Abstract
BACKGROUND
Longitudinal associations between depressive symptoms and blood pressure have been inconsistent. Most studies have examined incident hypertension as an outcome, and few have examined effect modification.
METHODS
This study examined moderating influences of sex and age on coincident trajectories of depressive symptoms and blood pressure among 2,087 participants from the Baltimore Longitudinal Study of Aging (aged 19–97 years; 53% men; 74% white). Participants underwent clinical blood pressure measurement and completed the Center for Epidemiological Studies–Depression (CES-D) scale on up to 14 occasions (mean = 3.8; SD = 2.6) over up to 29 years (mean = 7.8; SD = 6.4). CES-D was log-transformed (CES-D(log)) for analyses.
RESULTS
Mixed-effects regression revealed that prospective relations of CES-D(log) to diastolic blood pressure differed by age in women (b = 0.095; P = 0.001) but not men; greater CES-D(log) attenuated the expected age-related decline in diastolic blood pressure. Across all testing sessions, greater CES-D(log) was associated significantly with higher average systolic blood pressure for women (b = 2.238; P = 0.006) but not men. Age-stratified analyses showed that greater CES-D(log) was associated significantly with higher average systolic (b = 3.348; P = 0.02) and diastolic (b = 1.730; P < 0.03) blood pressure for older adults (≥58.8 years at first visit). In the younger age cohort, sex moderated the relation of CES-D(log) to systolic blood pressure (b = −3.563; P = 0.007); greater CES-D(log) in women, but lesser CES-D(log) in men, was associated with higher systolic blood pressure.
CONCLUSIONS
Results demonstrate sex and age differences in the relation between depressive symptoms and blood pressure. Findings suggest the potential importance of preventing, detecting, and lowering depressive symptoms to prevent hypertension among women and older adults.
Keywords: age factors, blood pressure, depressive symptoms, hypertension, longitudinal studies, older adults, repeated measures, sex factors, women.
Depression and depressive symptoms are established risk factors for cardiovascular disease (CVD),1 but the mechanisms underlying this relation are unclear. Hypertension may be a mechanism through which depressive symptoms influence CVD pathogenesis.2 Prior literature has identified inconsistent longitudinal associations between depressive symptoms and blood pressure (BP).3–7 Longitudinal studies have typically focused on incident hypertension as an outcome,4,5 including a recent meta-analysis of 9 prospective studies.8 Few studies have examined the prospective longitudinal covariation of depressive symptoms and BP.6,7
There are well-documented sex and age differences for both depressive disorder (and symptoms) and BP. However, these factors are not typically examined as concomitant effect modifiers in the context of a longitudinal study of depressive symptom–BP associations. The potential importance of sex and age in moderating depressive symptom–BP associations is suggested by several findings. First, women are twice as likely as men to experience depression starting in adolescence.9 Second, community studies have found a lower prevalence of clinical depression and depressive symptoms among elderly people than among younger adults in Western societies,10,11 although older adults appear to be at greater risk for depression biologically.10 Third, the prevalence of hypertension, particularly isolated systolic hypertension, increases with age.12 However, this age pattern differs between sexes: until age 45, more men than women have hypertension, but after age 64, more women than men have hypertension.12 Accordingly, this study examined the potential moderating influences of sex and age on prospective relations of coincident trajectories of depressive symptoms and BP.
METHODS
Participants
Participants were community-dwelling volunteers enrolled in the Baltimore Longitudinal Study of Aging (BLSA), a prospective open-cohort study initiated by the National Institute on Aging in 1958.13 Volunteers return every 2–3 years to Harbor Hospital in Baltimore, Maryland, for physiological and psychological testing. Beginning in 1979, participants were administered the Center for Epidemiologic Studies–Depression (CES-D) scale.14 Participant data were excluded from analyses after participants sustained stroke (n = 131), heart failure (n = 75), or dementia diagnoses (n = 92). Depression is a common consequence of stroke, and stroke damage to brain tissue may lead to depression.15 Second, heart muscle is often too stiff or weak to effectively pump blood in those with heart failure.16 Both conditions obscure the relation between BP and depressive symptoms. The final analysis sample included 2,087 participants (n = 1,095 men and 992 women) aged 19 to 97 years. Baseline sample characteristics are listed in Table 1, and the number of participants per visit is presented in Table 2. The BLSA uses continuous enrollment procedures, which results in participants having varied numbers of visits and follow-up times. On average, participants had 3.8 visits (SD = 2.6; range = 1–14 visits) and 2.3 years (SD = 1.0 years) between visits. Over the course of data collection, 628 participants died, and 382 withdrew or were lost to follow-up. Before 2002, institutional review board approval was obtained from the Johns Hopkins Bayview Medical Center. Afterward, it was obtained from the MedStar Research Institute. All procedures followed were in accordance with the appropriate institution’s guidelines, and all participants provided written informed consent. Institutional review board approval for data analyses was obtained from the University of Maryland, Baltimore County.
