Abstract
Limitations imposed by conventional analytical technologies for cell biology, such as flow cytometry or microplate imaging, are often prohibitive for the kinetic analysis of single-cell responses to therapeutic compounds. In this paper, we describe the application of a microfluidic array to the real-time screening of anti-cancer drugs against arrays of single cells. The microfluidic platform comprises an array of micromechanical traps, designed to passively corral individual non-adherent cells. This platform, fabricated in the biologically compatible elastomer poly(dimethylsiloxane), PDMS, enables hydrodynamic trapping of cells in low shear stress zones, enabling time-lapse studies of non-adherent hematopoietic cells. Results indicate that these live-cell, microfluidic microarrays can be readily applied to kinetic analysis of investigational anti-cancer agents in hematopoietic cancer cells, providing new opportunities for automated microarray cytometry and higher-throughput screening. We also demonstrate the ability to quantify on-chip the anti-cancer drug induced apoptosis. Specifically, we show that with small numbers of trapped cells (~300) under careful serial observation we can achieve results with only slightly greater statistical spread than can be obtained with single-pass flow cytometer measurements of 15,000 – 30,000 cells.
Keywords: microfluidics, Lab-on-a-Chip, cell array, apoptosis, anti-cancer drugs, cytotoxicity, cytometry, multitrap nanophysiometers
INTRODUCTION
Functional cell-based assays are becoming an important part of post-genomic biomedical research and future patient-tailored therapies.1,2 Validation of potential therapeutic targets, revealed by biochemical and genetic screens, requires the development of assays that provide information on both spatial and temporal interrelationships in signalling networks.2 Microfluidic devices are now being considered as an emerging technology in cell biology, promising both reductions in the cost of equipment and higher-throughput information.3 Importantly, as only low cell numbers and reagent volumes are required for such microfluidic analyzers, the ability to monitor single-cell signalling dynamics of rare subpopulations, such as those associated with cancers, provides the possibility of developing personalized therapeutics in which drug dosages and combinations of therapies can be patient-defined to treat individual disease.4,5
In both natural tumour suppression and cancer treatment, cancer treatment cell death through apoptosis is one of the most important events, resulting in the elimination of abnormal malignant cells and reducing the tumour size.6 Controlled cell death is, however, a stochastic process, often initiated and executed by multiple signalling pathways.2,6 As our understanding of these processes improve, the multiple mechanisms controlling cell demise continues to reveal increasingly complicated networks of molecular signalling.2,7
Although current conventional end-point assays are responsible for major advances in understanding cell signalling, there are still many areas of cancer cell biology that will benefit from further technological improvements.2,8 Most importantly, in the exploration of novel therapeutic targets in oncology, there is still a need for novel bioassays allowing both multivariate and real-time analysis of drug mechanisms.7
Here we have performed a kinetic, non-invasive analysis of tumour cell death using human promyelocytic leukaemia (HL60) and histiocytic leukaemia (U937) cell lines within a microfluidic device. Such suspension cells represent a particular challenge for conventional time-lapse imaging. They are non-adherent and their dislodgement during image acquisition renders conventional single-cell analysis difficult. One particular advantage of this microfluidic platform lies in its ability to enable the kinetic and multivariate analysis of signalling events in non-adherent hematopoietic cells, providing a method whereby the position of the cell is registered and maintained over extended periods of time (a feat that would be difficult with conventional technologies).
The microfluidic device consists of an array of mechanical traps fabricated in biologically compatible elastomer poly(dimethylsiloxane), PDMS. Computer simulations were utilized to model the flow behaviour around cell traps, indicating that isolated, trapped cells experience shear stress forces that are one to two orders of magnitude lower than what would be encountered by adherent cells not shielded by the traps. Not only does our approach avoid extensive preparative procedures for the cell, but also the device and trap design offer reductions in cell and reagent consumption when compared to more conventional assays, as the volume of the entire cell trap region is less than 20 nl.
Using this system, we show that low-dose continuous labelling with organic fluorochromes such as SYTO62 and SYTOX Green2,7,8 allows for a straightforward adaptation of existing microfluidic platforms9 for a real-time single-cell analysis. We demonstrate how the combined use of real-time fluorescent imaging and state-of-the-art microfluidic platforms can be a cost-effective solution for automated drug screening routines, as this technology provides the tools for the kinetic analysis of investigational anti-cancer compounds. We also postulate that our microchip technology can effectively supplement conventional techniques such as flow cytometry especially for ultra-low number, real-time monitoring of cellular parameters.
