Abstract
Peripheral T-cell lymphomas (PTCL) are a diverse group of rare non-Hodgkin lymphomas (NHL) that carry a poor prognosis and are in need of effective therapies. Alisertib (MLN8237) an investigational agent that inhibits Aurora A Ser/Thr kinase has shown activity in PTCL patients. Here we demonstrate that aurora A and B are highly expressed in T-cell lymphoma cell lines. In PTCL patient samples aurora A was positive in 3 of 24 samples and co-expressed with aurora B. Aurora B was positive in tumor cells in 22 of 32 samples. Of the subtypes of PTCL, aurora B was over-expressed in PTCL (NOS) [73%], T-NHL (100%), ALCL (Alk-Neg) [100%] and AITL [100%]. Treatment with MLN8237 inhibited PTCL cell proliferation in CRL-2396 and TIB-48 cells with an IC50 of 80-100 nM. MLN8237 induced endo-reduplication in a dose and time dependent manner in PTCL cell lines leading to apoptosis demonstrated by flow cytometry and PARP-cleavage at concentrations achieved in early phase clinical trials. Moreover, inhibition of HisH3 and aurora A phosphorylation was dose dependent and strongly correlated with endo-reduplication. The data provide a sound rationale for aurora inhibition in PTCL as a therapeutic modality and warrants clinical trial evaluation.
Keywords: Aurora kinases, Peripheral T-cell lymphoma, Immunohistochemistry, MLN8237 (Alisertib), Cell proliferation, Apoptosis
Introduction
Peripheral T-cell lymphomas (PTCL) are rare (∼10-15% of non-Hodgkin Lymphomas (NHL)) and heterogeneous lymphoid malignancies comprised of several subtypes [1, 2]. Patients with PTCL have a poor prognosis due to a very aggressive disease course coupled with a lack of effective therapies [3]. Aggressive histology PTCL has an inferior event free survival and overall survival relative to their B-cell counterparts as reported by multiple investigators [4]. The increased expression of multidrug resistance proteins (MRPs) and p53 [1] are attributed to be the cause of chemo-resistance but PTCLs are most likely to respond to anti-proliferative therapies designed based on pathobiology and biologically relevant targets [5, 6].
Aurora kinases (A and B) are a highly conserved family of oncogenic serine/threonine protein kinases that have critical regulatory roles throughout mitotic phase of the cell cycle. Aurora A localizes to centrosomes and the proximal mitotic spindle during mitosis. It is critical to bipolar spindle formation but also appears to participate in centrosome maturation and separation, mitotic entry, chromosome alignment and cytokinesis [6, 7]. Aurora B is a ‘chromosomal passenger protein’ and localizes to the centromere regions in the early stages of mitosis. Later in mitosis it re-localizes from the centromeres to the microtubules at the spindle equator and promotes the completion of cytokinesis [8]. Aurora B is thus essential for chromosomal segregation. Inhibition of Aurora B prevents proper alignment of chromosomes to the spindle plate, inhibits cytokinesis, and results in the formation of multi-nucleated cells [9]. Over-expression of aurora kinases has been observed in a variety of malignancies [7] and associated with a poor prognosis in mantle cell lymphoma [10]. Additionally, increased aurora A expression results in a higher degree of chromosomal aneuploidy, initiating oncogenesis and tumor progression [11]. The oncogenic potential of aurora A has been described specifically in NHL with increased expression correlating with rapidly dividing histological subtypes [12]. Up-regulation of Aurora A in PTCL has been also noted [13, 14] making this protein kinase an attractive therapeutic target in not only aggressive B-NHL, but specifically in those of non-skin T-cell lineages.
MLN8237 (alisertib) is an ATP-site competitive small molecule inhibitor with selectivity for aurora A over aurora B in in vitro kinase assays. MLN8237 induced in vitro growth inhibition associated with mitotic spindle abnormalities, polyploidy, apoptosis and mitotic catastrophe [7]. These effects have been noted in a broad range of tumor cell lines grown in culture including those originating from HTLV-1 infected adult T-cell leukemia [15]. Further, high levels of in vivo activity were noted in xenograft models of B [10] and T-cell lymphoid malignancies [15]. Preliminary data from a phase II clinical trial using single agent MLN8237 in patients with relapsed refractory aggressive B-and T-NHL has demonstrated activity with 4 confirmed complete responses in 6 evaluable PTCL patients [16]. In this study, we demonstrate that PTCL cell lines and patient samples over-express aurora A and B in different cellular compartments. MLN8237 inhibits cell proliferation by cell cycle arrest, induces polyploidy and promotes apoptosis in PTCL cell lines associated with inhibition of both Aurora A and B activity as assessed by downstream signaling. Taken together, our results suggest that inhibition of aurora kinases represents a novel therapeutic strategy for PTCL patients.
