Abstract
BACKGROUND
Elevated blood pressure, elevated angiotensin II (ANG II), and ANG II suppression with high salt (HS) diet all contribute to vascular dysfunction. This study investigated the interplay of HS diet and vascular function in a high renin model of hypertension.
METHODS
Male Sprague-Dawley rats were subjected to 2 kidney–1 clip (2K1C) Goldblatt hypertension for 4 weeks and compared with sham-operated controls.
RESULTS
Middle cerebral arteries (MCA) of 2K1C rats and sham-operated controls fed normal salt (NS; 0.4% NaCl) diet dilated in response to acetylcholine (ACh) and reduced partial pressure of oxygen (PO2). Switching to HS (4% NaCl) diet for 3 days to reduce plasma renin activity (PRA) eliminated vasodilation to ACh and reduced PO2 in sham-operated controls, with no effect on vasodilation in 2K1C rats. AT1 receptor blockade (losartan, 20mg/kg/day; 1 week) eliminated vasodilator responses to ACh and reduced PO2 in 2K1C rats fed NS or HS diet. ANG II infusion (5ng/kg/min, intravenous) for 3 days to prevent salt-induced reductions in plasma ANG II restored vascular relaxation in MCA of sham-operated controls fed HS diet. Copper/zinc superoxide dismutase expression and total superoxide dismutase activity were significantly higher in arteries of 2K1C rats fed HS diet vs. sham-operated controls.
CONCLUSIONS
These results suggest that the sustained effects of elevated ANG II levels in 2K1C hypertension maintain endothelium-dependent vasodilatation via AT1 receptor–mediated preservation of antioxidant defense mechanisms despite significant elevations in blood pressure and salt-induced suppression of PRA.
Keywords: angiotensin II, blood pressure, cerebral circulation, endothelial dysfunction, hypertension, oxidant stress, salt, sodium, superoxide.
Two kidney–1 clip (2K1C) Goldblatt hypertension is an excellent experimental model to study renal vascular hypertension, a serious and prevalent cardiovascular disease. Animals with 2K1C hypertension exhibit substantial elevations in plasma renin activity (PRA) and circulating angiotensin II (ANG II) levels that reach peak levels around 4 weeks after clipping.1,2 In contrast with 2K1C hypertension, several other forms of hypertension are characterized by low renin3 concomitant with endothelial dysfunction.4,5
One of the most devastating consequences of hypertension and high salt (HS) diet is stroke. Dysregulation of cerebral vascular relaxation can have other disastrous consequences, and vascular-related factors (including oxidant stress) have been proposed to contribute to several varieties of cognitive dysfunction and dementia, including Alzheimer’s disease.6 Importantly, endothelial dysfunction has been shown to be a powerful prognostic indicator of adverse cardiovascular events, including stroke, independent of blood pressure.7
Although the ability of supraphysiological levels of ANG II to cause endothelial dysfunction and increase vascular oxidant stress is well known,8 there is increasing evidence that salt-induced suppression of plasma ANG II also leads to endothelial dysfunction, vascular oxidant stress, and impaired vascular relaxation.9–12 Dahl salt-sensitive (SS) rats, a rodent model of chronically lowered renin-angiotensin system (RAS) activity,13 exhibit a similar vascular phenotype, even when they are normotensive and maintained on a normal salt (NS) diet.14 In light of these disparate observations, this study addressed the following questions: (i) what is the effect of short-term exposure to HS diet on cerebral vascular function in rats with 2K1C Goldblatt hypertension; and (ii) do the elevated PRA and increased circulating ANG II levels in 2K1C hypertension exacerbate or ameliorate salt-induced vascular dysfunction in cerebral arteries?
METHODS
Experimental animal groups
Male Sprague-Dawley rats were anesthetized by intramuscular injection of ketamine (75mg/kg), acepromazine (2.5mg/kg), and anased (10mg/kg). The left kidney was accessed through a left lateral incision, and a 0.20-mm silver clip was placed around the renal artery. 2K1C rats and their respective sham-operated controls in which the renal artery was exposed and cleared without clipping were aged 11–12 weeks at the time of the isolated vessel experiments and were studied 4 weeks after clipping or sham-operation. Other groups of rats anesthetized with the same anesthetic cocktail were fitted with indwelling catheters for blood sampling and arterial pressure measurement in the conscious undisturbed animal.13–16 Rats used for chronic recording were allowed a 3-day recovery period before the experiment.