MATERIALS AND METHODS
Microfluidic Chips
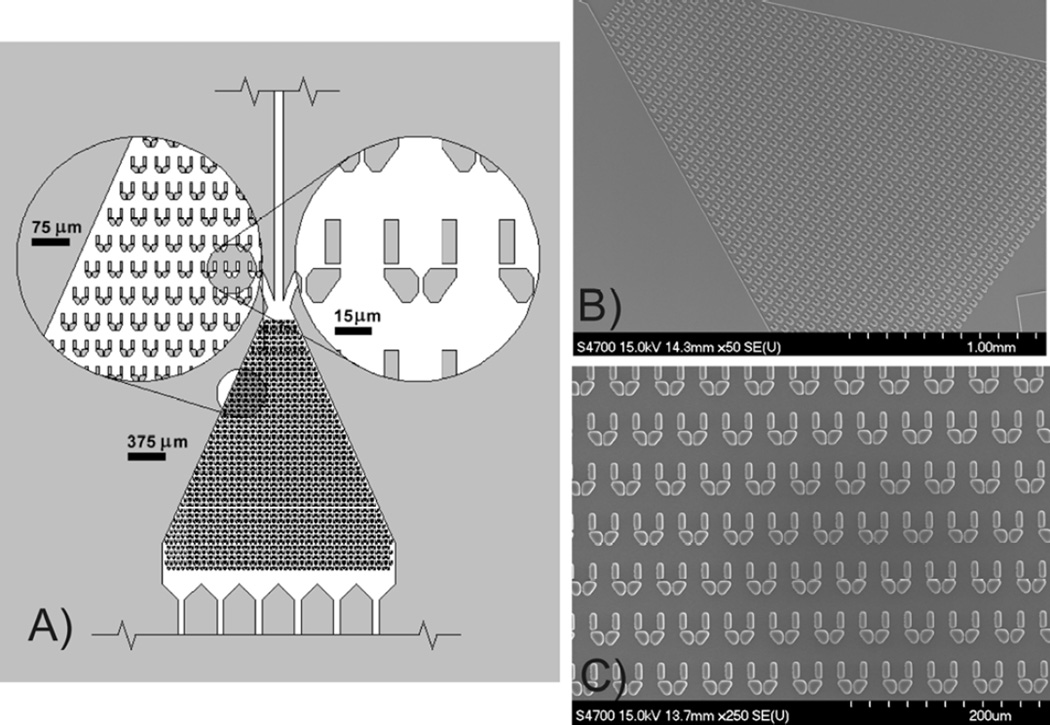
The microfluidic array cytometer consisted of a microfluidic chip fabricated in PDMS (Sylgard 184, Dow Corning) and comprised 440 mechanical traps, as shown in Figure 1. The dimensions of each cell trap were 18 µm (w), 20 µm (l) and 10 µm (d). Microchips were fabricated according to standard soft lithography procedures (refer to Supplementary Data).10,11 Briefly, microfluidic devices were made by casting a PDMS pre-polymer against a negative relief pattern developed in SU-8 that had been spun-coated on silicon wafers. A mixture of elastomer base and curing agent (10:1 ratio w/w) was degassed to remove any residual air bubbles and poured on SU-8 relief pattern to achieve a device thickness of 3–5 mm. PDMS was thermally cured at 70°C for 2 hours.9 Microfluidic chips were then sealed to quartz cover slips using conformal (reversible) bonding procedures10,11, and interfaced with computer-controlled, pressure-driven pumps using standard microfluidic inter-connects.9
Figure 1.
Microfluidic live-cell array (Array Cytometer). A) CAD schematic of chip layout showing a triangular microculture chamber, containing cell trapping array. B–C) SEM images of the array of PDMS cell traps.
Computer Simulations
In order to estimate the velocities throughout the flow domain and the shear stress over the cell surface, we created 2D and 3D models of a microfluidic device, with isolated single cells inside traps. This was achieved by solving the Navier–Stokes equation using finite element method simulations (Comsol 3.3). Boundary conditions consisted of a flow rate of 100 nL/min (average velocity 1.85 mm/s) at the inlet and pressure set to 0 at the outlets. The side-walls of the channels were set to a no slip condition. The complete device structure was modelled in 2D in order to estimate the different flow conditions throughout the length of the device. Single traps were also simulated in 3D for different flow conditions (due to the position of the trap in the device). Both velocities throughout the flow domain and, subsequently, the shear stress components at the boundaries of the domain were calculated.
Chip Loading and Induction and Visualization of Apoptosis
Microfluidic devices were primed with 70% ethanol (v/v) to help wet the device and reduce the nucleation and persistence of air bubbles. Ethanol priming also served as a sterilization technique in long-term cell culture experiments. Following the wetting of the PDMS surface, phosphate buffered saline, PBS, was flushed through the system for 30 s followed by RPMI 1640 culture medium,7 all at a flow rate of 2 µl min−1.
