Abstract
Purpose.
This study tested the hypothesis that intraocular pressure (IOP) elevations, induced by controlled increase of intraocular volume, are correlated with the biomechanical responses of the posterior sclera.
Methods.
Porcine globes were tested within 48 hours postmortem. The first group of globes (n = 11) was infused with 15 μL of phosphate-buffered saline at three different rates to investigate rate-dependent IOP elevations. The second group (n = 16) was first infused at the fast rate and then underwent inflation tests to investigate the relationship between IOP elevations (ΔIOP) and scleral strains. The strains in the superotemporal region of the posterior sclera were measured by ultrasound speckle tracking. Linear regression was used to examine the association between ΔIOP due to micro-volumetric infusion and the scleral strains at a specific inflation pressure.
Results.
The average ΔIOP was 14.9 ± 4.3 mm Hg for the infusion of 15 μL in 1 second. The ΔIOP was greater for the faster infusion rates but highly correlated across different rates (P < 0.001). A significant negative association was found between the ΔIOP and the tangential strains in both the circumferential (R2 = 0.54, P = 0.003) and meridian (R2 = 0.53, P = 0.002) directions in the posterior sclera.
Conclusions.
This study showed a substantial increase in IOP, with a large intersubject variance during micro-volumetric change. A stiffer response of the sclera was associated with larger IOP spikes, providing experimental evidence linking corneoscleral biomechanics to IOP fluctuation. In vivo measurement of corneoscleral biomechanics may help better predict the dynamic profile of IOP.
Keywords: scleral biomechanics, IOP fluctuation, glaucoma posterior segment
This study reports a significant association between the strains in the posterior sclera and IOP elevations during short-term micro-volumetric changes. These results provide experimental evidence linking corneoscleral biomechanics to dynamic IOP.
Introduction
Glaucoma is one of the leading causes of blindness in the United States and worldwide and is a major public health problem because of its significant visual and economic consequences.1 Elevated intraocular pressure (IOP) is known to be a primary risk factor for glaucomatous optic neuropathy. Although steady-state IOP and its determinants have been the longstanding focus of the glaucoma field, it is well documented that the eye experiences frequent IOP fluctuations during daily activities such as blinking, eye movement, postural change, fluid intake, or Valsalva maneuver.2–7 An early study showed that forcibly squeezing or rubbing the eye could raise IOP to over 80 mm Hg.3 Another study showed that postural change from sitting to supine position could raise IOP by 2 mm Hg in one individual but over 10 mm Hg in another.2,4 These short-term, rapid IOP elevations, whose mechanisms likely differ from steady-state, appear to vary substantially among individuals and may be linked to glaucoma susceptibility.7–9
Experimental studies have suggested potentially detrimental influences of rapid IOP fluctuations, particularly those of a repetitive nature. Brief but rapid IOP spikes were shown to cause accumulative damage to retinal ganglion cells in vitro and in an animal model, whereas the same level and total duration of IOP elevation with a slow rate of change was found to be harmless.10 Although clinical studies of the association between intervisit IOP fluctuations and glaucoma progression have reported disparate findings, it is generally recognized that large fluctuation is a characteristic of glaucoma and has certain diagnostic value. In a perspective review of the results reported in several large clinical trials,11 it was found that IOP fluctuation was associated with glaucoma progression in patients with low IOPs and moderately advanced disease12–14 but was not a significant risk factor in patients with high IOPs and earlier stage of glaucoma.15 These findings suggest the need to study the complex interactions between patient-specific characteristics and IOP fluctuations in order to better understand their role in glaucoma progression.
Although the detailed physiological processes involved in short-term IOP variations are not fully characterized, it is currently understood that IOP variations can be caused by aqueous flow changes associated with aqueous production or outflow facility; changes in blood flow (volume or pressure); and/or intraocular fluid displacement associated with external forces acting on the eye (e.g., blinking, eye rubbing, or eye movement). For example, when the body changes from a sitting to supine position, there is an increase in episcleral venous pressure which impairs aqueous outflow, leading to IOP elevation.16 There may also be rapid blood filling in the intraocular blood vessels (i.e., choroidal vessels) in the supine position,17 suggested by the fact that the postural effect on IOP is almost instantaneous.18,19 Changes in both aqueous flow and blood flow effectively alter the total volume within the corneoscleral shell, which presumably is the direct cause of IOP variation.
From a biomechanical point of view, the parameters of dynamic IOP (i.e., peak magnitude and rate of change) are influenced by the biomechanical properties of the corneoscleral shell. We have previously shown that a stiffened cornea resulted in significantly greater IOP elevations for a given intraocular volume increase by using an experimental porcine eye model.20 Those results suggested that the extensibility of the cornea could modulate the whole eye's response to microintraocular volume changes. It is thus reasonable to hypothesize that the sclera, which occupies five-sixths of the outer shell, could also significantly affect the characteristics of short-term IOP elevations.
Inflation tests with surface strain measurements in animal models or human cadaveric eyes have yielded important data for better understanding scleral biomechanics in association with glaucoma progression and IOP elevation.21–24 In the present study, we used inflation tests in combination with an ultrasound speckle tracking method25 to measure tangential (i.e., tensile) and radial (i.e., compressive) strains through the thickness of the posterior sclera. Our primary hypothesis was that the increase in IOP during controlled volume infusion was negatively correlated with the tangential strains of the posterior sclera.