One group of sham-operated controls and a corresponding group of 2K1C rats maintained on an NS diet (0.4% NaCl; Dyets, Bethlehem, PA) were switched to an HS diet (4% NaCl; Dyets, Bethlehem PA) 3–5 days before the isolated vessel experiment to reduce plasma ANG II levels. To further test of the role of ANG II in modulating responses to vasodilator stimuli, other groups of 2K1C rats fed NS diet or switched to HS diet for 3 days were given the ATR1 blocker losartan (20mg/kg/day for 1 week) in the drinking water before the acute experiments. Because infusion of a low dose of ANG II (5ng/kg/min intravenously) prevents salt-induced reductions in plasma ANG II levels,16,17 another group of HS-fed sham-operated controls received a continuous intravenous infusion of a subpressor dose of ANG II (5ng/kg/min) for 3 days before the experiment. All rats were housed with free access to food and water. All protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Cannulated middle cerebral artery (MCA) preparation
On the day of the experiment, animals were anesthetized with sodium pentobarbital (60mg/kg, intraperitoneally) or a ketamine (75mg/kg), acepromazine (2.5mg/kg), and anased (10mg/kg) cocktail. Mean arterial pressures (MAPs) in rats used for the isolated vessel experiments were evaluated by cannulating a carotid artery and were consistent with patterns obtained in the conscious rats (NS 2K1C = 154±6mm Hg (n = 6); HS 2K1C = 155±6mm Hg (n = 7); NS sham = 122±5mm Hg (n = 6); HS sham = 137±5mm Hg (n = 6)).
The brain was removed and immersed in physiological salt solution (PSS) with the following ionic composition: NaCl (119.0mM), potassium chloride (4.7mM), calcium chloride (1.6mM), monosodium phosphate (1.18mM), magnesium sulfate (1.17mM), sodium bicarbonate (24.0mM), D-glucose (5.5mM), and ethylenediaminetetraacetic acid (EDTA) (0.03mM). The MCA was carefully excised, cannulated with glass micropipettes, and extended to its approximate in situ length. Side branches were ligated to prevent leaks, and the artery was continuously perfused and superfused with PSS (37 °C) equilibrated with a 21% oxygen (O2)/5% carbon dioxide (CO2)/74% nitrogen (N2) gas mixture. Intraluminal pressure was maintained at 80mm Hg, and internal diameter was measured with video micrometer (model IV-550; FOR-A, Tokyo, Japan). Vessels lacking resting tone were not studied.
Response to acetylcholine (ACh), reduced partial pressure of oxygen (PO2), and calcium ion (Ca2+)–free solution
Diameter changes in response to a classic endothelium-dependent vasodilator agonist ACh (1 µM) and the physiological vasodilator stimulus of reduced PO2 were assessed in each group. The single dose of ACh was used to minimize the duration of the experiment and was identical to that previously used to demonstrate salt-induced endothelial dysfunction.10,11 However previous studies have shown that HS diet eliminates vasodilator responses to multiple doses of ACh in Sprague-Dawley rats9 and congenic rats carrying a normally functioning renin allele from the Brown Norway rat in the Dahl SS genetic background.18
To evaluate vessel responses to reduced PO2, the artery was allowed a minimum 30-minute equilibration period at 21% O2, after which the perfusion and superfusion solutions were simultaneously equilibrated with a 0% O2/5% CO2/95% N2 gas mixture for 10 minutes. Under these conditions, the PO2 of PSS equilibrated with 21% O2 is approximately 140mm Hg, and PO2 in the perfusate and superfusate decreases to 35–45mm Hg during equilibration with 0% O2. At the end of the experiment, resting tone and maximum diameter were assessed by superfusion with Ca2+-free PSS.9–11,19
Plasma renin activity (PRA)
For measurement of PRA, arterial blood (2ml) was withdrawn by spontaneous bleeding from the arterial catheter in the undisturbed, chronically cannulated rats. The blood was collected in chilled tubes containing potassium EDTA 50 μl/ml and 300 mmol/l Na4EDTA. Samples were centrifuged at 4 °C, and the plasma was frozen and stored at −80 °C. PRA (nanograms angiotensin I formed per milliliter per sample per hour) was measured in the Physiology Department Assay Core facility as described previously.13
Western blots and superoxide dismutase (SOD) activity
In addition to removing cerebral arteries to evaluate vessel responses to vasodilator stimuli, resistance arteries (100–300 µm) supplying the small intestine of the same rats were isolated to provide tissue to evaluate the expression of copper (Cu)/zinc (Zn) SOD, manganese SOD, endothelial nitric oxide synthase (eNOS), and phosphorylated eNOS (Ser-1177) by Western blotting.15,20 All values were normalized as percentage β-actin. As a complement to SOD expression, total SOD activity was measured in mesenteric arteries of 2K1C rats and sham-operated controls fed HS diet using a Cayman Chemical SOD-KIT (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions.
Statistical methods
Data are presented as mean ±SEM. Differences between multiple means were determined using analysis of variance with a Newman–Keuls test post hoc. P < 0.05 was considered to be statistically significant.
RESULTS
Arterial blood pressure and PRA
Table 1 compares MAP and PRA in sham-operated controls and 2K1C rats fed NS and HS diets. MAP, measured by chronic in-dwelling catheters in conscious rats, was significantly elevated (P < 0.05) in 2K1C rats fed an NS or HS diet vs. corresponding sham-operated controls. PRA was significantly elevated in 2K1C vs. sham-operated controls fed an NS diet. Short-term HS diet caused a significant reduction of PRA in 2K1C rats vs. 2K1C rats fed an NS diet and also reduced PRA by approximately 47% in sham-operated controls.
Table 1.