The chip was positioned on the microscope stage and cell loading was performed by placing an aliquot of cell suspension (30 µl, 2.5×105 cell/ml) in the loading reservoir. Both U937 and HL60 cells were used in the study. The output port was connected to a computer-controlled syringe pump (Harvard Apparatus, Holliston, MA, USA), adjusted to provide a continuous negative pressure (nominal withdrawal mode at 0.5–1 µl/min). Cell loading was confirmed microscopically, using bright-field imaging. To induce mitochondrial pathway apoptosis, cells were continuously perfused with staurosporine (STS; Sigma; 0.1–2.0 µM). Cell perfusion during experiments was performed using a flow rate of 100–250 nl/min. Cell events, including death, were recorded using a series of fluorescent markers, including SYTOX Green (30 nM), propidium iodide, PI (0.5 µg/ml), and red SYTO 62 (10 nM).7 Cells were incubated at 37°C on-chip under 5% CO2 and were imaged every minute for at least three hours.2,13
Chip Imaging and Data Analysis
Fluorescence images were acquired using a motorized Zeiss Axiovert 200M epifluorescence microscope. Objective lenses (×4, ×20, ×40) and appropriate fluorescence filters, i.e., 475nm excitation/535 nm emission for SYTOX Green, 525/595 nm for PI and 630/695 nm for SYTO 62, were used for obtaining multicolor images, which were overlaid, as required. Automated time-lapse image acquisition was used for periods of up to four hours under the control of Axiovision software (Zeiss). CellProfiler, an open source image analysis platform (based at the Broad Institute Imaging Platform and available freely at http://www.cellprofiler.org/), was used for quantitative imaging cytometry analysis. Results shown are representatives of three independent experiments. The Student's t-test was applied for comparison between groups using SPSS 11 (Chicago, IL, USA) with significance set at p<0.05.
Flow cytometry
Flow cytometry was performed using a BD FACSCalibur (BD Biosciences) analyser, equipped with a 15 mW Argon-ion and 20 mW red diode lasers. Logarithmic amplification scale using following configuration of band-pass (BP) filters was applied: (i) 488 nm excitation line: FL1 (525 BP for collection of: SYTOX Green fluorescence signals); (ii) 635 nm excitation line: FL4 (675 BP for collection of: SYTO62 fluorescence signals). Acquisition was performed in 1024 channels resolution scale using CellQuest Pro software (Becton Dickinson). A typical run used a sample with ~5,000 cells, so that with a triplicate experiment involved ca. 15,000 cells. The maximum sample size was always <10,000 cells (although clearly in some experiments this meant a total of 30,000 cells were used). Data analysis was performed using Summit v3.1 (Dako Cytomation, Fort Collins, CO, USA) and an open access WinMDI ver.2.8 (http://facs.scripps.edu/software.html) software.
RESULTS
Microfluidic Cell Array Performance
We have demonstrated the feasibility of using a PDMS-based cell array that consisted of a cell culture microchamber with an array of 440 mechanical traps9 (Figure 1) for the hydrodynamic positioning and mechanical trapping of single hematopoietic derived U937 and HL60 cells. We found that this device was suitable for the rapid screening of anti-cancer compounds by using negative pressure-driven flow with the output port connected to a computer-controlled syringe pump. Consistent with earlier reports, electrostatically bonded and vacuum-sealed PDMS devices maintained adequate integrity for up to 24 hours12, providing a convenient technique which alleviated the need for oxygen plasma treatment of PDMS (the latter being required for longer-term incubations).10
We found that the ethanol wash followed by RPMI was effective in wetting the device and removing bubbles. Subsequent cell loading was always performed under saturating concentration of cells (typically above 2.5×105 cells/ml) with trapping efficiency between 10–20% for both U937 and HL60 cells. Over 85% of hydrodynamically trapped U937 and HL60 cells retained their position during the course of the short-term experiments (ca. 3 hours). More extensive cell dislodgement, resulting mostly from cell migration and/or cell death was, however, observable during longer incubation periods.