Methods
Sample Preparation
Twenty-seven porcine eyes were obtained within 24 hours of slaughter (SiouxPreme Packing Co., Sioux City, IA). The animals were approximately 6 months old, and one eye per animal was used. The globes were stored in phosphate-buffered saline (PBS) at 4°C prior to use. All measurements were completed within 48 hours postmortem.
The globes were divided into two groups. The first group (n = 11) was used to investigate the IOP elevations at three different infusion rates (fast, intermediate, and slow, as described below). The second group (n = 16) was used to investigate the relationship between scleral strains and IOP elevations at the fast infusion rate.
Measurement of Ocular Dimensions
The dimensions of all experimental eyes were assessed using a customized A-mode ultrasonography system described previously.26 The eyes were immersed in saline during the measurements. Ultrasound echoes along the anterior-posterior, nasal-temporal, and superior-inferior directions of the eye were acquired. Assuming sound velocities of 1605 m/s (cornea and sclera),27,28 1540 m/s (aqueous and vitreous humor),29 and 1645 m/s (lens),28,29 the dimensions of the eyes were determined from the measurement of the time of flight of the ultrasound echoes.
Volume Controlled Infusion
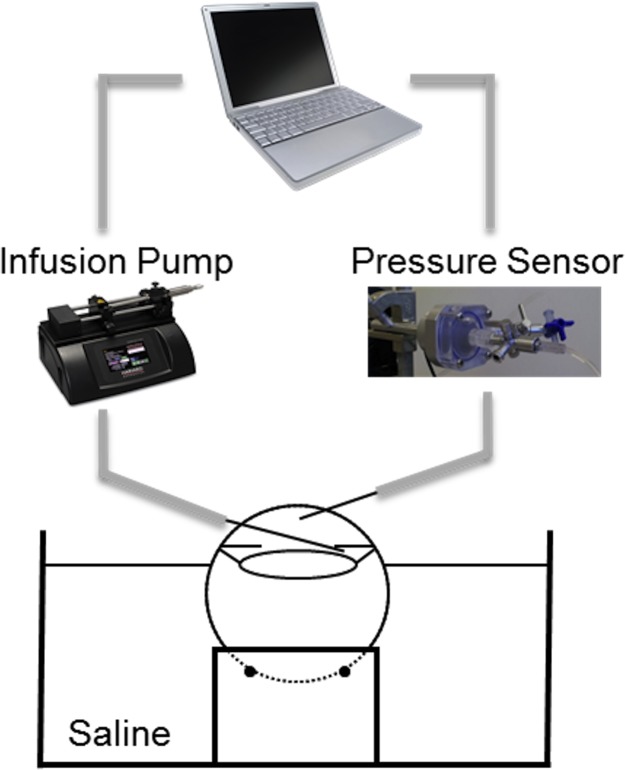
The globes were placed on a holder (Fig. 1) to allow unrestricted volume expansion during infusion. The globes were immersed in saline up to the limbus, and corneas were kept moist by continuous dripping of Optisol GS (Bausch & Lomb, Rochester, NY) to avoid excessive corneal swelling.
Figure 1.
Experimental setup for controlled volume infusion in porcine eyes.
A 20-G needle (Exelint, Inc., Los Angeles, CA) was inserted into the posterior chamber of the eye for infusion of PBS by using a programmable infusion pump (UltraPhD, Harvard Apparatus, Boston, MA) controlled by a customized LabVIEW software (LabVIEW; National Instruments, Austin, TX). Infusion of the posterior chamber has been shown to minimize the “washout” effect observed in non-human eyes, which manifests as a decrease in the resistance to aqueous outflow with the volume of perfusion.30 Another 20-G needle, inserted through the cornea into the anterior chamber, was connected to a pressure sensor (TAM-A; Harvard Apparatus) that recorded the continuous pressure data by using the LabVIEW program, allowing synchronous pressure and infusion volume measurements. The infusion volume was validated prior to each measurement by infusing 15 to 30 μL of PBS into a microcentrifuge tube and measuring the volume by precision pipetting.
Any residual choroidal blood in the eye was removed by two brief infusions prior to measurements. The eye was then perfused using the programmable infusion pump to establish a stable baseline IOP of 15 mm Hg, and the corresponding steady-state infusion rate was recorded for each eye as the outflow rate. For the first group of globes (n = 11), three infusion flow rates were selected to simulate fast (IOP rising in seconds), intermediate (IOP rising in 10s of seconds), and slow (IOP rising in minutes) short-term IOP elevations. These rates were chosen based on two considerations. First, the reported data of IOP fluctuations observed in the human eye during blinking, postural change, ocular pulse, fluid intake, and other physiologic conditions provided physiologically relevant nominal values of the magnitude of IOP elevations and the time scale within which such elevations are achieved. Table 1 summarizes the current literature of IOP fluctuations under different physiologic conditions. Telemetric IOP monitoring in several other species were also conducted in the past, reporting similar findings regarding the effects of blinking and eye movement on IOP.31–34 Second, our initial tests with infusions at a wide range of rates and total volumes provided the basis for defining the meaningful ranges that best simulate the short-term IOP elevations seen in the living eye.
Table 1.