Materials and Methods
Cells and reagents
Peripheral T-cell Lymphoma (PTCL) murine cell lines TIB-48 and CRL-2396 were purchased from ATCC and maintained in RPMI 1640 medium (Mediatech, VA) supplemented with 10% fetal bovine serum, 2 mM sodium pyruvate and 100 units/ml penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. MLN8237 was kindly provided by Millennium Pharmaceuticals Inc (Cambridge, MA). The compound was dissolved at 5 mM in distilled water as a stock solution, and then further diluted to desired concentrations for in vitro experiments. Nocodazole was purchased from Calbiochem (La Jolla, CA). Anti-Aurora A (ab1287) and anti-Aurora B (ab2254) antibodies were purchased from Abcam (Cambridge, MA). Anti-phospho-Aurora A (Thr288) (C39D8), anti-phospho-Histone H3 (Ser10) (6G3), anti-Histone H3 and anti-GAPDH (14C10) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-PARP (H-250) was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin antibody was from Sigma (St Louis, MO).
T-cell Lymphoma cell proliferation assay
T-cell Lymphoma cells were seeded at 8,000 per well in 96-well culture plates and allowed to grow for 24 hr followed by the desired treatment with increasing concentrations of the indicated agents for 4 days. Viable cell densities were determined using a CellTiter 96 Cell Proliferation Assay (Promega, Madison, WI). The studies were performed in triplicates × 4 and IC 50 values were estimated by Calcusyn software (Biosoft, UK).
Apoptosis assay
Using Annexin V staining to detect apoptosis, treated cells were harvested at 24 hr and rinsed with cold PBS once. After centrifugation for 5 min, cells were resuspended in 500 μl of 1× Annexin V binding buffer (BioVision, Mountain View, CA, Annexin V-FITC Reagent Kit, Cat.#1001-1000) and then added 1μl of Annexin V-FITC and 1μl of Propidium Iodide (BioVision, Annexin V-FITC Reagent Kit). After incubation for 5 min at room temperature in the dark, the samples were analyzed by flow cytometry. All studies were performed in triplicate.
Cell cycle analysis
Cells were treated with different concentrations of MLN8237 for 48 hr and then were centrifuged at 1,500 × g for 5 min at 4°C and resuspended in PBS, fixed by drop-wise addition of ice-cold ethanol (100%) to a final concentration of 70%, and incubated for 30 min on ice. Fixed cells were pelleted and treated with 100μl of RNase A (0.2 mg/ml in PBS) for 5 min at room temperature, then suspended in 1 ml ddH2O. After staining with 4 μg/ml propidium iodide, the DNA content was determined using a Becton Dickson flow cytometer and the cell cycle profile was analyzed by ModFit software. Cell aggregates were gated out of the analysis, based on the width of the propidium iodide fluorescence signal. Each profile was compiled from 10,000 gated events.
Immunoblotting
The cells were lysed in NP-40 lysis buffer containing 50 mM Tris.Cl (pH 7.4), 0.15 M NaCl, 0.5% NP-40, 1 mM DTT, 50 mM Sodium Fluoride, and 2 μl/ml Protease inhibitor cocktail (Sigma, St. Louis, MO). Protein concentrations were determined using the BioRad protein assay kit (Hercules, CA) and 50 μg of protein was resolved by electrophoresis on a 10% SDS-PAGE gel. The proteins were then transferred onto a nitrocellulose membrane and non-specific binding was blocked by incubating with 5% non-fat milk in TBST buffer (0.01 M Tris-Cl, 0.15 M NaCl, 0.5% Tween-20, pH 8.0) at room temperature for 1 hr. The membrane was subjected to the indicated antibodies and the proteins were detected by a LI-COR Odyssey Infrared Imaging System.
