Abstract
Lung cancer is the primary cause of cancer-related mortality worldwide and although improvements in treatment have been achieved over the last few years, long-term survival rates for lung cancer patients remain poor. Therefore, there is an imperative need for molecularly targeted agents that will achieve long-term disease control. Numerous downstream molecular pathways, such as EGF/RAS/RAF/MEK/ERK and PI3K/AKT/mTOR are identified as having a key role in the pathogenesis of various forms of human cancer, including lung cancer. PI3K/AKT/mTOR signal pathway is an important intracellular signal transduction pathway with a significant role in cell proliferation, growth, survival, vesicle trafficking, glucose transport, and cytoskeletal organization. Aberrations in many primary and secondary messenger molecules of this pathway, including mutations and amplifications, are accounted for tumor cell proliferation, inhibition of apoptosis, angiogenesis, metastasis and resistance to chemotherapy-radiotherapy. In this review article, we investigate thoroughly the biological role of PI3K pathway in lung cancer and its contribution in the development of future therapeutic strategies.
Keywords: lung cancer, PI3K pathway inhibitors, molecular pathways
1. Introduction
Lung cancer is still the leading cause of cancer-related mortality worldwide. It was estimated that in the United States alone, more than 220,000 new cases and 157,000 deaths occurred due to lung cancer in 2010 [1]. Lung cancer can be classified into two main subtypes: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) with many differences found between these two subtypes concerning the histological type, biological behavior, prevalence, prognosis and response to therapy.
Non-small cell lung cancer accounts for more than 80% of all lung cancer cases. The most common histological types are adenocarcinoma (AC), squamous cell carcinoma (SCC) and large cell lung carcinoma (LCLC). Only a small percentage of patients with NCLC will show early stage disease at the time of presentation and surgery remains the best therapeutic option for these patients [2]. The majority of NSCLC patients are diagnosed at advanced stage with inoperable locally advanced tumors or metastatic disease and the treatment is mainly focused on controlling the disease and sustain life quality, and commonly includes a combination of radio and chemotherapy. However, commonly administered chemotherapy provides no radical treatment for patients with advanced stage disease and has reached a plateau in efficacy with a median survival of 8–10 months [3].
Small cell lung cancer accounts for approximately 13% of all lung cancer cases and is highly associated with tobacco smoking [4,5]. A combination of a platinum agent (cisplatin or carboplatin) and etoposide and in some cases also radiotherapy is the mainstay method in management of SCLC patients [5,6] and although most patients initially respond to chemotherapy, disease recurrence is the most probable outcome and consequently the overall 5-year survival in patients with SCLC is less than 5% [6].
Even though major progress in the understanding of cancer biology and treatment of lung cancer has been achieved over the last few years, the survival rates for both NSCLC and SCLC patients is still disappointing [6,7]. The deregulation of many signaling pathways such as EGF/RAS/RAF/MEK/ERK and PI3K/AKT/mTOR is considered to play a critical role in oncogenesis and cancer progression [8]. Therefore, numerous components of these survival pathways may act as potential molecular targets for cancer treatment and the addition of new targeted agents with better tolerability, availability for chronic treatment and better selectivity to conventional chemotherapy has already produced definitive results. Novel therapeutic approaches are urgently needed for this common disease.