Parameters of Short-Term, Rapid IOP Elevations Observed in Human Eyes
|
Physiologic Conditions |
Typical IOP Elevation, mm Hg |
Typical Duration to Reach Peak |
References |
| Blinking/lid squeezing | 10–15/80–100 | Hundreds of ms/s | 3 |
| Sitting to supine position | 2–9 (higher in glaucoma) | 10s of s | 2, 4, 8, 19, 35, 36 |
| Head tilt to inversion | 20–30 | 10s of s | 5, 19 |
| Valsalva maneuver | −4 to 10 | s to 10s of s | 6, 9, 37 |
| Ocular pulse | 2–8 | s | 38–40 |
| Fluid intake | 1–5 (over 6 in some POAGs) | Min | 7, 41 |
POAG, primary open-angle glaucoma.
The targeted flow rates used in this study are specified in Table 2. The order in which the infusions were carried out was randomized, and each infusion was repeated twice. After each infusion, a withdrawal at the same rate as the infusion was implemented to restore the IOP to baseline. The globes were allowed to equilibrate for at least 10 minutes between two infusions. One additional fast infusion was repeated at the end of all experimental infusions to confirm that the tissue response was not altered due to multiple infusions.
Table 2.
Rate and Duration of the Three Different Infusions With the Same Total Infusion Volume of 15 μL
|
Parameter |
Fast |
Intermediate |
Slow |
| Rate | 15 μL/s | 1 μL/s | 0.1 μL/s |
| Duration | 1 s | 15 s | 150 s |
For the second group of globes (n = 16), only the fast infusion (i.e., 15 μL/s) was used and repeated twice.
Inflation Tests With Ultrasound Speckle Tracking
After the infusion experiments, the corneas of the second group of eyes were trephined, and the scleral shells were prepared for inflation tests. The scleral shells were mounted on a custom-built pressurization chamber (Fig. 2). The anterior sclera was clamped between two O-rings with a screw threaded through the opening in the shell (Fig. 2).
Figure 2.
Schematic of the inflation testing setup.
The surface of the sclera was cleaned with dissecting scissors, and the shell was immersed in a saline bath. The superotemporal region of the posterior pole was positioned at the apex of the sclera mount. The sclera shell was first preconditioned with 10 cycles of pressurization from 5 to 30 mm Hg in 60 seconds and then allowed to equilibrate at 5 mm Hg for 15 minutes. The pressure inside the scleral shell was then gradually increased from 5 to 45 mm Hg in steps of 0.2 mm Hg (for pressures between 5 and 15 mm Hg), 1 mm Hg (for pressures between 15 and 25 mm Hg), and 2 mm Hg (for pressures between 25 and 45 mm Hg). During inflation, a 55-MHz ultrasound probe (Vevo 660; Visualsonics, Toronto, ON) was positioned above the apex of the scleral mount (i.e., the posterior pole region in the superotemporal quadrant adjacent to the optic nerve head). Two inflations were carried out to acquire the radiofrequency data of ultrasound scans either along the meridian or circumferential directions (as shown in Fig. 3) in a randomized order. Fifteen minutes of equilibration was allowed between the two inflations.
Figure 3.

Location of the ultrasound scans (left, C: circumferential, M: meridian) and definition of radial and tangential strains superimposed on a sample ultrasound image of the posterior sclera (right).
The vector field of displacements within the cross-sections was computed using a previously validated ultrasound speckle tracking algorithm.25 The tangential and radial strains (as defined in Fig. 3) were computed from the displacement data, and the average strains within a pre-selected region of interest were used for further analysis. Due to the large thickness of the porcine posterior sclera (often greater than 1.2 mm), high-frequency ultrasound signals were typically attenuated to a noticeable extent in the deeper sclera. Therefore only the outer half was used for analysis in this study.
Statistical Analysis
Statistical analysis was performed using SAS version 9.3 software (SAS Institute, Inc., Cary, NC). Pearson correlation coefficients were used to evaluate the association between IOP increases at different infusion rates, as well as the association between scleral strains at different inflation pressures. Linear regression was used to examine the association between the change in IOP and the measured strains at a selected pressure level. Sensitivity analysis was conducted to confirm the conclusions using linear mixed model for repeated measures with the volume of the eye considered as a covariate.
Results
The mean volume of all 27 porcine eyes was 7.3 ± 0.7 mL (range: 5.9–9.1 mL). The axial length was 21.7 ± 0.8 mm; the diameter along the nasal-temporal direction was 25.5 ± 0.8 mm; and the diameter along the superior-inferior direction was 25.1 ± 1.0 mm. The outflow rate at baseline pressure (15 mm Hg) was 3.4 ± 1.0 μL/min.
The change in IOP from baseline (ΔIOP) after infusion of 15 μL of PBS at rates of 15 μL/s (fast), 1 μL/s (intermediate), and 0.1 μL/s (slow) is shown in Table 3. There was a large variance in ΔIOP among the tested eyes at the same infusion rate and there was a significant decrease in ΔIOP from the fast to intermediate to slow infusions. ΔIOP from the last additional fast infusion differed by <1.5 mm Hg from earlier fast infusions in the eye, suggesting minimal changes in the tissue response after multiple micro-volumetric infusions.
Table 3.