Immunohistochemistry analysis
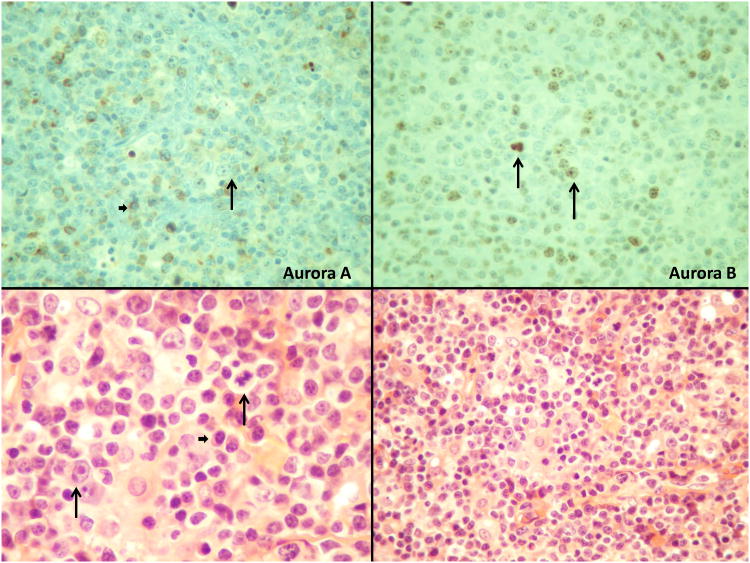
Immunohistochemistry (IHC) was performed on PTCL patient biopsies (SWOG 0350) [17] using Aurora A rabbit polyclonal antibody (Calibiochem, Gibbstown, NJ, Cat#PC742) diluted 1:40, and Aurora B rabbit polyclonal antibody (Abcam, Cambridge, MA, Cat# ab2254) diluted 1:40. Tissue sections were stained on a Discovery XT Automated Immunostainer (Ventana Medical Systems, Inc, Tucson, Arizona). All steps were performed using VMSI validated reagents. Aurora A and B were detected separately using a goat anti-Rabbit secondary antibody. Following staining on the instrument, slides were dehydrated through graded alcohols to xylene and cover slipped with mounting medium (Richard Allan, UT, Cat#4112). After review of the H&E stained sections for confirmation of tumor, the sections were reviewed for aurora A and B staining in the tumor cells and to assess non-tumor cell and non-specific staining. Tumor cells, when positive, showed nuclear staining and in rare cases nucleolar staining. Tumor cell positivity ranged from only rare to >95%. Cytoplasmic staining of plasma cells and small lymphocytes was frequent. Non-specific staining was infrequent. A total of 32 samples were used for aurora A and B analysis by IHC. Of these, there was insufficient tissue for aurora A in 8 cases, allowing analysis of the remaining 24. Aurora B was studied in 32 samples. Positive staining was defined as nuclear or nucleolar and in some cases, mitotic figures were also positive. Since T cell lymphomas may be morphologically heterogeneous, only the large cells were considered malignant. This may underestimate the total number of malignant cells involved.
Results
Aurora A and B are highly expressed in T-cell lymphoma patients and cell lines
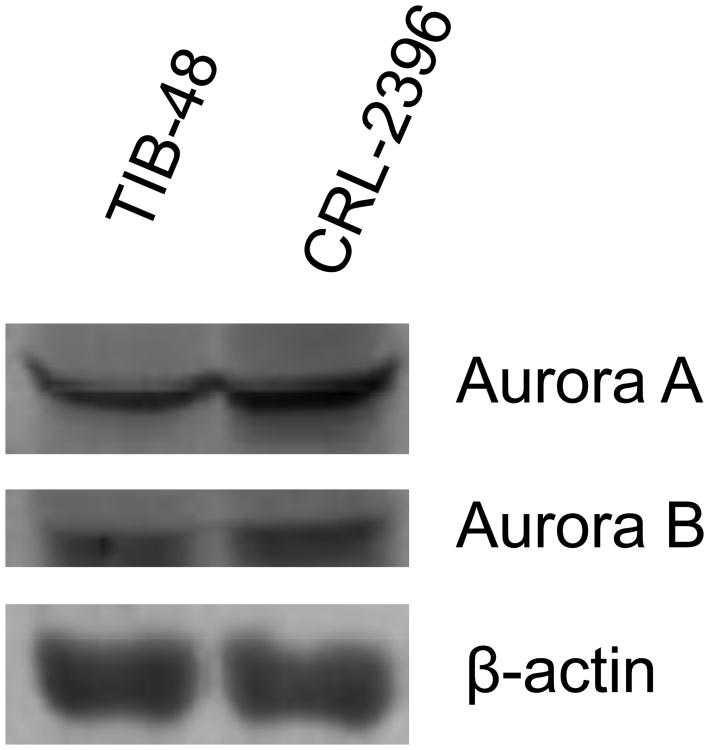
Aurora A and B are over-expressed in numerous human malignancies [7] and high level of aurora A and B correlates to poor prognosis and survival in mantle cell lymphoma (MCL) [10]. Previously, we demonstrated that auroras are over-expressed in PTCL [NOS - not otherwise specified] by gene expression profiling (GEP) analysis [13]. Western blotting analysis of the 2 PTCL cell lines TIB-48 and CRL-2396 indicated expression of both Auroras (Figure 1A). To confirm that auroras are expressed in human PTCL, IHC was performed for aurora A and B expression (Figure 1B). Aurora A was positive in 3 of 24 samples, and co-expressed with aurora B in all 3 cases. Aurora B was positive in the tumor cells in 22 of 32 samples. The positivity ranged from present in only rare tumor cells to >95% of tumor cells (Figure 1B). There was no correlation between the percent of aurora A positive tumor cells and the percent of aurora B positive tumor cells. IHC staining for aurora A and B by PTCL subtype demonstrated over-expression of aurora B in PTCL (NOS) [73%], mature T-NHL (100%), ALCL (Alk-Neg) [100%] and AITL [100%]. In contrast, aurora A expression was rare.