Table 1.
Characteristics of study sample at first assessment
| Variable | Mean | SD | Min | Max |
|---|---|---|---|---|
| Age, y | 57.0 | 16.8 | 19 | 94 |
| Education, y | 16.3 | 2.6 | 8 | 22 |
| Sex, % male | 52.5 | |||
| Race, % white | 74.0 | |||
| Alcohol use, %a | 9.9 | |||
| Cigarette smoking, ever % | 51.7 | |||
| Body mass index, kg/m2 | 25.9 | 4.3 | 16.7 | 48.8 |
| Antihypertensive use, current % | 22.5 | |||
| Antidepressant use, current % | 2.6 | |||
| Cardiovascular disease, present % | 15.7 | |||
| Diabetes, present % | 6.1 | |||
| Depressive symptoms, CES-D | 7.0 | 6.9 | 0 | 50 |
| Depressed (CES-D ≥ 16), % | 11.0 | |||
| Systolic blood pressure, mm Hg | 127.4 | 19.7 | 86 | 210 |
| Diastolic blood pressure, mm Hg | 78.9 | 11.0 | 40 | 118 |
Abbreviations: CES-D, Center for Epidemiologic Studies–Depression Scale; Max, maximum; Min, minimum.
aPercentage reporting consumption of ≥2 alcoholic drinks per day. One drink was classified as 12 oz of beer, 4–5 oz of wine, or 2 oz of spirits.
Table 2.
Sample size by number of visits with concurrent assessment of blood pressure and depressive symptoms
| No. of repeat administrations | No. (% of sample) |
|---|---|
| 1 | 2,087 (100) |
| 2 | 1,593 (76.3) |
| 3 | 1,238 (59.3) |
| 4 | 953 (45.7) |
| 5 | 707 (33.9) |
| 6 | 497 (23.8) |
| 7 | 339 (16.3) |
| 8 | 217 (10.4) |
| 9 | 132 (6.3) |
| 10 | 69 (3.3) |
| ≥11 | 39 (1.9) |
Depressive symptoms
At each visit, participants completed the CES-D14 as a paper and pencil inventory in a quiet room. CES-D scores of BLSA participants follow a U-shaped trajectory with age, with depressive symptom levels highest in young adulthood, with a decrease during middle adulthood, and with a subsequent increase in older adulthood.17 The CES-D was designed for use in general population surveys to measure current depressive symptomatology levels and to examine the relation between depressive symptoms and other variables. In this 20-item questionnaire, participants rate how frequently they have experienced each item in the past week on a scale from 0 (rarely/less than 1 day) to 3 (most or all of the time/5–7 days). The domains covered include depressive mood, feelings of guilt or worthlessness, loss of appetite, sleep disturbance, and psychomotor retardation. Higher scores indicate more depressive symptoms.
The CES-D has been used in many population surveys to estimate prevalence rates of depressive symptoms.18 It demonstrates strong reliability and validity for assessing depressive symptoms in the general population14,18,19 and concurrent validity with other scales used to measure depressive symptoms.14 Internal validity is strong across sex and age subgroups14,19 and reflects a single large higher-order factor of depression.18
Blood pressure
At each visit, trained nursing staff measured brachial BP in both arms with a mercury sphygmomanometer using an appropriately sized cuff while the participant sat in an upright position. Measurement was conducted after a 5-minute quiet rest period and at least 90 minutes after a nonstandardized breakfast. Systolic BP (SBP) and diastolic BP (DBP) were determined by Kortokoff phase I and phase V, respectively. BP values used are the average of the second and third measurements on both the right and left arms, and the mean of the BP values for each arm was used in analyses.