Change in IOP Associated With Fast, Intermediate, and Slow Infusions of 15 μL Total Volume in Porcine Eyes
|
Δ IOP, mm Hg |
Infusion Rate |
||
|
Fast |
Intermediate |
Slow |
|
| Mean | 14.9 | 12.2 | 8.0 |
| Standard deviation | 4.3 | 3.3 | 2.54 |
| Minimum | 9.6 | 7.8 | 5.4 |
| Maximum | 23.8 | 19.2 | 13.7 |
Strong correlations in ΔIOP were found between the fast infusion rate and the intermediate (R = 0.99, P < 0.001) or slow infusion rates (R = 0.89, P < 0.001) (Fig. 4a). The ΔIOP was also correlated between the intermediate and slow infusion rates (R = 0.93, P < 0.001). Figure 4b shows an approximately linear relationship between ΔIOP and the infused volume at different infusion rates.
Figure 4.
(a) Strong correlations in ΔIOP between different infusion rates; (b) Average ΔIOP as a function of the infused volume at different infusion rates (baseline IOP was 15 mm Hg for these tests).
Because the IOP elevations under different infusion rates were highly correlated in the same eye, only fast infusion was used in the second group of the eyes with inflation tests in order to reduce the possibility of unwanted tissue property change due to prolonged experimental time and many repeated IOP elevations in the same eye.
Figure 5 presents the tangential and radial strains measured at different inflation pressures in the superotemporal region of the posterior sclera. Only the even-numbered eyes, according to the testing sequence, were included in the plot for visual clarity. Both tangential and radial strains showed a consistent trend as the inflation pressure increased (e.g., a sample with a smaller tangential strain at a lower pressure also had a smaller tangential strain at a higher pressure). The Pearson correlation coefficients ranged from 0.94 to 0.99 (all P values < 0.001) for tangential strains and 0.74 to 0.99 (all P values ≤ 0.002) for radial strains along the circumferential direction measured at inflation pressures above 14 mm Hg, showing that these strains were highly correlated. Similar results were observed in the meridian tangential strains with Pearson correlation coefficients ranging from 0.98 to 0.99 (all P values < 0.001) and meridian radial strains with Pearson correlation coefficients ranging from 0.98 to 0.99 (all P values < 0.001). The tangential strains along the circumferential and meridian directions were also strongly correlated with each other (R ranged from 0.76–0.86; P ≤ 0.002). The meridian tangential strains were significantly higher than the circumferential tangential strains at an inflation pressure of 44.6 mm Hg (1.26% ± 0.52% vs. 1.05% ± 0.54%, P = 0.04).
Figure 5.
Circumferential tangential (a) and radial (b) strains at different inflation pressures in the superotemporal region of the posterior sclera in the even-numbered porcine eyes.
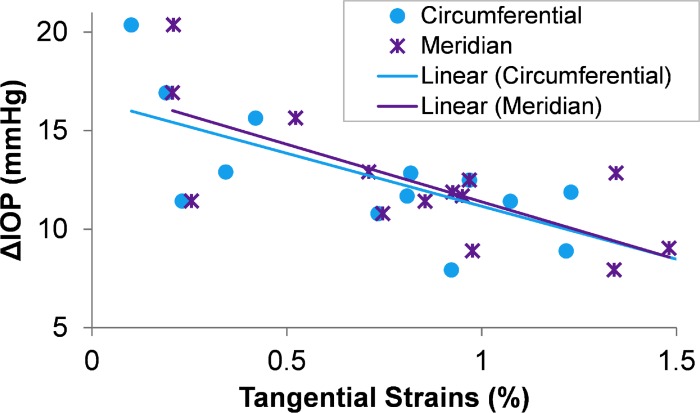
Given that the tangential strains were strongly correlated at different inflation pressures, the strains measured at 20.6 mm Hg were used in evaluating the relationship between ΔIOP and scleral biomechanics. This inflation pressure level was chosen because it is in the middle of the range of the tested pressure levels (5–45 mm Hg). Figure 6 shows the association between ΔIOP and the circumferential and meridian tangential strains measured at 20.6 mm Hg. A significantly negative association was found between ΔIOP and the tangential strains in both the circumferential (R2 = 0.54, P = 0.003) and meridian (R2 = 0.53, P = 0.002) directions. The regression lines using either circumferential strains or meridian strains closely coincided with each other (Fig. 6). Significant negative associations were also found between ΔIOP and tangential strains at other pressure levels (see Supplementary Table S1). In addition, we fit the strain/inflation pressure data to a three-parameter oblique asymptotic function and investigated the correlations between the fitting coefficients and the ΔIOP. The results showed a consistent association between ΔIOP and the parameters of scleral biomechanical properties (See Supplementary Table S2).
Figure 6.
Correlation between ΔIOP from fast infusion and the tangential strains measured in the superotemporal region of the posterior sclera along either circumferential or meridian direction at an inflation pressure of 20.6 mm Hg.