Figure 1.
Figure 1A: Aurora A and B are highly expressed in Peripheral T-cell Lymphoma cells. Total protein was isolated from cultured TIB-48 and CRL-2396 cells. Immunoblotting analysis was performed using anti-Aurora A and B antibodies, respectively. β-actin was used as a loading control. Figure 1B: IHC analysis of Aurora A and Aurora B in a representative PTCL patient specimen. (A). Aurora A is negative in tumor cells but does show reactivity in small lymphocytes and plasma cells. (B). Aurora B is positive in nuclei and nucleoli of tumor cells as well as in a mitotic figure. (C). and (D). Illustrate the histology of the tumor (H&E) staining. Note the many small lymphocytes, plasma cells and vascularity (Long arrows point to the tumor cells and a mitotic figure; short arrows point to plasma cells).
Small lymphocytes were often noted to be at least faintly positive, more often with aurora B than aurora A with predominant cytoplasmic staining. Additionally, a subset of plasma cells was also noted to be positive with aurora A and aurora B, in a cytoplasmic pattern of staining. There was no apparent correlation of plasma cell staining with their number or the morphologic diagnosis.
MLN8237 inhibits Aurora A and B activity in PTCL cell lines
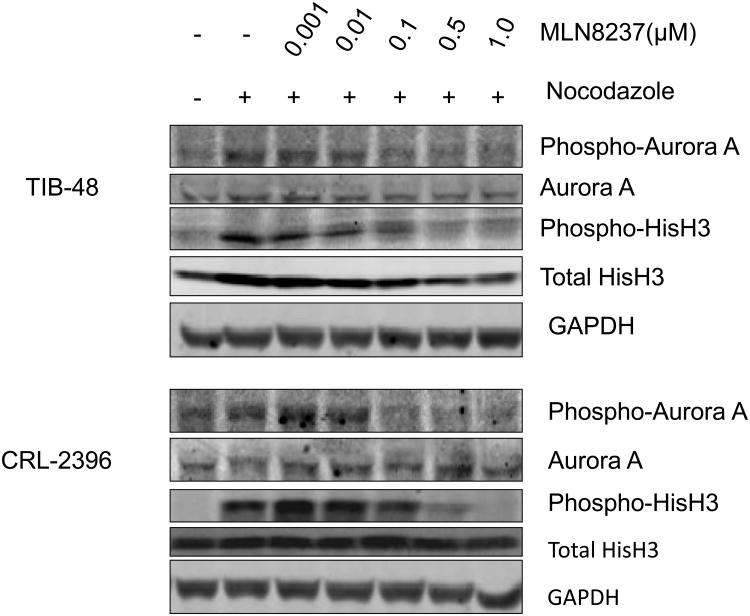
MLN8237 is a selective ATP-site competitive small molecule inhibitor with more Aurora A than B specificity in in vitro enzyme assays [18]. Exposure of MLN8237 to aggressive B-NHL cell lines induces an “Aurora inhibitory” phenotype [10]. However, no pre-clinical studies of MLN8237 have been performed in T-NHL cells. Here, we evaluated the effect of MLN8237 on Aurora A activity in two PTCL cell lines by detection of Aurora A auto-phosphorylation on Thr-288 (pT288) (Figure 2). Aurora A activity depends on auto-phosphorylation of T288 in the activation loop. TIB-48 and CRL-2396 cells were treated with nocodazole to cause a cell cycle synchrony and induce maximal phosphorylation of Aurora A on T288 reflecting increased Aurora A activity. Treatment of these cells with MLN8237 at 0.1 μM completely inhibited Aurora A auto-phosphorylation on T288. Total Aurora A protein level was unchanged upon MLN8237 treatment, indicating that the decreased pT288 was due to inhibition of phosphorylation and not Aurora A degradation or down-regulation (Figure 2). Structurally related Aurora B activity was also evaluated in these cells by detection of phosphorylated Histone H3 (pHisH3) on Ser10, a direct downstream substrate of Aurora B kinase.4 Similar to Aurora A, Aurora B activity was also suppressed by MLN8237 due to inhibition of pHisH3, while total Histone H3 protein level was unaffected (Figure 2). The inhibition pattern was dose-dependent and maximal inhibition was observed at 0.5 μM of MLN8237 (Figure 2). These data indicate that MLN8237 inhibits both Aurora A and B activity in PTCL cell lines.