Covariables
A number of common risk factors affect both depressive symptoms and BP; these variables were adjusted statistically to ascertain the independent effect of depressive symptoms on BP. Age and education were assessed in years. Race was coded white vs. nonwhite. Alcohol use was averaged over visits and then coded as a dichotomous variable reflecting ≥2 drinks per day. One drink was defined as 12 oz of beer, 4–5 oz of wine, or 2 oz of spirits. Time-varying covariables were assessed as follows. Smoking was collapsed into “ever/never” having smoked at least 100 cigarettes in one’s life before that visit. Body mass index was calculated by dividing measured weight by measured height squared (kg/m2). Use of antihypertensive and antidepressant medications was coded as current “yes/no.” Medical diseases such as CVDs and diabetes were assessed during the medical interview. A cardiovascular morbidity index (absent/present) reflected history of coronary artery disease, myocardial infarction, or peripheral artery disease.
Data analyses
Mixed-effects regression analyses were conducted to examine longitudinal relations of depressive symptoms with BP. Mixed-effects regression accounts for the lack of uniformity between measurement intervals both within and across participants that is present in the BLSA.20 Mixed-effects models calculate the unique effect of each predictor adjusted for all other predictors in the model and include both fixed and random effects. Moreover, they take into account the correlation among repeated measurements on the same individual and are unaffected by randomly missing data. In all analyses, age (at each visit) was assigned as a random effect to index time.20 McArdle and Bell21 recommend using age to index time when participants’ study entry time is random, as is the case in the BLSA. Models also included linear and quadratic main and interactive longitudinal (i.e., age) terms.
Separate models were constructed for SBP and DBP as the dependent measure. All analyses included age, education, and body mass index as continuous covariables, and sex, race, alcohol consumption, smoking, cardiovascular morbidity, diabetes mellitus, antihypertensive use, and antidepressant use as categorical covariables. Because CES-D scores were highly positively skewed, CES-D was log10-transformed and treated as continuous (CES-D(log)). CES-D(log) and age were centered to reduce the effects of multicollinearity in interaction terms.
Three sets of analyses were conducted. The first set of models examined all participants and used backward elimination of nonsignificant higher-order interactions22 from CES-D(log)2 × Age2 × Sex (P > 0.04). The second set of models was computed to better understand the significant interactions of BP and sex. Accordingly, sex-stratified models were computed, with subsequent backward elimination of nonsignificant interactions.22 The third set of models further examined significant age interactions. These models were stratified by age at first visit based on a median split (<58.8 years vs. ≥58.8 years), followed by backward elimination of nonsignificant interaction terms. Because interactions involving the quadratic CES-D(log) term were nonsignificant in all analyses, they were removed in all final models.
Statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC), and graphs were created using R version 2.15.0 (Vienna, Austria). Graphs illustrate significant relations using the prototypical values of predictors.
RESULTS
All participants
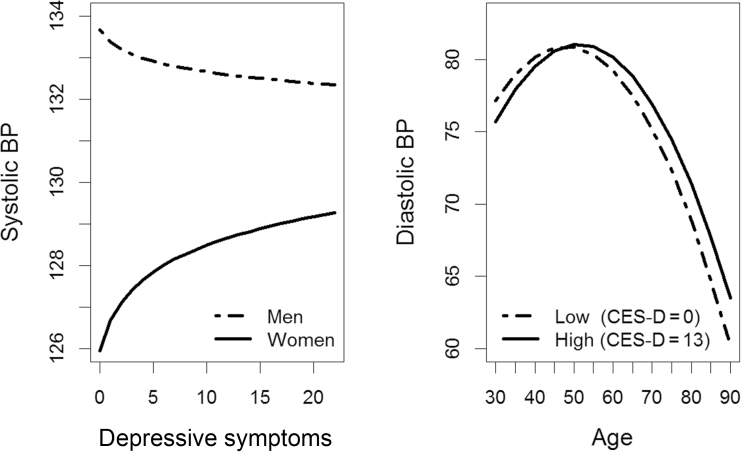
In models analyzing all participants, there was a significant interaction between CES-D(log) and sex for SBP (b = −3.399; P = 0.002). Across all testing visits, women with greater CES-D(log) had higher average SBP, whereas men with lower CES-D(log) had higher average SBP (Figure 1). For DBP, results revealed a significant longitudinal association between CES-D(log) and age (b = 0.070; P = 0.001). In general, those with lower CES-D(log) had relatively greater increases in DBP until about age 50 years, after which they displayed a more pronounced decrease in DBP. In contrast, those with greater CES-D(log) had lower DBP until about age 50 years but then showed lesser decline over time (Figure 1).