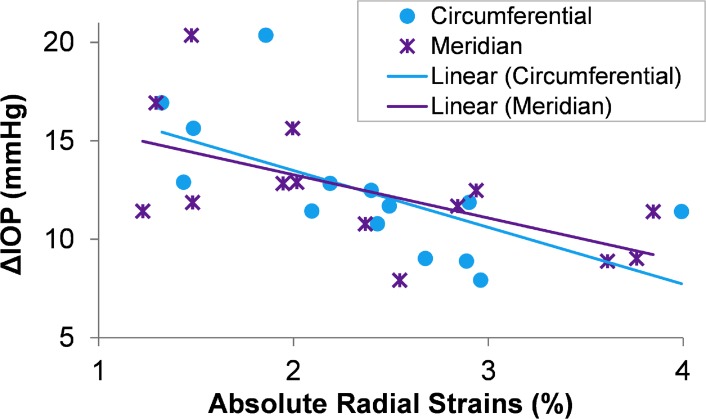
The relationships between ΔIOP and radial strains were also examined. The radial strains were recorded as negative values due to their compressive nature (Fig. 5b), and the absolute values are indicative of the stiff or compliant response (i.e., a smaller absolute strain suggests less compression and a stiffer response). A significant negative association was found between ΔIOP and the absolute radial strains measured at 20.6 mm Hg of inflation (Fig. 7), either along the circumferential (R2 = 0.39, P = 0.02) or meridian (R2 = 0.36, P = 0.02) directions. The negative association suggested that a smaller ΔIOP was associated with a larger absolute strain in the radial direction (i.e., greater compression). The absolute radial strains were also strongly correlated with the tangential strains along the circumferential direction (R ranged from 0.58–0.77; P < 0.05) and along the meridian direction (R ranged from 0.58 to 0.66; P < 0.05).
Figure 7.
Correlation between ΔIOP from fast infusion and the absolute radial strains measured in the superotemporal region of the posterior sclera along either circumferential or meridian direction at an inflation pressure of 20.6 mm Hg.
We also found a negative correlation between eye volume and ΔIOP (R = −0.61, P = 0.02). Eye volume was then considered as a covariate to re-examine the relationship between ΔIOP and scleral strains. The results showed that considering eye volume as a covariate did not change the outcome of the correlations reported above, although P values were slightly increased.
Discussion
Dynamic fluctuation of IOP is poorly characterized and difficult to study but clinically relevant. In this study, we used an experimental porcine eye model to examine the relationship between rapid IOP increase during controlled volumetric change in the eye and the mechanical responses of the sclera. In support of the original hypothesis, our results showed a significant negative association between the magnitude of IOP increase due to microvolumetric change (ΔIOP) and the tangential strains (i.e., stretch) in the superotemporal region of the posterior sclera. We also found a significant negative association between the ΔIOP and the absolute radial strains (i.e., compression) in the same region of the sclera. Combined with our previous findings,20 these results suggest that corneoscleral biomechanics could significantly influence the dynamic profile of IOP.
In the present study, the induced volume change was 15 μL, which was approximately 1/500th of the total volume of the porcine eye (i.e., approximately 7.3 mL). Our data showed that this small portion of volume change could result in significant IOP elevations from a baseline of 15 mm Hg to a peak pressure at 24.6 to 38.8 mm Hg in the porcine eye, for a rate of volume change at 15 μL per second. Volume displacement or change at such a rate could likely occur during eye movement, lid squeezing, Valsalva maneuver, and head inversion. Our data revealed a wide range of ΔIOP, suggesting markedly different responses of individual eyes to micro-volumetric fluctuations.
The determining factors for the differential responses to microvolumetric changes have not been well studied in the past. The present study examined the relationship between the level of IOP increase and the biomechanical responses of the posterior sclera. We found that the tangential strain in the sclera was negatively associated with IOP increase, which suggested that a more compliant sclera could dampen IOP spikes during microvolumetric fluctuations and thus smoothen the IOP curves experienced by ocular components including the optic nerve head and the retinal ganglion cells. Conversely, a stiffer sclera resulting from genetic variations, age-associated glycation, or other pathophysiological processes, could result in more pronounced IOP spikes during normal physiological activities such as blinking, eye movement, or postural change. Based on the current finding, it is reasonable to expect that similar microvolumetric changes (i.e., 15 μL/s) would induce even higher IOP spikes in older human eyes than in the young porcine eyes reported in this study because of the age-associated stiffening of the corneoscleral tissue.21,24,42,43 The likelihood of larger IOP fluctuations may partially explain the age-associate increase in glaucoma risk while it is known that the steady-state IOP does not increase with age.44
We observed that different infusion rates resulted in different levels of IOP elevations, and the fast volume change was associated with a significantly higher IOP elevation even though the total volume change remained the same. This rate-dependent, viscoelastic response of the eye warrants further study. It is likely that the known viscoelastic behavior of the corneoscleral shell dominated such response,45,46 but the potential influence of outflow and vitreous humor should also be examined. The viscoelastic properties of the sclera were shown to alter in experimental glaucoma models47 or donor eyes of glaucoma patients.24 The influence of altered scleral viscoelastic properties on IOP fluctuation and susceptibility to glaucomatous damage would thus be of interest for future investigations. Although significantly different, the IOP elevations from different infusion rates were highly correlated in the same eye (Fig. 4). This result was the basis of only using the fast infusion in the second group of the eyes for investigating the relationship between ΔIOP and scleral strains to avoid lengthy experimental periods and potentially unwanted changes in tissue properties. More importantly, the strong correlation in ΔIOP between different infusion rates suggested that the biomechanical component of the fast IOP fluctuations was likely consistent with that of the longer-term, slower IOP fluctuations, which are concurrently subject to the regulations in aqueous outflow and choroidal blood volume/flow.