To evaluate influence of micro-environmental conditions on cell viability, U937 cells were loaded as described previously and perfused with RPMI 1640 medium supplemented with 0.25 µg/ml of propidium iodide (PI) for up to 24 hours. Over 90% of U937 cells maintained viability for up to 24 hours on chip perfusion. Results also corresponded well to those obtained in a conventional cell culture performed on a 96-well polystyrene plate, indicating that the microfluidic environment does not compromise viabilty.9
Computational Modelling of Hydrodynamic Conditions
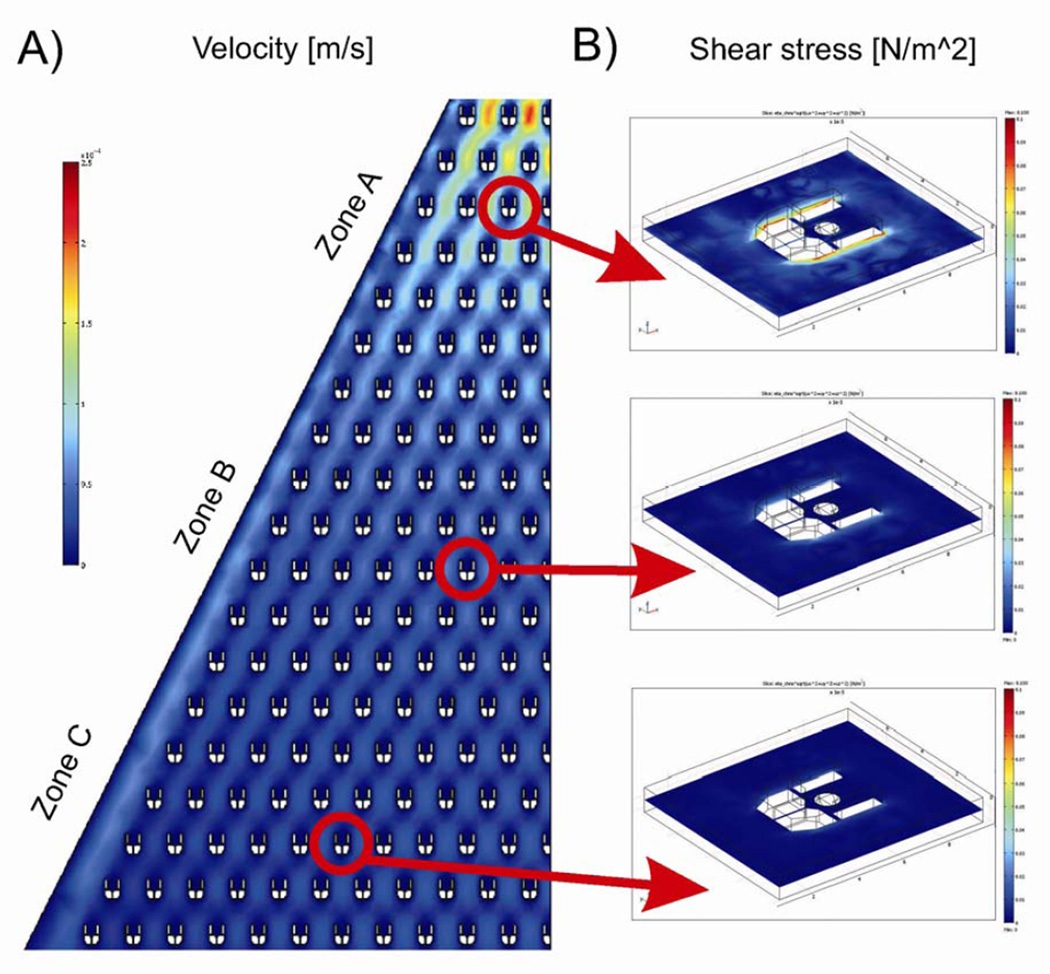
Numerical simulations were performed to estimate both the flow velocity throughout the device and the extent of shear stress exerted over the trapped cells.4 Firstly, a 2D model of the device was built and simulated for an average flow rate of 1.85 mm/s at the inlet to characterize the flow throughout the chip. Due to the device geometry, the flow velocity decreased non-linearly towards the outlet, with a substantial drop after the first ten rows of traps. In all cases, the flow velocity was estimated always to be an order of magnitude greater in the regions of the device outside the traps (velocities varying from 900 to 30 µm/s) when compared to that within the traps (velocities varying from 5 to less than 1 µm/s). The hydrodynamic conditions to which the immobilised cells were subjected in different regions of the chip were estimated in regions of different flow velocities, as shown in Figure 2(a). The cells were simulated as rigid (not deformable) spheres. The distribution of shear stress around the traps was obtained from a 3D model simulation, Figure 2(b), where different average flow velocities at the inlet were used (according to 2D model solutions). From these simulation results, the average shear stress observed outside the trapping structure varied between 0.09 and 0.01 dyne/cm2, whilst the average shear stress experienced by a spherical trapped cell of 9 µm in diameter varied between 0.05 and 0.007 dyne/cm2, depending upon the position of the trap in the device, Figure 2(b). These simulations show that, as a consequence of the nature of the flow and the device geometry, the cells are being maintained within a low shear-stress environment. We have previously explored signalling events associated with cells under a range of different flow induced mechanical loads.14 Our results indicate that the cells within this current device are experiencing stresses at least two orders of magnitude lower than values reported to have initiated cell signalling.