Figure 1.
Moderated associations of depressive symptoms with systolic and diastolic blood pressure (BP) for all participants. Abbreviation: CES-D, Center for Epidemiologic Studies–Depression Scale.
Sex-stratified models
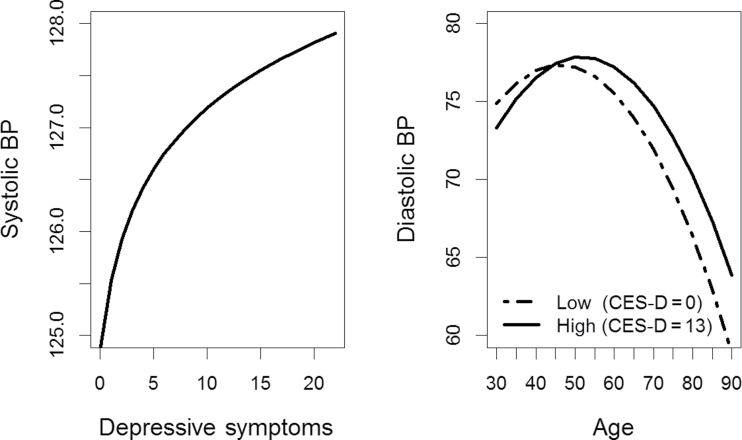
In sex-stratified models, results revealed significant associations between CES-D(log) and BP for women but not for men (SBP: b = −0.7033, P = 0.36; DBP: b = 0.5222, P < 0.27). Women with greater CES-D(log) had higher average SBP than women with relatively lower levels of symptoms across all testing sessions (b = 2.238; P = 0.006) (Figure 2). For DBP, results revealed a significant longitudinal association between CES-D(log) and age for women (b = 0.095; P = 0.001). In general, women with greater CES-D(log) generally had lower DBP until about age 45 years but then showed lesser decline over time. In contrast, women with lower CES-D(log) had relatively greater increases in DBP until about age 45 years, after which they displayed a more pronounced decrease in DBP (Figure 2).
Figure 2.
Associations of depressive symptoms with systolic and diastolic blood pressure (BP) for women. Abbreviation: CES-D, Center for Epidemiologic Studies–Depression Scale.
Age-stratified models
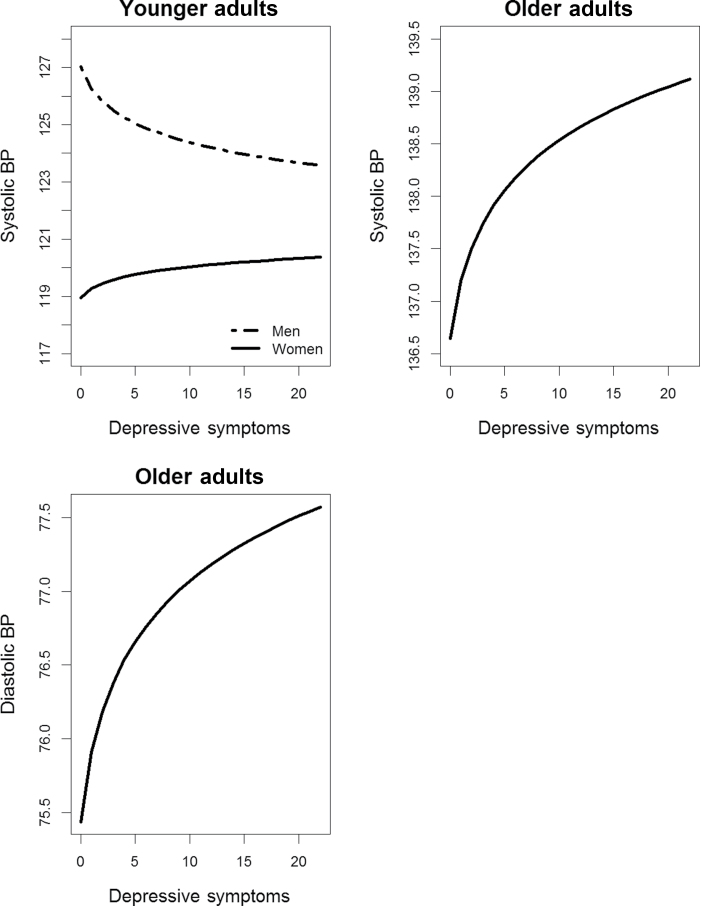
In age-stratified models, results for the younger group revealed a significant interaction between CES-D(log) and sex for SBP (b = −3.563; P = 0.007) but no significant terms of interest for DBP (b = 0.5344; P = 0.35). Similar to SBP results for all participants, across all testing sessions, younger women with greater CES-D(log) had higher average SBP, whereas younger men with lower CES-D(log) had higher average SBP (Figure 3). Additionally, significant associations were found between CES-D(log) and BP for the older group, regardless of longitudinal change. In general, older participants with greater CES-D(log) had higher average SBP (b = 3.3480; P = 0.02) (Figure 3) and DBP (b = 1.730; P < 0.03) (Figure 3) than older participants with relatively lower levels of symptoms. To view significant results from all models, please see Tables 3 and 4. To view all coefficient tables and graphs, please see the Supplementary Material online.