Our current understanding about the role of IOP fluctuation in glaucoma progression is hindered by the lack of a good clinical approach to characterize the fluctuation. Without continuous in vivo monitoring of IOP, it is difficult to determine what measurements at what intervals could best quantify IOP insults and compare patient outcome. If translated to in vivo, the ultrasound technique could provide a consistent method to predict the extent of IOP fluctuations at different time scales based on their correlations to corneoscleral biomechanics. This could help advance our understanding of the role of IOP fluctuation and identify new modifiable risk factors for glaucomatous optic neuropathy.
The tangential (i.e., tensile) and radial (i.e., compressive) strains were significantly correlated in the tested eyes and both types of strains were significantly correlated with ΔIOP, suggesting that the measurement of any of these two types of strains was sufficient in characterizing scleral mechanical responses in order to estimate the extent of IOP fluctuations. Our previous study48 showed a higher accuracy in measuring compressive strains using ultrasound speckle tracking due to the higher axial resolution and the additional phase information along that direction. In addition, compressive strains were found to be much larger than tensile strains in the sclera (i.e., 3–5 times larger),25 making it possible to accurately measure the strains without the need to inflate the tissue beyond the normal IOP. This is desirable when translating the ultrasound speckle tracking technique into a clinical tool for safe and accurate in vivo measurements of scleral biomechanics.
We also found that the strains were correlated at different inflation pressures (i.e., an eye with a higher strain at 15 mm Hg inflation would likely have a higher strain at 45 mm Hg). This correlation suggests that in future studies, the eyes need only be inflated to a pressure of 15 or 20 mm Hg, if the comparison of strains among different eyes is needed. The meridian tangential strains were slightly but statistically significantly larger than the circumferential tangential strains at an inflation pressure of 44.6 mm Hg, consistent with our previous findings25 and the known preferred collagen alignment in the circumferential direction in the peripapillary sclera.49,50
The eye volume was found to be negatively associated with ΔIOP, which suggests that a larger eye would have a smaller ΔIOP at a given applied volume increase if all other parameters are the same. This was an interesting yet expected outcome because a larger ocular coat would be expected to more easily accommodate an applied volume increase with its larger surface area and baseline volume.
One potential issue with infusion experiments is leakage associated with needle insertion. Prior to all experiments, we investigated the potential leakage using nonexperimental animal globes by infusing PBS dissolved with a red rhodamine dye. Under the same experimental setup conditions, we found no evidence of leakage throughout the experimental protocol based on visual inspection of the needle insertion site. In addition, in all but one of the experimental porcine eyes, the calculated outflow rate at 15 mm Hg was ≤4 μL/min. This was consistent with previous reports of an average outflow rate of 3.7 ± 0.2 μL/min in normal porcine eyes at 15 mm Hg51 suggesting minimal leakage in the present study.
This study is limited in the following aspects. First, we examined only the biomechanical response of the superotemporal region of the posterior sclera. Future studies should include a comprehensive analysis of the whole corneoscleral shell to understand if the biomechanical responses of different regions (e.g., the cornea, and the anterior/equatorial/posterior sclera) are consistent within the same eye or if any region dominates the response to microvolumetric changes. In a previous study, we found that corneal stiffening could significantly increase ΔIOP.20 Therefore, the intrinsic difference in corneal properties in the experimental eyes could have also influenced the ΔIOP response reported in this study. In addition, all corneas were swollen at the time of experimentation due to the storage of the whole globes in moist chambers during shipping. A previous study indicated that mild corneal swelling could stiffen the cornea,52 and this would suggest a larger ΔIOP response in the experimental eyes than in fresh eyes without corneal swelling. The details of corneal swelling's effect on corneal biomechanics however remain poorly understood, and future studies should systematically and quantitatively characterize such effects. Second, the current study used a two-dimensional speckle tracking algorithm which could be subject to the influence of some out-of-plane motion resulting in errors in the strain measurements. In the present study, the correlation coefficients for speckle tracking were high (generally over 0.8), suggesting minimal out-of-plane influence. Future studies will use three-dimensional speckle tracking capability to capture the full-field deformation of the sclera. Third, the current study examined only the elastic response during a pressure ramp. Future work should examine creep and other viscoelastic responses of the sclera to better understand potentially clinically relevant biomechanical behavior of this tissue.
In summary, this study reported a strong correlation between the strains in the superotemporal region of the posterior porcine sclera and the IOP elevations during short-term microvolumetric change. Our results suggested that the biomechanical responses of the posterior sclera had a strong association with the profile of dynamic IOP. More specifically, a more compliant posterior sclera (i.e., larger strains at the same inflation pressure) could better dampen IOP spikes, while a stiffer sclera (i.e., smaller strains) was associated with larger IOP spikes, which may be detrimental to ocular structures and contribute to glaucoma risk.
Supplementary Material
Acknowledgments
The authors thank Haiyan Gong, MD, PhD, Boston University, for helpful discussions on the infusion experiments.
Supported by U.S. National Institutes of Health Grant RO1EY020929. Xueliang Pan is also partially supported by Grant 8UL1TR000090-05 from the National Center for Advancing Translational Sciences.