Figure 2. MLN8237 potently inhibits Aurora A and B kinase activity in Peripheral T-cell Lymphoma cells.
TIB-48 and CRL-2396 cells were untreated or treated with 0.2 μg/ml nocodazole for 16hr, and then untreated or treated with the indicated concentrations of MLN8237 for 1hr. 50 μg of protein from each lysate was resolved by SDS-PAGE and immunoblotted with antibodies specific for Aurora A, histone H3, phosphorylated Aurora A (Thr288) and phosphorylated histone H3 (Ser10). GAPDH was used as a loading control.
MLN8237 induces endo-reduplication in PTCL cell lines
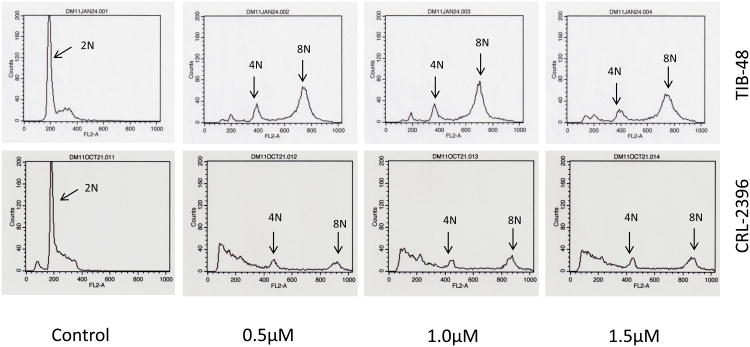
A number of publications have demonstrated that inhibition of Aurora A by siRNA knockdown or pharmacologic small molecular inhibition in tumor cells delays mitotic entry and progression, resulting in G2/M cell cycle arrest [19-22] and inhibition of Aurora B prevents cytokinesis [23-26] which leads to an endo-reduplication phenotype (polyploidy). The effect of MLN8237 on the cell cycle of PTCL cells was evaluated for DNA content using flow cytometry. Treatment of TIB-48 and CRL-2396 cells with MLN8237 at 0.5, 1.0 and 1.5 μM for 48 h dramatically increased 4N (G2/M phase) and 8N (endo-reduplication) cell population relative to control cells (Figure 3). There was a concomitant decrease in the G0/G1 phases within this population which almost completely disappears after treatment (Figure 3). Hence, there is a clear cell cycle progression effect and endo-reduplication in PTCL cells when treated with MLN8237 demonstrating a phenotype of Aurora inhibition.
Figure 3. MLN8237 induces polyploidy in Peripheral T-cell Lymphoma cell lines.
TIB-48 and CRL-2396 cells were treated with 0, 0.5, 1.0 and 1.5 μM of MLN8237 for 48 hr. Samples were harvested and stained with propidium iodide for DNA content analysis by flow cytometry. X-axis: DNA content, Y-axis: cell numbers.
MLN8237 inhibits PTCL cell proliferation and induces apoptosis
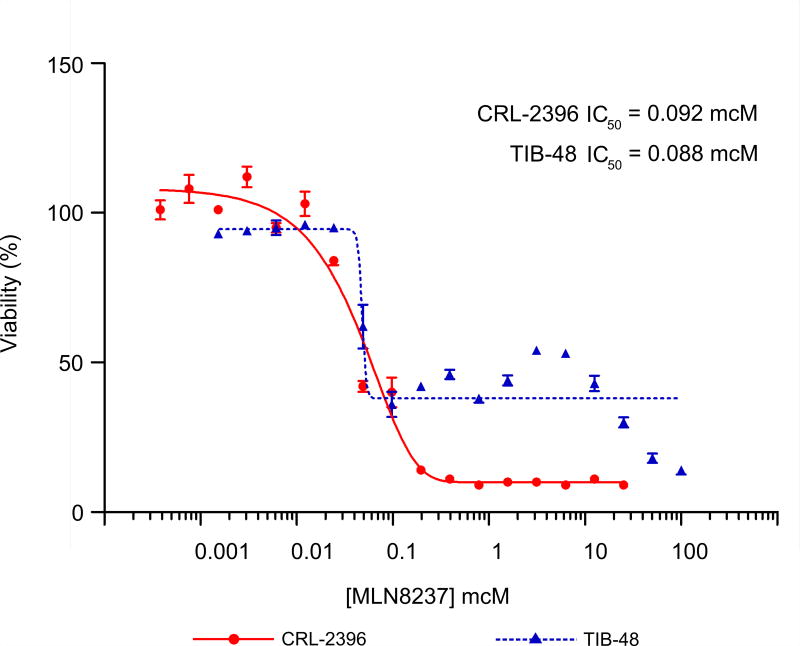
Aurora A and B have been reported to play a pivotal role in cell proliferation and survival in cancer cells [7]. To examine this in PTCL, MTS assays were performed to evaluate the growth of TIB-48 and CRL-2396 cell lines treated with MLN8237. Consistent with previous studies that inhibition of Aurora A and/or Aurora B suppresses cell proliferation, MLN8237 effectively inhibited the growth of these cells with IC50 values ranging from 80 to 100 nM (Figure 4).