Figure 3.
Associations of depressive symptoms with systolic and diastolic blood pressure (BP) for younger and older adults.
Table 3.
Summary of significant depressive symptom associations with systolic blood pressure from mixed-effects regression analyses
| Participant subgroupa | Significant CES-D(log) effects | Coefficient | F | P value |
|---|---|---|---|---|
| All participants | CES-D(log) × Sex | −3.3994 | 9.22 | 0.002 |
| CES-D(log) | 2.4350 | 8.90 | 0.003 | |
| Women only | CES-D(log) | 2.2379 | 7.54 | 0.006 |
| Men only | None | NA | NA | NA |
| Younger age group | CES-D(log) × Sex | −3.5630 | 7.37 | 0.007 |
| Older age group | CES-D(log) | 3.3478 | 5.42 | 0.02 |
Abbreviations: CES-D, Center for Epidemiologic Studies–Depression Scale; NA, not applicable.
aSeparate regression models were computed for each participant subgroup. Each contained all relevant main effects and interaction terms as detailed in the text. However, for space-saving purposes, we only report significant findings for each model in this table. Complete data are available in the Supplementary Material online.
Table 4.
Summary of significant depressive symptom associations with diastolic blood pressure from mixed-effects regression analyses
| Participant subgroupa | Significant CES-D(log) effects | Coefficient | F | P value |
|---|---|---|---|---|
| All participants | CES-D(log) × Age | 0.0695 | 10.77 | 0.001 |
| CES-D(log) | 1.7443 | 12.57 | 0.0004 | |
| Women only | CES-D(log) × Age | 0.0947 | 10.71 | 0.001 |
| CES-D(log) | 1.6159 | 11.16 | 0.001 | |
| Men only | None | NA | NA | NA |
| Younger age group | None | NA | NA | NA |
| Older age group | CES-D(log) | 1.7297 | 4.99 | 0.03 |
Abbreviations: CES-D, Center for Epidemiologic Studies–Depression Scale; NA, not applicable.
aSeparate regression models were computed for each participant subgroup. Each contained all relevant main effects and interaction terms as detailed in the text. However, for space-saving purposes, we only report significant findings for each model in this table. Complete data are available in the Supplementary Material online.
DISCUSSION
Using participants of a broad age range and a balance of men and women, this study examined the coincident trajectories of depressive symptoms and BP over 1–14 occasions spanning up to 29 years. The full spectrum of depressive symptoms was examined rather than categorical depression to examine risk associated with subclinical depressive symptoms without losing statistical power.23 Because few participants met suggested CES-D criteria (CES-D ≥ 16)14 for clinically significant depression, results suggest that even low levels of depressive symptoms are associated with BP levels. Alternatively, these findings may also reflect individual differences in the broader and stable construct of negative affectivity,24 rather than actual fluctuations in depressive symptoms per se, given the construct and measurement overlap between specific types of negative affect.25 Hence, stability of depressive symptoms may in part reflect a personality characteristic and propensity to experience negative emotions. Our results further indicate the presence of complex sex and age differences in the relation of depressive symptoms to BP.