Figure 2.
Computational modelling of hydrodynamic conditions inside microculture chamber. A) A 2D computer model of the flow velocity. Because of the shape of the overall device, the fluid flow velocity decreases nonlinearly towards the outlet with a substantial drop after first ten rows of traps. In all cases, the regions inside the traps are characterized by a velocity field much lower than in the remaining area of the microchamber. B) 3D simulation results of the shear stress exerted on a trapped cell in three sections of the chip: high velocity (zone A), medium velocity (zone B) and low velocity (zone C). All scale bars have been set to the same limits.
Quantification of Drug-induced Cytotoxicity
Tumour cell death is a stochastic process, often initiated and/or executed by multiple signalling pathways. We have recently shown that many organic fluorescent probes allow non-invasive tracking of intracellular events over extended time without compromising cell viability.7,8 The low-dose, continuous labelling procedure not only provides similar results to a standard end-point staining protocol, but also allows a straightforward adaptation for microfluidic platforms.2,7,8
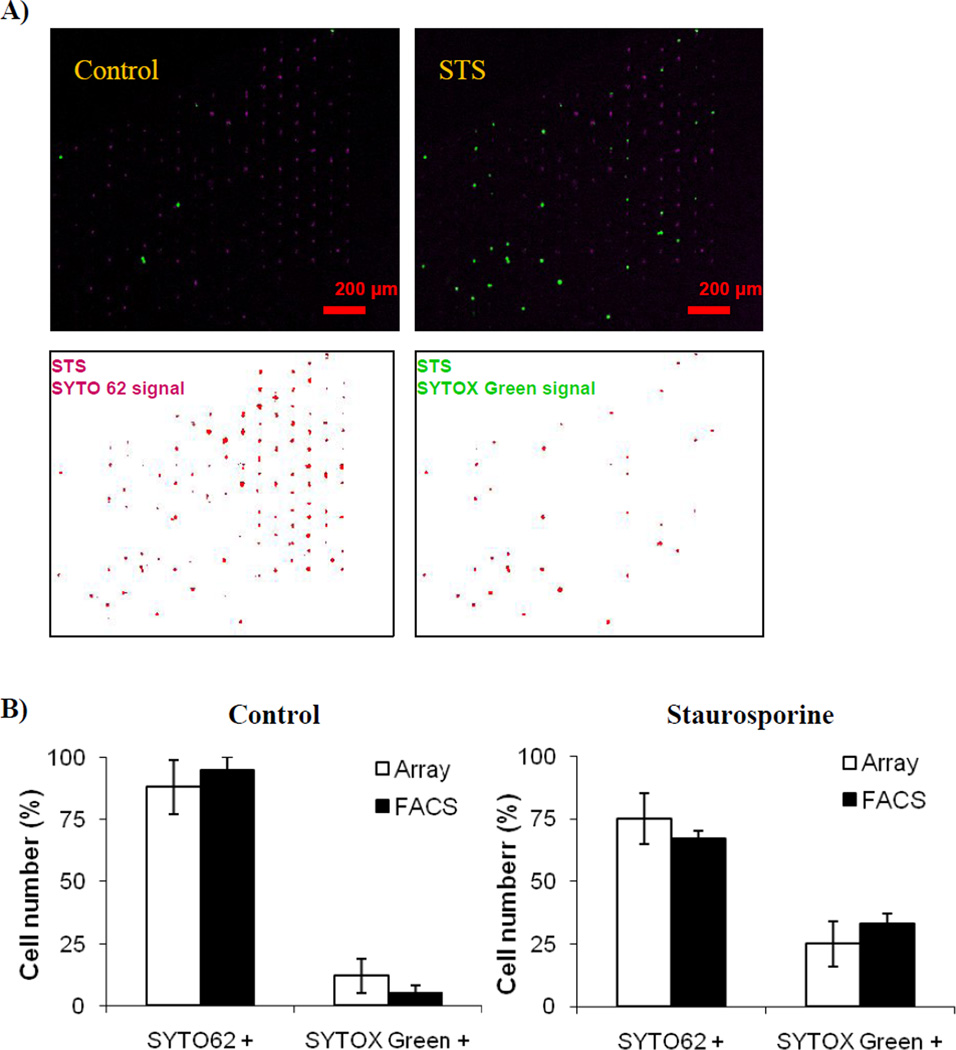
We demonstrated that it is possible to load HL-60 cells into the microfluidic device and continuously perfuse them with media containing SYTO62 and SYTOX green, two well-known apoptosis indicators. In addition, the exposure of the cells to 2 µM STS induced mitochondrial pathway apoptosis. The deconvolved images of a chip filled with STS-treated cells are depicted on lower panels of Figure 3(a). As expected, cells underwent apoptosis upon exposure to STS as shown in Figure 3. These results correlate well with the conventional gold standard of flow cytometry, as shown in Figure 3(b). Although the chip-to-chip variation of 8% achieved with populations of ~300 cells exceeded the 5% variation obtained when using conventional flow cytometers on 15,000–30,000 cells, the excellent correlation between the two platforms lends further support for the development of automated microfluidic cytometers.