Mean arterial blood pressure (MAP) and plasma renin activity (PRA) during normal salt (NS) or high salt (HS) diet in sham operated controls and rats with 2 kidney–1 clip (2K1C) hypertension
| Experimental Group | MAP (mm Hg) | PRA (ng angiotensin I/ml/h) |
|---|---|---|
| NS sham | 110±1 (7) | 2.92±0.48 (9) |
| HS sham | 121±2 (8) | 1.54±0.38 (11) |
| NS 2K1C | 133±4 (10)* | 4.51±0.54 (14)* |
| HS 2K1C | 143±7 (10)* | 0.92±0.20 (8)** |
*Significantly different (P < 0.05) vs. sham-operated controls on the same diet. **Significantly different (P < 0.05) from NS diet within the same experimental group. Numbers in parentheses indicate the number of animals.
Response of MCA to ACh and reduced PO2
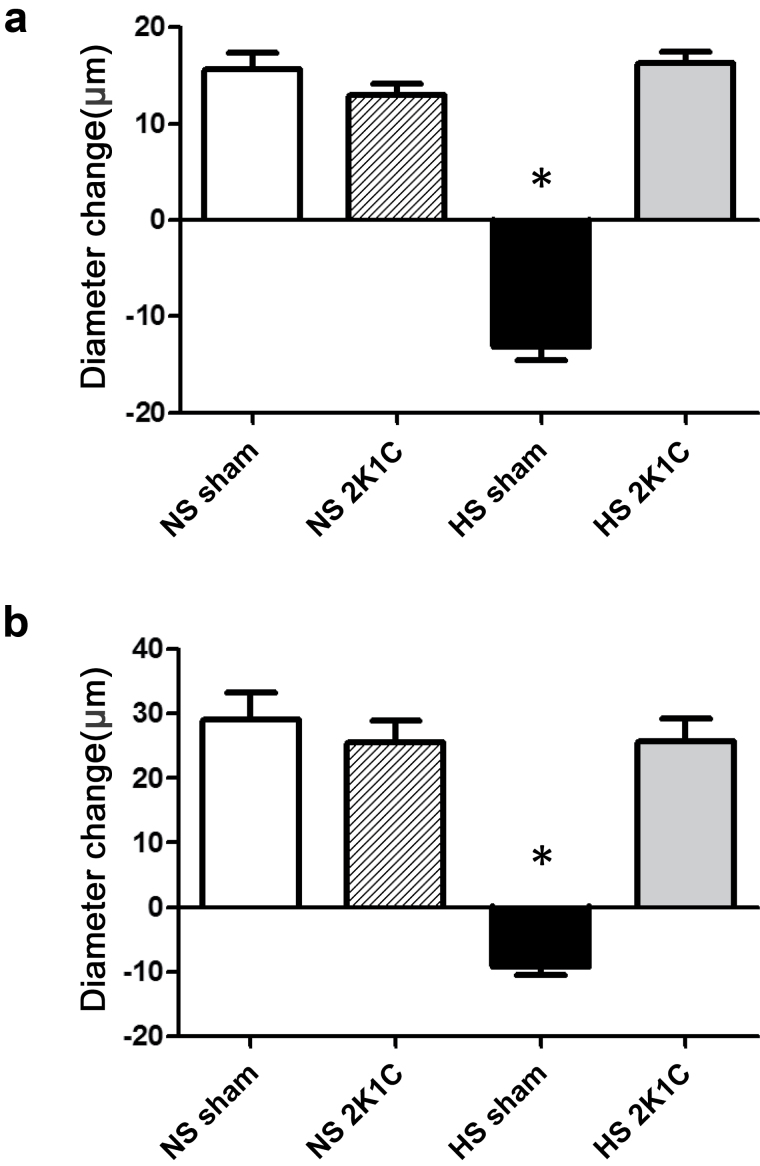
MCA of sham-operated controls fed an NS diet dilated in response to ACh and reduced PO2. These responses were converted to a paradoxical constriction in animals fed an HS diet (Figure 1). MCA of 2K1C rats fed an NS diet dilated in response to ACh and reduced PO2, despite the elevated blood pressure. However, in contrast with sham-operated controls, short-term HS diet did not eliminate vascular relaxation in response to ACh or reduced PO2 in 2K1C rats, despite the significant elevation in arterial pressure. Active resting tone, calculated as [(Dmax − Drest)/Dmax] × 100, where Dmax is maximum diameter in Ca2+-free solution and Drest is resting control diameter, was similar in isolated MCA from all the groups (NS sham-operated controls = 46±3% (n = 6); NS 2K1C = 48±2% (n = 6); HS sham-operated controls = 51±2% (n = 6); HS 2K1C = 47±3% (n = 6)), showing that any differences in vascular relaxation were not due to differences in initial tone of the artery.
Figure 1.
Responses to acetylcholine (ACh; 1 µM) (a) and reduced partial pressure of oxygen (PO2) (b) in middle cerebral arteries of 2 kidney–1 clip (2K1C) rats and sham-operated controls fed normal salt (NS) or high salt (HS) diet. Data are summarized as mean change from resting diameter ± SEM for n = 6–8 per group. *P < 0.05 vs. NS sham and HS 2K1C rats.