Disclosure: H.J. Morris, None; J. Tang, None; B. Cruz Perez, None; X. Pan, None; R.T. Hart, None; P.A. Weber, None; J. Liu, None
References
- 1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson DR, Grant WM. The influence of position on intraocular pressure. Invest Ophthalmol. 1973; 12: 204–212 [PubMed] [Google Scholar]
- 3. Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969; 82: 637 [DOI] [PubMed] [Google Scholar]
- 4. Parsley J, Powell RG, Keightley SJ, Elkington AR. Postural response of intraocular-pressure in chronic open-angle glaucoma following trabeculectomy. Br J Ophthalmol. 1987; 71: 494–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinreb RN, Cook J, Friberg TR. Effect of inverted body position on intraocular pressure. Am J Ophthalmol. 1984; 98: 784–787 [DOI] [PubMed] [Google Scholar]
- 6. Vieira GM, Oliveira HB, de Andrade DT, Bottaro M, Ritch R. Intraocular pressure variation during weight lifting. Arch Ophthalmol. 2006; 124: 1251–1254 [DOI] [PubMed] [Google Scholar]
- 7. Susanna R Jr, Vessani RM, Sakata L, Zacarias LC, Hatanaka M. The relation between intraocular pressure peak in the water drinking test and visual field progression in glaucoma. Br J Ophthalmol 2005; 89: 1298–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kiuchi T, Motoyama Y, Oshika T. Relationship of progression of visual field damage to postural changes in intraocular pressure in patients with normal-tension glaucoma. Ophthalmology. 2006; 113: 2150–2155 [DOI] [PubMed] [Google Scholar]
- 9. Schuman JS, Massicotte EC, Connolly S, Hertzmark E, Mukherji B, Kunen MZ. Increased intraocular pressure and visual field defects in high resistance wind instrument players. Ophthalmology. 2000; 107: 127–133 [DOI] [PubMed] [Google Scholar]
- 10. Resta V, Novelli E, Vozzi G, et al. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007; 25: 2741–2754 [DOI] [PubMed] [Google Scholar]
- 11. Caprioli J, Varma R. Intraocular pressure: modulation as treatment for glaucoma. Am J Ophthalmol. 2011; 152: 340–344 e342 [DOI] [PubMed] [Google Scholar]
- 12. Caprioli J, Coleman AL. Intraocular pressure fluctuation—a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008; 115: 1123–1129 [DOI] [PubMed] [Google Scholar]
- 13. Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011; 118: 1766–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hong S, Seong GJ, Hong YJ. Long-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedure. Arch Ophthalmol. 2007; 125: 1010–1013 [DOI] [PubMed] [Google Scholar]
- 15. Bengtsson B, Leske MC, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007; 114: 205–209 [DOI] [PubMed] [Google Scholar]
- 16. Friberg TR, Sanborn G, Weinreb RN. Intraocular and episcleral venous-pressure increase during inverted posture. Am J Ophthalmol. 1987; 103: 523–526 [DOI] [PubMed] [Google Scholar]
- 17. Longo A, Geiser MH, Riva CE. Posture changes and subfoveal choroidal blood flow. Invest Ophthalmol Vis Sci. 2004; 45: 546–551 [DOI] [PubMed] [Google Scholar]
- 18. Krieglstein G, Waller W, Leydhecker W. The vascular basis of the positional influence of the intraocular pressure. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978; 206: 99–106 [DOI] [PubMed] [Google Scholar]
- 19. Linder BJ, Trick GL, Wolf ML. Altering body position affects intraocular pressure and visual function. Invest Ophthalmol Vis Sci. 1988; 29: 1492–1497 [PubMed] [Google Scholar]
- 20. Liu J, He XY. Corneal stiffness affects IOP elevation during rapid volume change in the eye. Invest Ophthalmol Vis Sci. 2009; 50: 2224–2229 [DOI] [PubMed] [Google Scholar]
- 21. Girard MJA, Suh JKF, Bottlang M, Burgoyne CF, Downs JC. Scleral biomechanics in the aging monkey eye. Invest Ophthalmol Vis Sci. 2009; 50: 5226–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Myers KM, Cone FE, Quigley HA, Gelman S, Pease ME, Nguyen TD. The in vitro inflation response of mouse sclera. Exp Eye Res. 2010; 91: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fazio MA, Grytz R, Bruno L, et al. Regional variations in mechanical strain in the posterior human sclera. Invest Ophthalmol Vis Sci. 2012; 53: 5326–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012; 53: 1714–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang J, Liu J. Ultrasonic measurement of scleral cross-sectional strains during elevations of intraocular pressure: method validation and initial results in posterior porcine sclera. J Biomech Eng. 2012; 134: 091007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He XY, Liu JA. Quantitative ultrasonic spectroscopy method for noninvasive determination of corneal biomechanical properties. Invest Ophthalmol Vis Sci. 2009; 50: 5148–5154 [DOI] [PubMed] [Google Scholar]
- 27. Silverman RH, Patel MS, Gal O, et al. Effect of corneal hydration on ultrasound velocity and backscatter. Ultrasound Med Biol. 2009; 35: 839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thijssen JM, Mol HJM, Timmer MR. Acoustic parameters of ocular-tissues. Ultrasound Med Biol. 1985; 11: 157–161 [DOI] [PubMed] [Google Scholar]
