOI] [PubMed] [Google Scholar]
- 17.Brodey BB, Rosen CS, Brodey IS, Sheetz B, Unutzer J. Reliability and acceptability of automated telephone surveys among Spanish- and English-speaking mental health services recipients. Ment Health Serv Res. 2005 Sep;7(3):181–184. doi: 10.1007/s11020-005-5786-1. [DOI] [PubMed] [Google Scholar]
- 18.Lorig K, Ritter PL, Villa F, Piette JD. Spanish diabetes self-management with and without automated telephone reinforcement: two randomized trials. Diabetes Care. 2008;31(3):408–414. doi: 10.2337/dc07-1313. [DOI] [PubMed] [Google Scholar]
- 19.Piette JD. Patient education via automated calls: a study of English- and Spanish-speakers with diabetes. Am J Prev Med. 1999;17(2):138–141. doi: 10.1016/s0749-3797(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 20.Piette JD, McPhee SJ, Weinberger M, Mah CA, Kraemer FB. Use of automated telephone disease management calls in an ethnically diverse sample of low-income patients with diabetes. Diabetes Care. 1999;22(8):1302–1309. doi: 10.2337/diacare.22.8.1302. [DOI] [PubMed] [Google Scholar]
- 21.Schillinger D, Hammer H, Wang F, et al. Seeing in 3-D: Examining the reach of diabetes self-management support strategies in a public health care system. Health Education and Behavior. 2008;35(5):664–682. doi: 10.1177/1090198106296772. [DOI] [PubMed] [Google Scholar]
- 22.Tanke ED, Leirer VO. Automated telephone reminders in tuberculosis care. Med Care. 1994;32:380–389. doi: 10.1097/00005650-199404000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar U, Handley MA, Gupta R, et al. Use of an interactive, telephone-based, self-management support program to identify adverse events among ambulatory diabetes patients. Journal of General Internal Medicine. 2007;23(4):459–465. doi: 10.1007/s11606-007-0398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piette JD. Interactive voice response systems in the diagnosis and management of chronic disease. Am J Manag Care. 2000;6(7):817–827. [PubMed] [Google Scholar]
- 25.Balas EA, Jaffrey F, Kuperman G. Electronic communication with patients. JAMA. 1997;278:152–159. al. e. [PubMed] [Google Scholar]
- 26.Perrine MW, Mundt JC, Searles JS, Lester LS. Validation of daily self-reported alcohol consumption using interactive voice response (IVR) technology. J Stud Alcohol. 1995;56(5):487–490. doi: 10.15288/jsa.1995.56.487. [DOI] [PubMed] [Google Scholar]
- 27.Schroder KE, Johnson CJ. Interactive voice response technology to measure HIV-related behavior. Curr HIV/AIDS Rep. 2009 Nov;6(4):210–216. doi: 10.1007/s11904-009-0028-6. [DOI] [PubMed] [Google Scholar]
- 28.Midanik LT, Greenfield TK. Reports of alcohol-related problems and alcohol dependence for demographic subgroups using interactive voice response versus telephone surveys: the 2005 US National Alcohol Survey. Drug Alcohol Rev. 2010 Jul;29(4):392–398. doi: 10.1111/j.1465-3362.2009.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker JA, Foushee HR, Black BC, Roth DL. Agreement between prospective interactive voice response self-monitoring and structured retrospective reports of drinking and contextual variables during natural resolution attempts. J Stud Alcohol Drugs. 2007 Jul;68(4):538–542. doi: 10.15288/jsad.2007.68.538. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Bracha Y, Tipnis A. Automated depression screening in disadvantaged pregnant women in an urban obstetric clinic. Arch Womens Ment Health. 2007;10(4):163–169. doi: 10.1007/s00737-007-0189-5. [DOI] [PubMed] [Google Scholar]
- 31.Kobak KA, Greist JH, Jefferson JW, Mundt JC, Katzelnick DJ. Computerized assessment of depression and anxiety over the telephone using interactive voice response. MD Comput. 1999 May-Jun;16(3):64–68. [PubMed] [Google Scholar]