Effect of 2K1C hypertension and dietary salt intake on SOD expression, eNOS expression, eNOS phosphorylation, and total SOD activity
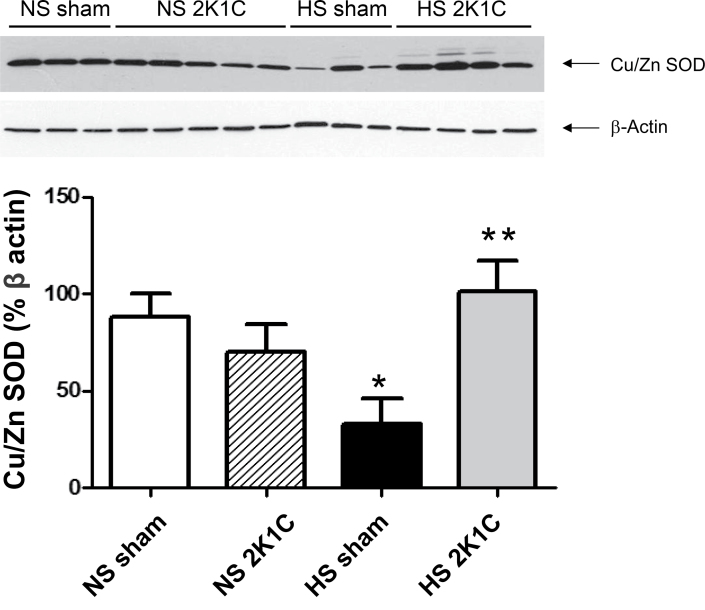
HS diet downregulated Cu/Zn SOD expression significantly in vessels of sham-operated controls but not 2K1C rats, and Cu/Zn SOD expression was significantly higher in arteries of 2K1C rats fed an HS diet vs. sham-operated controls fed an HS diet (Figure 2). Consistent with the latter observation, total SOD activity was significantly higher in 2K1C rats fed an HS diet (39.8±3.80U/mg protein; n = 8) compared with sham-operated controls fed an HS diet (25.4±1.80U/mg protein; n = 10). There were no significant differences in magnesium SOD expression, eNOS expression, or phosphorylation of eNOS (S1177) between any of the groups (not shown).
Figure 2.
Copper/zinc superoxide dismutase (Cu/Zn SOD) expression (% β-actin) in mesenteric arteries of 2 kidney–1 clip (2K1C rats) and sham-operated controls fed a normal salt (NS) or high salt (HS) diet. Data are summarized as mean ± SEM for n = 6–8 per group. *P < 0.05 vs. sham-operated controls fed an NS diet; **P < 0.05 vs. sham-operated control fed an HS diet.
Effect of losartan and low-dose ANG II infusion on vasodilator responses
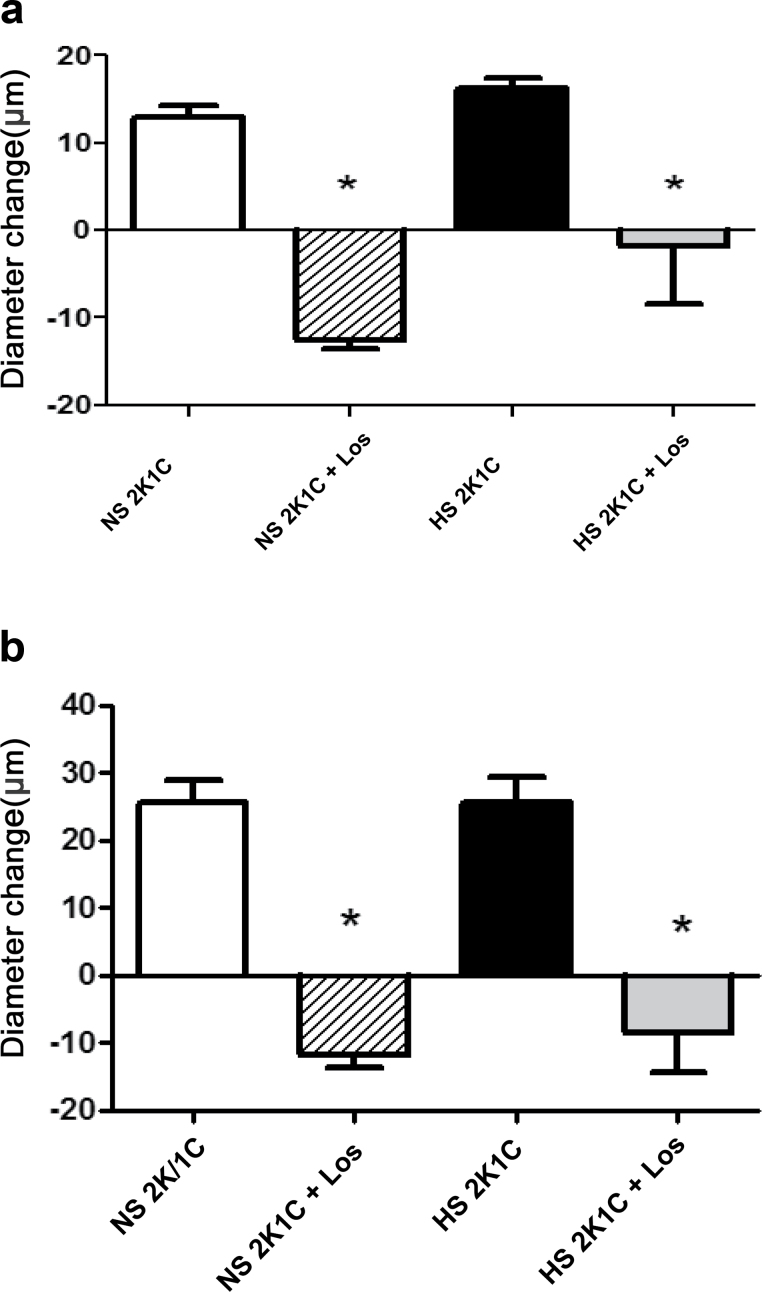
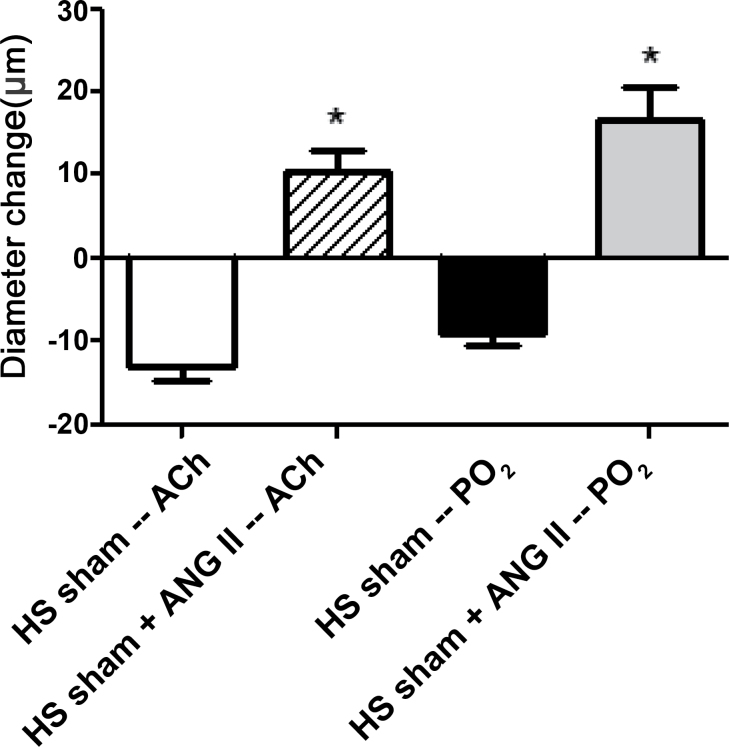
AT1 receptor blockade with losartan eliminated vascular relaxation in response to ACh and reduced PO2 in 2K1C rats fed either an NS or HS diet (Figure 3). MAP was significantly lower (P < 0.05) in losartan-treated 2K1C rats fed an NS diet (96±2mm Hg; n = 5) vs. untreated 2K1C rats (133±3mm Hg; n = 10), but not in losartan-treated 2K1C rats fed an HS diet (126±6mm Hg; n = 11) vs. nontreated 2K1C rats fed an HS diet (143±7mm Hg; n = 10). As previously reported in intact Sprague-Dawley