Figure 3.
On-chip analysis of drug induced apoptosis. Human HL-60 cells were perfused with 2 µM of Staurosporine (STS) for 2 hours in the presence of apoptosis marker SYTO 62 and plasma membrane permeability marker SYTOX Green. A) On-chip quantification of apoptosis using SYTO 62 and SYTOX Green. Composite images were collected at time 0 and 2 hours (upper panels). Automated chip analysis for STS treated samples is shown in lower panels. B) Quantification and comparison of data obtained by array chip and conventional flow cytometer. Parallel samples were separately analyzed using either conventional flow cytometer (BD FACS Calibur, BD Biosciences) or microfluidic cell array. Note excellent correlation between results from these two technologically dissimilar platforms (R2 ≥ 0.85; p < 0.05 for Pearson and Lee linear correlation test).
Dynamic Quantification of Tumour Cell Death
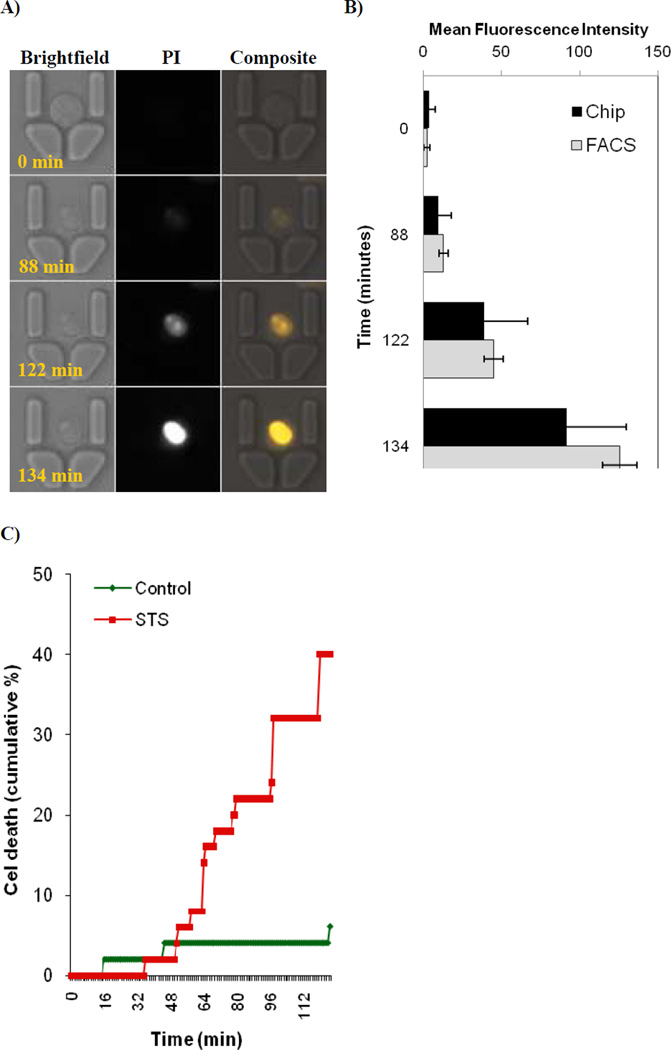
In our kinetic on-chip analysis of tumour cell death, HL60 cells were loaded onto a chip and continuously perfused with media containing 2 µM PI and STS, and images were acquired every minute for 3 hours. Figure 4 presents representative images of single cells entrapped on a chip. In contrast to standard end-point analysis, on-chip kinetic image analysis allows observation of early plasma membrane permeabilization, an event seen at ca. 90 mins in Figure 4(a), which is followed by a gradual increase in PI permeability. Quantification of 250 cells analyzed in 1 minute intervals provided a stratified cell survival curve, as presented in Figure 4(b). This allows the tracking of differences in stochastic cell death sensitivity that could remain undetectable with conventional end-point analysis such as flow cytometry. The control chip consisted of HL60 cells perfused with complete RPMI1640 medium supplemented only with 0.5 µg/ml of propidium iodide (PI).