First, the prospective relations of CES-D(log) to DBP differed as a function of increasing age in women (but not men) such that higher CES-D(log) was associated with lower DBP before approximately age 45 years but were thereafter associated with lesser decline in DBP. Similarly, Nabi and colleagues6 noted a more rapid age-related increase in hypertension in those participants with more depressive episodes over time. Yet, important methodological differences between these and other available studies preclude direct comparison. In that regard, most previous longitudinal studies did not find significant interactive relations of sex4,5 or age5 and depressive symptoms with respect to incident hypertension. However, whereas this study assessed the relation of concurrent depressive symptoms and BP, previous studies have assessed the effect of depressive symptoms on future BP or incident hypertension. Furthermore, whereas this study evaluated depressive symptoms and BP continuously, prior studies categorized depression and/or hypertension.4,5
The present findings further demonstrated that across all testing sessions, higher CES-D(log) was significantly associated with greater average SBP for women but not for men. Räikkönen and colleagues found similar results for SBP in a sample of middle-aged women over 10 years.7 Yet, in contrast, most prior studies, albeit with differing methodologies, have noted similar results for both men and women, with greater depressive symptoms associated with higher BP.4–6
Next, age-stratified analyses showed that across all testing sessions, greater CES-D(log) was significantly associated with both higher SBP and DBP for older adults (aged ≥58.8 years at first visit). Sex moderated the results across all testing sessions in the younger age cohort: greater CES-D(log) was associated with higher SBP in women, whereas lower levels of CES-D(log) were related to higher SBP in men. Although we could not identify any directly comparable studies, Jonas and colleagues4 found that for initially normotensive white adults aged 25–44 years at baseline, intermediate levels of baseline depressive symptoms predicted incident hypertension; however, for those aged 45–64 years at baseline, high levels of baseline depressive symptoms predicted incident hypertension. As noted earlier, most previous studies have reported similar results for both men and women, with greater depressive symptoms predicting higher BP.4–6 Although one prior study reported that greater depressive symptoms predicted lower BP, no sex differences were found.3
The above findings may reflect age and sex moderation of the more stable construct of negative affectivity on BP, rather than depression per se.24,25 In that regard, a meta-analysis conducted by Jorgensen and colleagues24 found that greater negative affectivity and defensiveness and lower anger-affect expression were associated with higher BP. Although not assessed here, men are more likely than women to underreport negative affect including depressive symptoms;26 it is conceivable that defensiveness or lesser affect expression may, in part, explain the relation of lower levels of depressive symptoms to higher BP in younger men noted herein.
Several similar mechanisms may underlie sex and age differences in the relations between depressive symptoms and BP. Depression and depressive symptoms are associated with hypothalamic-pituitary-adrenal (HPA) axis dysregulation and autonomic imbalance, and this altered activity may heighten risk for increased BP.1 Women are more likely than men to become depressed in response to stressors, particularly chronic stress.27 Additionally, women may be more likely than men to experience certain stressful events, such as work–family tensions,28 that may chronically activate the HPA axis. Older adults may also experience unique stressors that contribute to depressive symptoms and CVD risk.29,30 Furthermore, stressful events occurring in late life have been associated with differential HPA axis changes as compared with early life events.31
Second, depressive symptoms have a well-known association with inflammatory factors32 that are posited to play a role in the development of hypertension.33 Inflammatory processes may influence CVD development more in women than in men;34 indeed circulating C-reactive protein levels are higher in women than in men at all ages.34 Thus, it may be that even low levels of depressive symptoms affect inflammatory process, leading to hypertension in women more than men across the lifespan. As well, the aging process is associated with chronic low-grade systemic inflammation;35 this age-related inflammation may put older adults at greater risk for greater depressive symptoms and BP. Moreover, age-related arterial stiffening may leave older adults more vulnerable to the effects of depressive symptoms on BP.36,37
Finally, common biobehavioral risk factors such as physical inactivity, obesity, smoking, and poor sleep that are associated with both depressive symptoms and CVD may have heightened effects on women than on men.38 The attenuation of age-related decline in DBP may be a reflection of these mechanisms associated with depressive symptoms and BP (e.g., HPA dysregulation, inflammation, and biobehavioral risk factors) reducing the impact of increases in arterial stiffening with age on DBP.