Figure 4. MLN8237 inhibits cell proliferation in Peripheral T-cell Lymphoma cell lines.
TIB-48 and CRL-2396 cells were exposed to varying concentrations of MLN8237 for 4 days. Cell viability was assessed by MTS analysis. Points are the means of triplicate determinations ± SD. The IC50 of MNL8237 ranged from 80-100 nM.
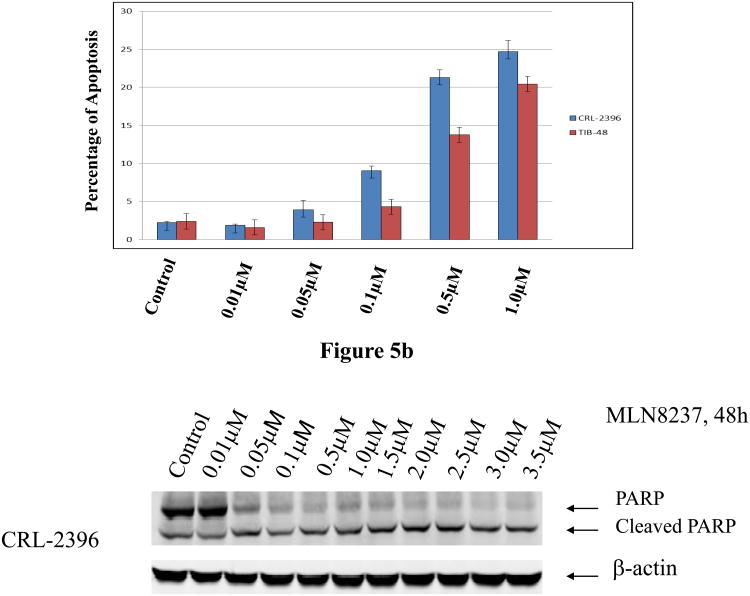
It is also known that apoptosis is induced in several cancers after aurora A or B inhibition. Flow cytometry assays following Annexin V and PI staining were used to examine apoptosis in TIB-48 and CRL-2396 cells treated with MLN8237 at 10 nM, 50 nM, 100 nM, 500 nM and 1.0 μM for 48 h. As expected, MLN8237 induced apoptosis at concentrations >100 nM, suggesting that induction of apoptosis is dose-dependent (Figure 5A). These results were confirmed by demonstrating an increased level of cleaved PARP in treated TIB-48 and CRL-2396 cells (Figure 5B). PARP cleavage was observed even at the concentration of MLN8237 as low as 50 nM. Together, the data demonstrate that Aurora A and B inhibition with MLN8237 leads to inhibition of cell proliferation and induction of apoptosis in PTCL cells.
Figure 5. Apoptosis is induced by MLN8237 in Peripheral T-cell Lymphoma cells.
(A) TIB-48 and CRL-2396 cells were treated with vehicle (control) or MLN8237 at 0.01, 0.05, 0.1, 0.5 and 1.0 μM for 48 hr. Apoptosis was detected by flow cytometric analysis based on propidium iodide and annexin V staining. The graph represents the mean percentage of apoptosis ± S.D. (n=3). (B). TIB-48 and CRL-2396 cells were treated with MLN8237 at 0.01 μM, 0.05 μM, 0.1 μM, 0.5 μM, 1.0 μM, 1.5 μM, and 2.0 μM for 48 hr. Apoptosis was evaluated by immunoblotting to detect PARP cleavage with anti-PARP antibody. β-actin was used as a loading control.