Figure 4.
Dynamic analysis of drug induced cytotoxicity using microfluidic cell array. Human HL-60 cells were perfused with 2 µM of Staurosporine for up to 3 hours in the presence of plasma membrane permeability marker propidium iodide (PI). Time-lapse images were collected every minute. Note the stochastic nature of anti-cancer drug action. A) Typical time-resolved images of an HL-60 cell undergoing apoptosis after perfusion with Staurosporine, with time points indicated at the lower left corner. Note the gradual increase in plasma membrane permeability to PI that represents initial destabilization of plasma membrane structure during apoptosis. B) Comparison between mean fluorescence intensity distributions achieved by both chip imaging and flow cytometry (FACS). Note higher SD values obtained by cell imaging as compared to FACS. C) Quantification of results from time-lapse images. Results represent cumulative percentage from two independent chips where a total of 250 cells were analysed. Note the stochastic nature of cell death.
DISCUSSION
Innovative microfluidic technologies can bring substantial improvements to conventional methods of cell screening.9,15,16 Such devices promise greatly reduced equipment costs, important for both personalized diagnostics (bench-to-bed approach) and drug discovery pipelines.4,7,17 Most importantly, as only low cell numbers and reagent volumes are required by microfluidic analyzers, single-cell studies of rare subpopulations will finally appear within reach. This is especially true for studies on progenitor cells, stem cells and other patient-derived primary samples.4,9,18
We show that, when used with high-content image analysis platforms, microfluidic live-cell arrays, of the type described in this paper are useful for multiparameter studies on a single-cell level.9,14,18 The PDMS-based cell array, as described, not only reduces the complexity of conventional cell culture protocols but also enables time-resolved studies on hydrodynamically trapped hematopoietic (non-adherent) cells. The latter feature is of particular advantage for determining the kinetics of pharmaceutical efficacy in these non-adherent cell lines, allowing for sequential pharmacological stimulations and real-time analysis of cellular physiology while providing reductions in the use of reagents and sample.
Our study shows the application of live-cell microarrays to the kinetic analysis of investigational anti-cancer agents in hematopoietic cancer cells. We show how non-invasive tracking of intracellular events over extended time provides new information in the understanding of the process of apoptosis. Most importantly, the use of our device allows multiple serial observations of individual cells protected from the stress associated with shear flow. In contrast, the serial observation of cells by FACS produces only population averages, cannot identify whether there are separate populations of cells exhibiting a different time course,9 and subjects the entire population of cells to additional shear forces during each passage through the FACS.19
The microfluidic chip can be used to sample a greatly reduced number of cells when compared with FACS. This ability is of particular importance in studying the outcome of patient derived cancer cells, when exposed to therapeutic drugs, as these cells are often rare and difficult to collect and purify. Indeed, although Figure 3(b) seems to suggest that FACS with a 5% variation is statistically superior to the on-chip assay with an 8% chip-to-chip variation, it is important to consider the difference in the sizes of the two cell populations. If the differences in the two techniques were governed only by population-size statistics, and for a normal distribution, the difference between 300 cells and 15,000–30,000 would produce square-root-of-N uncertainties of 5.8% and 0.8-0.6%, respectively. This simple analysis would suggest that the on-chip device is operating closer to its statistical limit and that the FACS approach is subject to another, yet unidentified source of cell-to-cell variability (perhaps as a consequence of hydrodynamically induced apoptosis).19 We suggest that our technology is likely to become a useful adjunct technique to conventional flow cytometry especially in situations where ultra-low numbers of cells need to be quantified and continuously monitored.
The presented platform was adapted for the quantification (functional assays) on established cancer cell lines. An interesting point worth consideration, however, is the proliferative potential of cells cultured on-chip. This particular situation can be encountered with both established cell lines as well as patient derived cancer stem cells. Especially the proliferative potential of the latter can be relevant for performance of long-term on-chip assays. Only recent we have showed the successful on-chip implementation of dynamic cell proliferation, viability, and motility, techniques which are otherwise difficult to perform using conventional methods.20 We have investigated both patient-derived normal and cancerous stem cell responses to the small molecule kinase inhibitors approved for treatment of imatinib resistant leukaemia. By utilizing similar trapping array to presented in this work, we were able to track both the stem cell division symmetry, proliferation rate of cancer stem cells and even for the first time quantify cancer stem cell motility characteristic in response to therapeutic agents.20 These results provide further support to the notion that the microfluidic cell array environment is well equipped to support long-term cell culture and real-time analysis of cancer stem cells.20
In conclusion, we present the use of a microfluidic chip for the real-time analysis of events leading up to apoptosis in model cell lines. We show that for small numbers populations, as is appropriate for patient derived cancer cells), we can produce statistically robust information, including the analysis of membrane permeabilisation and cell death, leading to the generation of a stratified cell survival curve.