Study limitations include the convenience sample of primarily well-educated white participants. Although the sample’s homogeneity and nonrepresentative nature limits the generalizability of the study’s findings, it may also limit the effects of confounding variables. Next, participants were often recommended to receive antihypertensive medication after discovery of their hypertensive status in the BLSA. It is possible that depressive symptom and BP relations differ between treated and untreated hypertensive individuals, as well as between those who were and were not adherent to antihypertensive and antidepressant treatment.39 Additionally, some risk factors for both depressive symptoms and BP, such as sleep and physical activity, were not assessed, and smoking was assessed in a cursory manner. Finally, it is possible that the relational patterns between depression and BP may differ when using clinically defined depression (e.g., by psychiatric screening) as a predictor, rather than a larger range of depressive symptoms.
This study has numerous strengths as compared with previous studies.3–7 Depressive symptoms and BP were both analyzed continuously, instead of using dichotomized groups, which allowed for observing the effect of changes in depressive symptoms on BP at a more granular level; this also results in increased power to detect an effect.23 Additionally, this study also examined a wide age range and a balance of men and women. Finally, a large number of repeated, concurrent assessments of depressive symptoms and clinically measured BP were used, also resulting in increased power.
These findings indicate that that women and older adults with greater depressive symptoms are at increased risk for elevated BP, as compared with those with fewer depressive symptoms. Given the risk of CVD and mortality associated with higher BP,40 these results make plain the importance of evaluating these effect moderators on the depressive symptoms–BP relationship, even before BP values reach hypertension level.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURES
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
We thank the BLSA participants for their continued involvement with the study and all members of the BLSA study team. The National Institute on Aging Intramural Research Program of the National Institutes of Health (NIH) conducted this research. M.T.S. is a participant in the NIH Graduate Partnership Program and a graduate student at University of Maryland, Baltimore County.
REFERENCES
- 1. Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 2009; 12: 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scuteri A. Depression and cardiovascular risk: does blood pressure play a role? J Hypertens 2008; 26: 1738–1739 [DOI] [PubMed] [Google Scholar]
- 3. Hildrum B, Mykletun A, Holmen J, Dahl AA. Effect of anxiety and depression on blood pressure: 11-year longitudinal population study. Brit J Psychiatry 2008; 193: 108–113 [DOI] [PubMed] [Google Scholar]
- 4. Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med 1997; 6: 43–49 [DOI] [PubMed] [Google Scholar]
- 5. Patten SB, Williams JV, Lavorato DH, Campbell NR, Eliasziw M, Campbell TS. Major depression as a risk factor for high blood pressure: epidemiologic evidence from a national longitudinal study. Psychosom Med 2009; 71: 273–279 [DOI] [PubMed] [Google Scholar]
- 6. Nabi H, Chastang JF, Lefevre T, Dugravot A, Melchior M, Marmot MG, Shipley MJ, Kivimaki M, Singh-Manoux A. Trajectories of depressive episodes and hypertension over 24 years: the Whitehall II prospective cohort study. Hypertension 2011; 57: 710–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Räikkönen K, Matthews KA, Kuller LH. Trajectory of psychological risk and incident hypertension in middle-aged women. Hypertension 2001; 38: 798–802 [PubMed] [Google Scholar]
- 8. Meng L, Chen D, Yang Y, Zheng Y, Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens 2012; 30: 842–851 [DOI] [PubMed] [Google Scholar]
- 9. Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord 1993; 29: 85–96 [DOI] [PubMed] [Google Scholar]
- 10. Blazer DG, Hybels CF. Origins of depression in later life. Psychol Med 2005; 35: 1241–1252 [DOI] [PubMed] [Google Scholar]
- 11. Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V. Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R). Psychol Med 2010; 40: 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010; 121: e46–e215 [DOI] [PubMed] [Google Scholar]