rats,9,11,12,19 continuous intravenous infusion of ANG II to prevent salt-induced reductions in plasma ANG II levels16,17 restored vasodilator responses to ACh and reduced PO2 that were absent in noninfused controls fed an HS diet (Figure 4) without an increase in arterial blood pressure (anesthetized MAP = 104±4mm Hg; n = 7).
Figure 3.
Effect of losartan (Los) on responses to acetylcholine (ACh; 1 µM) (a) and reduced partial pressure of oxygen (PO2) (b) in middle cerebral arteries of 2 kidney–1 clip (2K1C) rats fed a normal salt (NS) or high salt (HS) diet. Data are summarized as mean change from resting diameter ± SEM for n = 6–8 per group. *P < 0.05 vs. untreated 2K1C rats fed same diet.
Figure 4.
Restoration of responses to acetylcholine (ACh; 1 µM) and reduced partial pressure of oxygen (PO2) in middle cerebral arteries of high salt (HS)–fed sham-operated control rats receiving continuous intravenous infusion of a low dose of angiotensin II (ANG II). Data are summarized as mean change from resting diameter ± SEM for n = 6–8 per group. *P < 0.05 vs. noninfused sham-operated controls fed an HS diet.
DISCUSSION
Vascular relaxation is impaired not only in human hypertension21 but also in response to elevated intravascular pressure in normotensive animals.22 An HS diet also leads to impaired vascular relaxation and endothelial dysfunction in normotensive rats,9−12,19 mice,23 and healthy human volunteers.24 An HS diet reduces circulating ANG II levels in humans25 and other species,16,17,26,27 and salt-induced ANG II suppression is a crucial factor contributing to loss of vascular relaxation in normotensive rats fed an HS diet.9−12,19 However, to our knowledge, there have been no studies of the effects of short-term elevations in dietary salt intake on PRA and vascular reactivity in 2K1C hypertension.