Discussion
Aurora kinases are validated oncologic targets that have attracted much attention over the past few years. Numerous ATP-site competitive Aurora SMIs are currently in early clinical development [7]. Alisertib (MLN8237) has demonstrated anti-tumor activity in a phase II study of aggressive B-and T-cell NHL [16]. Previously we demonstrated over-expression of Aurora in PTCL (NOS) by gene expression profiling [13]. More recently, gene expression profile studies on extra-nodal NK/T-cell lymphoma, nasal type (ENKTL) identified aurora A to be over-expressed. Targeted inhibition of aurora A by a SMI induced significant growth arrest in NK-cell lines, providing a rationale for evaluation of aurora inhibitors in NK-cell malignancies [14]. Here we show by Western blotting analysis that aurora A and B are expressed in T-NHL cell lines TIB-48 and CRL-2396 (Figure 1A). IHC analysis of PTCL patients for aurora A expression showed positivity in 3 of 24 samples and co-expression with aurora B. In contrast, aurora B showed strong positivity in 22 of 32 tumor samples (Figure 1B). Of the T-cell lymphoma subtypes, aurora B is over-expressed in PTCL (NOS) [73%], T-NHL (100%), ALCL (Alk-Neg) [100%] and AITL [100%] implicating a predominant aurora B expression compared to aurora A. These data will be confirmed in the ongoing SWOG S1108 trial of Alisertib (MLN8237) in relapsed/refractory PTCL, where response to therapy will be correlated with Aurora B expression.
Pre-clinical studies have shown that MLN8237 overcomes resistance to microtubule targeted agents such as taxanes and vinca alkaloids and is synergistic when combined with rituximab in aggressive B-NHL [27]. MLN8237 potently inhibits Aurora A and B activity, as measured by a decrease in Ser10 histone H3 phosphorylation and Aurora A activity by decreased auto-phosphorylation on Thr288 in T-NHL cell lines (Figure 2). These inhibitory events were associated with endo-reduplication (Figure 3). Together the data confirm that MLN8237 inhibits aurora A and B at concentrations ≥ 0.5 μM achieved clinically at 50 mg BID the maximum tolerated dose determined in early phase clinical trials [16]. Moreover, the dose at which maximal inhibition of histone H3 phosphorylation on Ser10 (0.5 μM) was five times higher than dose required to inhibit aurora A auto-phosphorylation (0.1 μM), indicating MLN8237 is more efficient in inhibiting Aurora A compared to Aurora B (Figure 2). Furthermore, MLN8237 inhibited cell proliferation of both PTCL cell lines with an IC50 ranging from 80-100 nM (Figure 4) which is consistent with inhibition of aurora A phosphorylation (Figure 2). By flow cytometry MLN8237 induced a dose dependent apoptosis of 20-25% in CRL-2396 and 18-20% in TIB-48 cell lines at ≥ 0.5 μM respectively (Figure 5A). However, PARP-cleavage analyzed at 48 hr of MLN8237 treatment was induced at 0.05 μM and completed at 0.5 μM (Figure 5B). Together, the data indicate that in PTCL, inhibition of aurora activity with MLN8237 (alisertib) leads to a dose and time dependent apoptosis at concentrations achieved in clinical trials.
Conclusions
Our findings indicate that in patients with PTCL expression of aurora B predominates over aurora A, the significance of which is under active investigation. Our data demonstrate that Alisertib (MLN8237) inhibits cell proliferation by suppressing aurora A and B activity, induces endo-reduplication and subsequent apoptosis in T-NHL cell lines. A phase II study is ongoing evaluating the efficacy of Alisertib (MLN8237) in relapsed/refractory PTCL (SWOG S1108).
Acknowledgments
We wish to thank the Lymphoma SPORE (1 P5O CA B080501A1) for funding this project and the SWOG tissue bank for providing PTCL samples for analysis from S0350. We also wish to thank Millennium: The Takeda Oncology Company (Boston, MA) for providing Alisertib (MLN8237).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foss FM, Zinzani PL, Vose JM, Gascoyne RD, Rosen ST, Tobinai K. Peripheral T-cell lymphomas. Blood. 2011;117(25):6756–67. doi: 10.1182/blood-2010-05-231548. [DOI] [PubMed] [Google Scholar]
- 2.Dunleavy K, Piekarz RL, Zain J, Janik JE, Wilson WH, O'Connor OA, Bates SE. New strategies in peripheral T-cell lymphoma: understanding tumor biology and developing novel therapies. Clin Cancer Res. 2010;16(23):5608–17. doi: 10.1158/1078-0432.CCR-09-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage JO. The aggressive peripheral T-cell lymphomas: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87(5):511–9. doi: 10.1002/ajh.23144. [DOI] [PubMed] [Google Scholar]
- 4.Savage KJ. Therapies for peripheral T-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2011;2011:515–24. doi: 10.1182/asheducation-2011.1.515. [DOI] [PubMed] [Google Scholar]