- 13. Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Lakatta EG. Normal Human Aging: The Baltimore Longitudinal Study of Aging. United States Government Printing Office: Washington, DC, 1984 [Google Scholar]
- 14. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur 1977; 1: 385–401 [Google Scholar]
- 15. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry 2002; 52: 253–264 [DOI] [PubMed] [Google Scholar]
- 16. Hess OM, Carroll JD. Clinical assessment of heart failure. In Libby P, Bonow RO, Mann DL, Zipes DP. (eds), Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 8th edn Saunders Elsevier: Philadelphia, 2008, pp. 561–581 [Google Scholar]
- 17. Sutin AR, Terracciano A, Milaneschi Y, An Y, Ferrucci L, Zonderman AB. The trajectory of depressive symptoms across the adult life span. JAMA Psychiatry 2013: 1–9 http://archpsyc.jamanetwork.com/article.aspx?articleid=1696350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol 2006; 62: 123–146 [DOI] [PubMed] [Google Scholar]
- 19. Clark VA, Aneshensel CS, Frerichs RR, Morgan TM. Analysis of effects of sex and age in response to items on the CES-D scale. Psychiatry Res 1981; 5: 171–181 [DOI] [PubMed] [Google Scholar]
- 20. Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press: New York, 2003 [Google Scholar]
- 21. McArdle JJ, Bell RQ. An introduction to latent growth models for developmental data analysis. In Little TD, Schnabel KU, Baumert J. (eds), Modeling Longitudinal and Multilevel Data: Practical Issues, Applied Approaches, and Specific Examples. Lawrence Erlbaum Associates Publishers: Mahwah, NJ, 2000, pp. 69–281 [Google Scholar]
- 22. Morrell CH, Pearson JD, Brant LJ. Linear transformations of linear mixed-effects models. Am Statistician 1997; 51: 338–343 [Google Scholar]
- 23. Bennette C, Vickers A. Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med Res Methodol 2012; 12: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jorgensen RS, Johnson BT, Kolodziej ME, Schreer GE. Elevated blood pressure and personality: a meta-analytic review. Psychol Bull 1996; 120: 293–320 [DOI] [PubMed] [Google Scholar]
- 25. Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull 2005; 131: 260–300 [DOI] [PubMed] [Google Scholar]
- 26. Addis ME. Gender and depression in men. Clin Psychol 2008; 15: 153–168 [Google Scholar]
- 27. Hammen C. Stress and depression. Annu Rev Clin Psychol 2005; 1: 293–319 [DOI] [PubMed] [Google Scholar]
- 28. Dixon JP, Dixon JK, Spinner JC. Tensions between career and interpersonal commitments as a risk factor for cardiovascular disease among women. Women Health 1991; 17: 33–57 [DOI] [PubMed] [Google Scholar]
- 29. Buckley T, McKinley S, Tofler G, Bartrop R. Cardiovascular risk in early bereavement: a literature review and proposed mechanisms. Int J Nurs Stud 2010; 47: 229–238 [DOI] [PubMed] [Google Scholar]
- 30. Bruce ML. Psychosocial risk factors for depressive disorders in late life. Biol Psychiatry 2002; 52: 175–184 [DOI] [PubMed] [Google Scholar]
- 31. Gerritsen L, Geerlings MI, Beekman AT, Deeg DJ, Penninx BW, Comijs HC. Early and late life events and salivary cortisol in older persons. Psychol Med 2010; 40: 1569–1578 [DOI] [PubMed] [Google Scholar]
- 32. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Medic 2009; 71: 171–186 [DOI] [PubMed] [Google Scholar]
- 33. Montecucco F, Pende A, Quercioli A, Mach F. Inflammation in the pathophysiology of essential hypertension. J Nephrol 2011; 24: 2334. [DOI] [PubMed] [Google Scholar]
- 34. Low CA, Thurston RC, Matthews KA. Psychosocial factors in the development of heart disease in women: current research and future directions. Psychosom Med 2010; 72: 842–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exper Gerontol 2004; 39: 687–699 [DOI] [PubMed] [Google Scholar]
- 36. Franklin SS. Arterial stiffness and hypertension: a two-way street? Hypertension 2005; 45: 349–351 [DOI] [PubMed] [Google Scholar]
- 37. Baune BT, Stuart M, Gilmour A, Wersching H, Heindel W, Arolt V, Berger K. The relationship between subtypes of depression and cardiovascular disease: a systematic review of biological models. Transl Psychiatry 2012; 2: e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moller-Leimkuhler AM. Higher comorbidity of depression and cardiovascular disease in women: a biopsychosocial perspective. World J Biol Psychiatry 2010; 11: 922–933 [DOI] [PubMed] [Google Scholar]
- 39. Bautista LE, Vera-Cala LM, Colombo C, Smith P. Symptoms of depression and anxiety and adherence to antihypertensive medication. Am J Hypertens 2012; 25: 505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, the National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.