ACKNOWLEDGMENTS
Grant sponsor: Supported by BBSRC, EPSRC and Scottish Funding Council, funded under RASOR (DW, SF and JMC), NIH Grant U01AI061223 (JPW). The authors thank Dr Joanna Skommer (QMRI, MRC, Edinburgh, UK) and Dr Zbigniew Darzynkiewicz (Brander Cancer Research Institute, NYMC, USA) for providing cell lines, reagents and helpful discussions, and David Schaffer and Allison Price (Vanderbilt Institute for Integrative Biosystems Research and Education) for the production of device masters and their editorial assistance, respectively.
REFERENCES
- 1.Martin RM, Leonhardt H, Cardoso MC. Cytometry A. 2005;67A:45–52. doi: 10.1002/cyto.a.20172. [DOI] [PubMed] [Google Scholar]
- 2.Wlodkowic D, Skommer J, Darzynkiewicz Z. Cytometry A. 2008;73:496–507. doi: 10.1002/cyto.a.20535. [DOI] [PubMed] [Google Scholar]
- 3.Andersson H, van den Berg A. Curr Opin Biotechnol. 2004;15:44–49. doi: 10.1016/j.copbio.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Di Carlo D, Wu LY, Lee LP. Lab Chip. 2006;6:1445–1449. doi: 10.1039/b605937f. [DOI] [PubMed] [Google Scholar]
- 5.Svahn HA, van den Berg A. Lab Chip. 2007;7:544–546. doi: 10.1039/b704632b. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Wlodkowic D, Skommer J, Faley S, Darzynkiewicz Z, Cooper JM. Exp Cell Res. 2009;315:1706–1714. doi: 10.1016/j.yexcr.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wlodkowic D, Darzynkiewicz Z. Cytometry A. 2008;73A:877–879. doi: 10.1002/cyto.a.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faley S, Seale K, Hughey J, Schaffer DK, VanCompernolle S, McKinney B, Baudenbacher F, Unutmaz D, Wikswo JP. Lab Chip. 2008;8:1700–1712. doi: 10.1039/b719799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sia SK, Whitesides GM. Electrophoresis. 2003;24:3563–3576. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 11.McDonald JC, Whitesides GM. Acc Chem Res. 2002;35:491–499. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- 12.Khademhosseini A, Yeh J, Eng G, Karp J, Kaji H, Borenstein J, Farokhzad OC, Langer R. Lab Chip. 2005;5:1380–1386. doi: 10.1039/b508096g. [DOI] [PubMed] [Google Scholar]
- 13.Wlodkowic D, Skommer J, Hillier C, Darzynkiewicz Z. Cytometry A. 2008;73:563–569. doi: 10.1002/cyto.a.20564. [DOI] [PubMed] [Google Scholar]
- 14.Yin H, Zhang X, Pattrick N, Klauke N, Cordingley H, Haswell SJ, Cooper JM. Anal. Chem. 2007;79:7139–7144. doi: 10.1021/ac071146k. [DOI] [PubMed] [Google Scholar]
- 15.Qin J, Ye N, Liu X, Lin B. Electrophoresis. 2005;26:3780–3788. doi: 10.1002/elps.200500113. [DOI] [PubMed] [Google Scholar]
- 16.Lee PJ, Gaige TA, Hung PJ. Lab Chip. 2009;9:164–166. doi: 10.1039/b807682k. [DOI] [PubMed] [Google Scholar]
- 17.Manz A, Dittrich PS. Nature Drug Discovery. 2006;5:210–218. doi: 10.1038/nrd1985. [DOI] [PubMed] [Google Scholar]
- 18.Sims CE, Allbritton NL. Lab Chip. 2007;7:423–440. doi: 10.1039/b615235j. [DOI] [PubMed] [Google Scholar]
- 19.Mollet M, Godoy-Silva R, Berdugo C, Chalmers JJ. Biotechnology and Bioengineering. 2008;100:260–272. doi: 10.1002/bit.21762. [DOI] [PubMed] [Google Scholar]
- 20.Faley SL, Copland M, Wlodkowic D, Kolch W, Seale KT, Wikswo JP, Cooper JM. Lab Chip. 2009 doi: 10.1039/b902083g. (in press) [DOI] [PubMed] [Google Scholar]