Similar to existing studies investigating the effect of a long-term HS diet on PRA in 2K1C Goldblatt hypertension,28,29 we found that a short-term HS diet reduced PRA in 2K1C rats. Consistent with existing studies of intact Sprague-Dawley rats, an HS diet also eliminated vascular relaxation in response to ACh and reduced PO2 in MCA of the normotensive sham-operated controls. By contrast, MCA of NS- and HS-fed 2K1C rats dilated to a similar extent in response to ACh and reduced PO2. Thus, HS-induced vascular defects were not present in 2K1C rats despite a substantial elevation of blood pressure, prolonged exposure to elevated plasma renin activity2 (Table 1) and elevated ANG II levels,1 and an HS diet—all of which are well-known to cause severe endothelial dysfunction.
At the integrative level, the most likely mechanism for the preservation of vascular relaxation in the hypertensive 2K1C rats fed either an NS or HS diet is activation of the AT1 receptor because administration of losartan eliminated the restored relaxation to both ACh and reduced PO2 in both groups. By contrast, vasodilator responses to ACh and reduced PO2 that were absent in sham-operated controls fed an HS diet were restored by infusion of ANG II at a dose previously shown to prevent salt-induced ANG II suppression.16,17,30
Because administration of losartan before the diet change eliminated vascular relaxation in 2K1C rats fed an HS diet, the maintenance of vasodilator responses to ACh and reduced PO2 in HS-fed 2K1C rats is most likely related to the persisting effects of AT1 receptor activation to upregulate antioxidant defenses (e.g., Cu/Zn SOD) (Figure 2) in response to the elevated ANG II levels after unilateral renal artery clipping. Those findings provide additional support for the hypothesis that tonic interaction of ANG II with the AT1 receptor plays an important role in maintaining normal vascular relaxation mechanisms. The most novel and important finding of this study is that these protective effects of AT1 receptor activation are manifest in the context of the prolonged elevations of ANG II levels with 2K1C hypertension1 and also during exposure to an HS diet.
The mechanisms by which PRA and ANG II maintain vascular relaxation in 2K1C rats fed an HS diet are most likely related to a paradoxical reduction in vascular oxidant stress. Continuous intravenous infusion of a low dose of ANG II to prevent the salt-induced decrease in plasma ANG II levels not only restores ACh-induced dilation that is lost in cerebral arteries of HS-fed Sprague-Dawley rats9–11,19 but also reduces vascular superoxide levels to NS values and maintains nitric oxide availability in small mesenteric arteries12 and aortas30 of HS-fed Sprague-Dawley rats. Low-dose ANG II infusion also prevents the downregulation of Cu/Zn SOD in cerebral arteries of Sprague-Dawley rats fed an HS diet.31 Consistent with the hypothesis that AT1 receptor activation maintains antioxidant defenses are recent findings that losartan blocks the protective effect of ANG II infusion to reduce vascular superoxide levels and restore arteriolar dilation in hamsters fed an HS diet.32
In this study, Cu/Zn SOD expression was significantly higher in arteries of 2K1C rats fed an HS diet vs. sham-operated controls fed an HS diet and was not downregulated by an HS diet in the 2K1C rats. Those observations are consistent with the hypothesis that earlier exposure to elevated PRA and ANG II in HS-fed 2K1C rats preserves vascular relaxation by preventing salt-induced downregulation of Cu/Zn SOD (and possibly other antioxidant enzymes). A role for normal plasma ANG II levels in maintaining vascular antioxidant defenses is consistent with reports that ANG II upregulates extracellular SOD expression in mouse aorta and human aortic smooth muscle cells33 and increases Cu/Zn SOD activity in aortas of extracellular SOD knockout mice.34 Consistent with the latter report, we also found that total SOD activity in arteries of 2K1C rats fed an HS diet was significantly higher than that in arteries of sham-operated controls fed an HS diet.
One limitation of our study was the use of small mesenteric arteries as a surrogate for SOD expression in the cerebral arteries. We sampled mesenteric arteries because they provided ample amounts of tissue for Western blots while minimizing animal use and conserving resources. However, previous studies have shown that an HS diet reduces nitric oxide levels and increases vascular superoxide levels in aortas30 and small mesenteric arteries12 and that these changes are prevented by low-dose ANG II infusion. Because the actions of an HS diet (±ANG II infusion) in mesenteric arteries12 are similar to those in cerebral arteries,9,18 those vessels should be representative of changes occurring in the cerebral vasculature. Nonetheless, verification of these changes in future studies of cerebral arteries is clearly warranted.
Another limitation of this study is that mechanisms other than preservation of antioxidant defenses could also contribute to the ability of AT1 receptor activation to preserve vascular function. The mechanisms by which AT1 receptor activation exerts its protective effect on vascular function in 2K1C rats fed an HS diet at a more reductionist (cellular and molecular) level also remain to be determined and are clearly worthy of further investigation.