- 5.Roncolato F, Gazzola A, Zinzani PL, Pileri SA, Piccaluga PP. Targeted molecular therapy in peripheral T-cell lymphomas. Expert Rev Hematol. 2011;4(5):551–62. doi: 10.1586/ehm.11.55. [DOI] [PubMed] [Google Scholar]
- 6.Mahadevan D, Fisher RI. Novel therapeutics for aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2011;29(14):1876–84. doi: 10.1200/JCO.2010.32.7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green MR, Woolery JE, Mahadevan D. Update on Aurora Kinase Targeted Therapeutics in Oncology. Expert Opin Drug Discov. 2011;6(3):291–307. doi: 10.1517/17460441.2011.555395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musacchio A. Spindle assembly checkpoint: the third decade. Philos Trans R Soc Lond B Biol Sci. 2011;366(1584):3595–604. doi: 10.1098/rstb.2011.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21(3):133–40. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi W, Cooke LS, Liu X, Rimsza L, Roe DJ, Manziolli A, et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochem Pharmacol. 2011;81(7):881–90. doi: 10.1016/j.bcp.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakhoum SF, Compton DA. Chromosomal instability and cancer: a complex relationship with therapeutic potential. J Clin Invest. 2012;122(4):1138–43. doi: 10.1172/JCI59954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikezoe T, Takeuchi T, Yang J, Adachi Y, Nishioka C, Furihata M, et al. Analysis of Aurora B kinase in non-Hodgkin lymphoma. Lab Invest. 2009;89(12):1364–73. doi: 10.1038/labinvest.2009.106. [DOI] [PubMed] [Google Scholar]
- 13.Mahadevan D, Spier C, Della Croce K, Miller S, George B, Riley C, et al. Transcript profiling in peripheral T-cell lymphoma, not otherwise specified, and diffuse large B-cell lymphoma identifies distinct tumor profile signatures. Mol Cancer Ther. 2005;4(12):1867–79. doi: 10.1158/1535-7163.MCT-05-0146. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal J, Weisenburger DD, Chowdhury A, Tsai MY, Srivastava G, Greiner TC, et al. Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic γδ T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro. Leukemia. 2011;25(2):348–58. doi: 10.1038/leu.2010.255. [DOI] [PubMed] [Google Scholar]
- 15.Tomita M, Mori N. Aurora A selective inhibitor MLN8237 suppresses the growth and survival of HTLV-1-infected T-cells in vitro. Cancer Sci. 2010;101(5):1204–11. doi: 10.1111/j.1349-7006.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedberg J, Mahadevan D, Jung J, Persky DO, Lossos IS, et al. Phase 2 Trial of Alisertib (MLN8237), An Investigational, Potent Inhibitor of Aurora A Kinase (AAK), in Patients (pts) with Aggressive B- and T-Cell Non-Hodgkin Lymphoma (NHL). American Society of Hematology, #95, 53rd Annual Meeting; December 2011. [Google Scholar]
- 17.Mahadevan D, Unger JM, Spier CM, Persky DO, Young F, Leblanc M, et al. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer. 2012 Jul 25; doi: 10.1002/cncr.27733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinel S, Barbault-Foucher S, Lott-Desroches MC, Astier A. Inhibitors of aurora kinases. Ann Pharm Fr. 2009;67:69–77. doi: 10.1016/j.pharma.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Hata T, Furukawa T, Sunamura M, Egawa S, Motoi F, Ohmura N, et al. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res. 2005;65(7):2899–905. doi: 10.1158/0008-5472.CAN-04-3981. [DOI] [PubMed] [Google Scholar]
- 20.Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114(5):585–98. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Ruderman JV. Aurora A, mitotic entry, and spindle bipolarity. Proc Natl Acad Sci. 2006;103(15):5811–6. doi: 10.1073/pnas.0601425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marumoto T, Hirota T, Morisaki T, Kunitoku N, Zhang D, Ichikawa Y, et al. Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells. 2002;7(11):1173–82. doi: 10.1046/j.1365-2443.2002.00592.x. [DOI] [PubMed] [Google Scholar]
- 23.Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161(2):267–80. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161(2):281–94. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honda R, Körner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14(8):3325–41. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murata-Hori M, Wang YL. The kinase activity of aurora B is required for kinetochore-microtubule interactions during mitosis. Curr Biol. 2002;12(11):894–9. doi: 10.1016/s0960-9822(02)00848-5. [DOI] [PubMed] [Google Scholar]
- 27.Mahadevan D, Stejskal A, Cooke LS, Manziello A, Morales C, Persky DO, et al. Aurora A inhibitor (MLN8237) plus vincristine plus rituximab is synthetic lethal and a potential curative therapy in aggressive B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2012;18(8):2210–9. doi: 10.1158/1078-0432.CCR-11-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]