- 29. Jansson F, Sundmark E. Determination of velocity of ultrasound in ocular tissues at different temperatures. Acta Ophthalmol. 1961; 39: 899 [DOI] [PubMed] [Google Scholar]
- 30. Gong H, Freddo TF. The washout phenomenon in aqueous outflow--why does it matter? Exp Eye Res. 2009; 88: 729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dinslage S, McLaren J, Brubaker R. Intraocular pressure in rabbits by telemetry. II: effects of animal handling and drugs. Invest Ophthalmol Vis Sci. 1998; 39: 2485–2489 [PubMed] [Google Scholar]
- 32. Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011; 52: 7365–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li R, Liu JH. Telemetric monitoring of 24 h intraocular pressure in conscious and freely moving C57BL/6J and CBA/CaJ mice. Mol Vis. 2008; 14: 745–749 [PMC free article] [PubMed] [Google Scholar]
- 34. Percicot CL, Schnell CR, Debon C, Hariton C. Continuous intraocular pressure measurement by telemetry in alpha-chymotrypsin-induced glaucoma model in the rabbit: effects of timolol, dorzolamide, and epinephrine. J Pharmacol Toxicol Methods. 1996; 36: 223–228 [DOI] [PubMed] [Google Scholar]
- 35. Jain MR, Marmion VJ. Rapid pneumatic and Mackey-Marg applanation tonometry to evaluate the postural effect on intraocular pressure. Br J Ophthalmol. 1976; 60: 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prata TS, De Moraes CG, Kanadani FN, Ritch R, Paranhos A Jr. Posture-induced intraocular pressure changes: considerations regarding body position in glaucoma patients. Surv Ophthalmol. 2010; 55: 445–453 [DOI] [PubMed] [Google Scholar]
- 37. Rafuse PE, Mills DW, Hooper PL, Chang TS, Wolf R. Effects of Valsalva's manoeuvre on intraocular pressure. Can J Ophthalmol. 1994; 29: 73–76 [PubMed] [Google Scholar]
- 38. Schwenn O, Troost R, Vogel A, Grus F, Beck S, Pfeiffer N. Ocular pulse amplitude in patients with open angle glaucoma, normal tension glaucoma, and ocular hypertension. Br J Ophthalmol. 2002; 86: 981–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trew DR, Smith SE. Postural studies in pulsatile ocular blood-flow. 1. Ocular hypertension and normotension. Br J Ophthalmol. 1991; 75: 66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trew DR, Smith SE. Postural studies in pulsatile ocular blood flow: II. Chronic open angle glaucoma. Br J Ophthalmol. 1991; 75: 71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar RS, de Guzman MH, Ong PY, Goldberg I. Does peak intraocular pressure measured by water drinking test reflect peak circadian levels? A pilot study. Clin Experiment Ophthalmol. 2008; 36: 312–315 [DOI] [PubMed] [Google Scholar]
- 42. Elsheikh A, Geraghty B, Rama P, Campanelli M, Meek KM. Characterization of age-related variation in corneal biomechanical properties. J R Soc Interface 2010; 7: 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knox Cartwright NE, Tyrer JR, Marshall J. Age-related differences in the elasticity of the human cornea. Invest Ophthalmol Vis Sci. 2011; 52: 4324–4329 [DOI] [PubMed] [Google Scholar]
- 44. Rochtchina E, Mitchell P, Wang JJ. Relationship between age and intraocular pressure: the Blue Mountains Eye Study. Clin Experiment Ophthalmol 2002; 30: 173–175 [DOI] [PubMed] [Google Scholar]
- 45. Glass DH, Roberts CJ, Litsky AS, Weber PA. A viscoelastic biomechanical model of the cornea describing the effect of viscosity and elasticity on hysteresis. Invest Ophthalmol Vis Sci. 2008; 49: 3919–3926 [DOI] [PubMed] [Google Scholar]
- 46. Downs JC, Suh JKF, Thomas KA, Bellezza AJ, Burgoyne CF, Hart RT. Viscoelastic characterization of peripapillary sclera: material properties by quadrant in rabbit and monkey eyes. J Biomech Eng. 2003; 125: 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Downs JC, Suh JKF, Thomas KA, Bellezza AJ, Hart RT, Burgoyne CF. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci. 2005; 46: 540–546 [DOI] [PubMed] [Google Scholar]
- 48. Tang JH, Liu J. Ultrasonic measurement of scleral cross-sectional strains during elevations of intraocular pressure: method validation and initial results in posterior porcine sclera. J Biomech Eng. 2012; 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Girard MJA, Dahlmann-Noor A, Rayapureddi S, et al. Quantitative mapping of scleral fiber orientation in normal rat eyes. Invest Ophthalmol Vis Sci. 2011; 52: 9684–9693 [DOI] [PubMed] [Google Scholar]
- 50. Hernandez MR, Luo XX, Igoe F, Neufeld AH. Extracellular matrix of the human lamina cribrosa. Am J Ophthalmol. 1987; 104: 567–576 [DOI] [PubMed] [Google Scholar]
- 51. Vaajanen A, Vapaatalo H, Oksala O. A modified in vitro method for aqueous humor outflow studies in enucleated porcine eyes. J Ocul Pharmacol Ther. 2007; 23: 124–131 [DOI] [PubMed] [Google Scholar]
- 52. Lau W, Pye D. Changes in corneal biomechanics and applanation tonometry with induced corneal swelling. Invest Ophthalmol Vis Sci. 2011; 52: 3207–3214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.