An HS diet is a risk factor not only for hypertension but also for vascular dysfunction in normotensive individuals. Even short-term elevations in dietary salt intake lead to significant reductions in endothelium-dependent vasodilation in healthy young human volunteers.24 An HS diet not only increases the risk for hypertension but also increases the risk for other adverse cardiovascular events, including death, even in the absence of an elevated blood pressure.35 The MCA investigated in this study are highly relevant to clinical problems in humans because an HS diet and endothelial dysfunction have been implicated in the pathogenesis of stroke and other cardiovascular pathologies in humans.7,35
The pathophysiological effects of high levels of ANG II in promoting oxidant stress and tissue damage are well known, and the therapeutic benefits of angiotensin-converting enzyme inhibitors and AT1 receptor blockers are indisputable. However, unexpected beneficial effects of ANG II infusion and detrimental effects of AT1 receptor blockade have been reported. For example, Takazawa et al.36 reported that ANG II infusion reduces glomerular injury in the early phase of anti-Thy-1.1 nephritis; and Maitland et al.37 reported that AT1 receptor blockers treatment increases tissue injury in the Dahl salt-sensitive model of low renin hypertension. In another study, Reed et al.38 reported that the effects of ANG II on the p38 and Akt signal transduction pathways and on ischemia-induced coronary collateral growth differed in WKY rats vs. JCR rats (a rodent model of metabolic syndrome), depending on the level of tissue oxidant stress and the dose of ANG II that was administered. In that study, low doses of ANG II were beneficial for coronary collateral growth, and high doses of ANG II were detrimental for coronary collateral growth in WKY.
The paradoxical effect of AT1 receptor activation to prevent salt-induced vascular dysfunction that we observed in a high renin model of hypertension is highly novel and can provide valuable insight into an unexpected role of ANG II in maintaining normal vascular reactivity under certain physiological and pathophysiological conditions. Future studies to determine how elevations in blood pressure and ANG II interact with the long-term effects of HS diet on vascular function in the 2K1C model Goldblatt hypertension could be of substantial benefit in understanding the relationships between salt, vascular function, and hypertension, especially in light of the well-known difficulties of remaining on a salt-restricted diet in humans.39
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
We thank Tianjian Huang and Lynn Dondlinger for their skillful assistance and the staff of the Physiology Department Biochemical Assay core for their measurements of plasma renin activity. We offer special thanks in loving memory to the late Dr Mary Pat Kunert for all her advice and assistance that was indispensible for completion of this study. This work was supported by an R-01 grant from the National Institutes of Health (HL-65289).
REFERENCES
- 1. Amiri F, Garcia R. Renal angiotensin II receptor regulation in two- kidney, one clip hypertensive rats: effect of ACE inhibition. Hyper tension 1997; 30: 337–344 [DOI] [PubMed] [Google Scholar]
- 2. Murphy WR, Coleman TG, Smith TL, Stanek KA. Effects of graded renal artery constriction on blood pressure, renal artery pressure, and plasma renin activity in Goldblatt hypertension. Hypertension 1984; 6: 68–74 [DOI] [PubMed] [Google Scholar]
- 3. Gordon RD, Laragh JH, Funder JW. Low renin hypertensive states: perspectives, unsolved problems, future research. Trends Endicronol Metab 2005; 16: 108–113 [DOI] [PubMed] [Google Scholar]
- 4. Zhou MS, Schulman IH, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension. Hypertension 2006; 47: 81–86 [DOI] [PubMed] [Google Scholar]
- 5. Duffy SJ, Biegelsen ES, Eberhardt RT, Kahn DF, Kingwell BA, Vita JA. Low-renin hypertension with relative aldosterone excess is associated with impaired NO-mediated vasodilation. Hypertension 2005; 46: 707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu X, Smith MA, Honda K, Aliev G, Moreira PI, Nunomura A, Casadesus G, Harris PL, Siedlak SL, Perry G. Vascular oxidative stress in Alzheimer disease. J Neurol Sci 2007; 257: 240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J AmCollCardiol 2003; 42: 1149–1160 [DOI] [PubMed] [Google Scholar]
- 8. Di Wang H, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res 2001; 88: 947–953 [DOI] [PubMed] [Google Scholar]
- 9. McEwen ST, Balus SF, Durand MJ, Lombard JH. Angiotensin II maintains cerebral vascular relaxation via EGF receptor transactivation and ERK1/2. AmJ Physiol Heart Circ Physiol 2009; 297: H1296–H1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart CircPhysiol 2003; 284: H1124–H1133 [DOI] [PubMed] [Google Scholar]
- 11. Weber DS, Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 2001; 280: H2196–H2202 [DOI] [PubMed] [Google Scholar]
- 12. Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res 2007; 44: 382–390 [DOI] [PubMed] [Google Scholar]
- 13. Cowley AW, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 2001; 37: 456–461 [DOI] [PubMed] [Google Scholar]