- 32.Piette JD, Weinberger M, McPhee SJ. The effect of automated calls with telephone nurse follow-up on patient-centered outcomes of diabetes care: a randomized, controlled trial. Med Care. 2000;38(2):218–230. doi: 10.1097/00005650-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Piette JD, Weinberger M, McPhee SJ, Mah CA, Kraemer FB, Crapo LM. Do automated calls with nurse follow-up improve self-care and glycemic control among vulnerable patients with diabetes? Am J Med. 2000;108(1):20–27. doi: 10.1016/s0002-9343(99)00298-3. [DOI] [PubMed] [Google Scholar]
- 34.Piette JD, Weinberger M, Kraemer FB, McPhee SJ. Impact of automated calls with nurse follow-up on diabetes treatment outcomes in a department of veterans affairs health care system: a randomized controlled trial. Diabetes Care. 2001;24(2):202–208. doi: 10.2337/diacare.24.2.202. [DOI] [PubMed] [Google Scholar]
- 35.Bender BG, Apter A, Bogen DK, et al. Test of an interactive voice response intervention to improve adherence to controller medications in adults with asthma. J Am Board Fam Med. 2010 Mar-Apr;23(2):159–165. doi: 10.3122/jabfm.2010.02.090112. [DOI] [PubMed] [Google Scholar]
- 36.Sherrard H, Struthers C, Kearns SA, Wells G, Chen L, Mesana T. Using technology to create a medication safety net for cardiac surgery patients: a nurse-led randomized control trial. Can J Cardiovasc Nurs. 2009;19(3):9–15. [PubMed] [Google Scholar]
- 37.Reid RD, Pipe AL, Quinlan B, Oda J. Interactive voice response telephony to promote smoking cessation in patients with heart disease: a pilot study. Patient Educ Couns. 2007 Jun;66(3):319–326. doi: 10.1016/j.pec.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Mahoney DF, Tarlow BJ, Jones RN. Effects of an automated telephone support system on caregiver burden and anxiety: findings from the reach for tlc intervention study. Gerontologist. 2003;43(4):556–567. doi: 10.1093/geront/43.4.556. [DOI] [PubMed] [Google Scholar]
- 39.Milton EC, Herman WH, Aiello AE, Danielson KR, Mendoza-Alvelarez MO, Piette JD. Validation of a type 2 diabetes screeening tool in rural Honduras. Diabetes Care. 2010;33(2):275–277. doi: 10.2337/dc09-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piette JD, Milton EC, Aiello AE, Mendoza-Avelares MO, Herman WH. Comparison of three methods for diabetes screening in a rural clinic in Honduras. Pan American Journal of Public Health. 2010;28(1):49–57. doi: 10.1590/s1020-49892010000700008. [DOI] [PubMed] [Google Scholar]
- 41.Stetler CB, Legro MW, Wallace CM, et al. The role of formative evaluation in implementation research and the QUERI experience. Journal of General Internal Medicine. 2006;21:S1–S8. doi: 10.1111/j.1525-1497.2006.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irwin M, Haydari K, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies of Depression Scale (CES-D) Archives of Internal Medicine. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 43.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- 44.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Heisler M, Piette JD. “I help you, and you help me”: facilitated telephone peer support among patients with diabetes. Diabetes Educ. 2005 Nov 1;31(6):869–879. doi: 10.1177/0145721705283247. 2005. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the Diabetes Care Profile. Evaluation and the Health Professions. 1996;19(2):209–231. doi: 10.1177/016327879601900205. [DOI] [PubMed] [Google Scholar]
- 47.American Diabetes Association Executive Summary: Clinical Practice Recommendations for Diabetes. Diabetes Care. 2010;33:S4–S10. [Google Scholar]
- 48.Arsie MP, Marchioro L, Lapolla A, et al. Evaluation of diagnostic reliability of DCA 2000 for rapid and simple monitoring of HbA1c. Acta Diabetologica. 2000;37(1):1–7. doi: 10.1007/s005920070028. [DOI] [PubMed] [Google Scholar]
- 49.Nathan DM, Balkau B, Bonora E, et al. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs/American Medical Association Health literacy: report of the council on scientific affairs. JAMA. 1999;281(6):552–557. [PubMed] [Google Scholar]