- 14. Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension 2005; 45: 687–691 [DOI] [PubMed] [Google Scholar]
- 15. Durand MJ, Moreno C, Greene AS, Lombard JH. Impaired relaxation of cerebral arteries in the absence of elevated salt intake in normotensive congenic rats carrying the Dahl salt-sensitive renin gene. Am J Physiol Heart Circ Physiol 2010; 299: H1865–H1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petersen MC, Munzenmaier DH, Greene AS. Angiotensin II infusion restores stimulated angiogenesis in the skeletal muscle of rats on a high-salt diet. Am J Physiol Heart Circ Physiol 2006; 291: H114–H120 [DOI] [PubMed] [Google Scholar]
- 17. Gross V, Kurth TM, Skelton MM, Mattson DL, Cowley A., Jr Effects of daily sodium intake and ANG II on cortical and medullary renal blood flow in conscious rats. Am J Physiol 1998; 274: R1317–R1323 [DOI] [PubMed] [Google Scholar]
- 18. Durand MJ, Lombard JH. Low-dose angiotensin ii infusion restores vascular function in cerebral arteries of high salt-fed rats by increasing copper/zinc superoxide dimutase expression. Am J Hypertens 2013; 26: 739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weber DS, Lombard JH. Elevated salt intake impairs dilation of skeletal muscle resistance arteries via angiotensin II suppression. Am J Physiol Heart Circ Physiol 2000; 278: H500–H506 [DOI] [PubMed] [Google Scholar]
- 20. Durand MJ, Lombard JH. Introgression of the Brown Norway renin allele onto the Dahl salt-sensitive genetic background increases Cu/Zn SOD expression in cerebral arteries. Am J Hypertens 2011; 24: 563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. New Engl J Med 1990; 323: 22–27 [DOI] [PubMed] [Google Scholar]
- 22. Huang A, Sun D, Kaley G, Koller A. Superoxide released to high intra-arteriolar pressure reduces nitric oxide-mediated shear stress-and agonist-induced dilations. Circ Res 1998; 83: 960. [DOI] [PubMed] [Google Scholar]
- 23. Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. AmJ Physiol 2007; 292: R1550–R1556 [DOI] [PubMed] [Google Scholar]
- 24. Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 2008; 51: 1525–1530 [DOI] [PubMed] [Google Scholar]
- 25. Singer D, Markandu N, Morton J, Miller M, Sagnella G, MacGregor G. Angiotensin II suppression is a major factor permitting excretion of an acute sodium load in humans. Am J Physiol 1994; 266: F89–F93 [DOI] [PubMed] [Google Scholar]
- 26. Krieger J, Liard J, Cowley A., Jr Hemodynamics, fluid volume, and hormonal responses to chronic high-salt intake in dogs. Am J Physiol 1990; 259: H1629–H1636 [DOI] [PubMed] [Google Scholar]
- 27. Cholewa BC, Mattson DL. Role of the renin-angiotensin system during alterations of sodium intake in conscious mice. Am J Physiol 2001; 281: R987–R993 [DOI] [PubMed] [Google Scholar]
- 28. Sato Y, Ando K, Ogata E, Fujita T. Salt sensitivity in Goldblatt hypertensive rats—role of extracellular fluid volume and renin-angiotensin system. Jpn Circ J 1991; 55: 165–173 [DOI] [PubMed] [Google Scholar]
- 29. Jackson CA, Navar LG. Arterial pressure and renal function in two-kidney, one clip Goldblatt hypertensive rats maintained on a high-salt intake. J Hypertens 1986; 4: 215–221 [DOI] [PubMed] [Google Scholar]
- 30. Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol Heart Circ Physiol 2006; 291: H929–H938 [DOI] [PubMed] [Google Scholar]
- 31. McEwen ST, Schmidt JR, Somberg L, Cruz Lde L, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation 2009; 16: 220–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Priestley JR, Buelow MW, McEwen ST, Weinberg BD, Delaney M, Balus SF, Hoeppner C, Dondlinger L, Lombard JH. Reduced angiotensin II levels cause generalized vascular dysfunction via oxidant stress in hamster cheek pouch arterioles. Microvasc Res 2013; [MVR(2013), http.//dx.doi.org/10.1016/jmvr.2013.04.004] (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fukai T, Siegfried MR, Ushio-Fukai M, Griendling KK, Harrison DG. Modulation of extracellular superoxide dismutase expression by angiotensin II and hypertension. Circ Res 1999; 85: 23–28 [DOI] [PubMed] [Google Scholar]
- 34. Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension 2006; 48: 473–481 [DOI] [PubMed] [Google Scholar]
- 35. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001; 37: 429–432 [DOI] [PubMed] [Google Scholar]
- 36. Takazawa Y, Maeshima Y, Kitayama H, Yamamoto Y, Kawachi H, Shimizu F, Matsui H, Sugiyama H, Yamasaki Y, Makino H. Infusion of angiotensin II reduces loss of glomerular capillary area in the early phase of anti-Thy-1.1 nephritis possibly via regulating angiogenesis-associated factors. Kidney Int 2005; 68: 704–722 [DOI] [PubMed] [Google Scholar]
- 37. Maitland K, Bridges L, Davis WP, Loscalzo J, Pointer MA. Different effects of angiotensin receptor blockade on end-organ damage in salt-dependent and salt-independent hypertension. Circulation 2006; 114: 905–911 [DOI] [PubMed] [Google Scholar]
- 38. Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol 2008; 28: 61–67 [DOI] [PubMed] [Google Scholar]
- 39. Neyses L, Dorst K, Michaelis J, Berres M, Philipp T, Distler A, Losse H, Vetter H, Epstein FH, Vetter W. Compliance with salt restriction as a limiting factor in the primary prevention of hypertension. J Hypertens Suppl 1985; 3: S87–S90 [PubMed] [Google Scholar]