2. PI3K Signaling Pathway
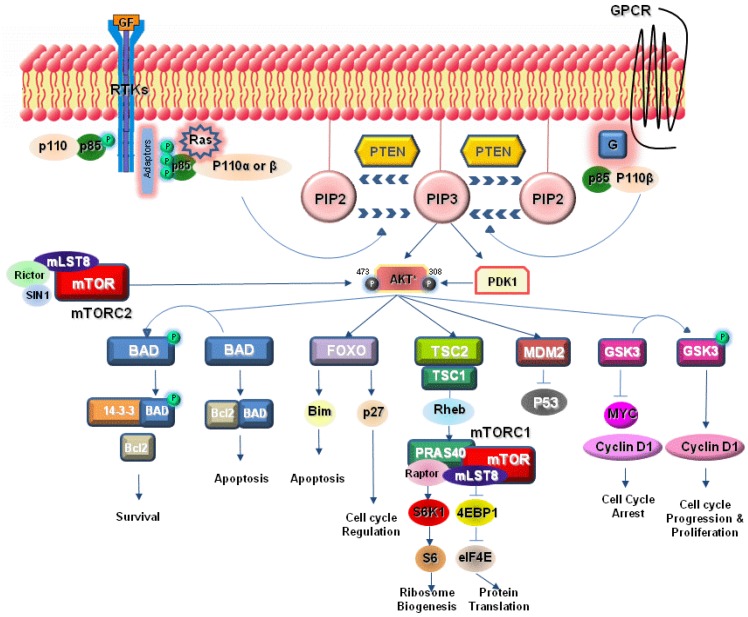
The phosphoinositide-3-kinase (PI3K) signaling pathway has a critical role in cell growth and survival [9]. Alterations of the PI3K/AKT/mTOR pathway can occur at many levels resulting in PI3K activation and malignant transformation. The PI3Ks are lipid kinases, which can be grouped into three classes based on their structure and function. Class IA PI3K is most closely related to human cancer [10]. Class IA PI3Ks are heterodimers consisting of a regulatory subunit (p85) and a catalytic subunit (p110). Three genes PIK3R1, PIK3R2 and PIK3R3 encode p85α, p85β and p85γ regulatory subunits whereas catalytic isoforms p110α, p110β, and p110δ are the products of three genes PIK3CA, PIK3CB and PIK3CD respectively [10,11,12]. Class IA PI3Ks are usually activated by receptors tyrosine kinase (RTKs) such as EGFR, IGF1-R and HER2/neu [13,14,15,16] and activation often occurs through recruitment of the enzymes to cell membranes via phosphotyrosine binding of the Src-homology 2 (SH2) domains present in the p85 regulatory subunit to the cytoplasmic domains of RTKs. PI3K can be activated also by Ras, which directly binds p110 [17] and the p110β catalytic subunit can be additionally regulated by G-protein coupled receptors [12]. Subsequently, the second messenger phosphatidylinositol-3,4,5-triphosphate (PIP3) is produced through phosphorylation by the activated PI3K of phosphatidyl-inositol-4,5-biphosphate (PIP2). The phosphatase and tensin homolog deleted on chromosome 10 (PTEN) dephosphorylates PIP3 to PIP2, acting thereby as a direct antagonist of PI3K. PIP3 transduces intracellular signaling by directly binding pleckstrin homology (PH) domains of various proteins [16], participating thus in the regulation of cell proliferation and survival, cytoskeletal organization, vesicle trafficking, cell adhesion and motility, angiogenesis and glucose transport [18,19]. Two such PH domain-containing kinases, phosphoinositide-dependent kinase 1 (PDK1) and the serine threonine kinase Akt, are recruited to the membrane via PIP3, where PDK1 activates Akt by phosphorylation at the threonine 308 [20,21]. Mammalian target of rapamycin complex 2 (mTORC2) contributes to the complete activation of Akt via phosphorylation at serine 473. Activated Akt promotes cell growth and survival with various mechanisms. Akt inhibits proapoptotic Bcl-2 family members BAD and BAX [11,16], phosphorylates forkhead box O transcription factors (FoxO), the glycogen synthase kinase 3 (GSK3) and negatively regulates the transcription factor NF-κB, leading to increased expression of antiapoptotic and cell survival signals [22]. The Akt-mediated phosphorylation of TSC2 protein, which combined with TSC1 protein forms a Ras homologue enriched in brain (Rheb) inhibiting complex, allows Rheb to be released and activated. Rheb then stimulates the mammalian target of rapamycin complex 1 (mTORC1), which phosphorylates the p70S6 kinase (S6K1) and the eukaryotic initiation factor 4E binding protein 1 (4EBP1), leading to increased protein synthesis (Figure 1).
Figure 1.
The PI3K/Akt/mTOR signaling pathway.
3. Activation of PI3K Pathway in Lung Cancer
The PI3K pathway is frequently deregulated in lung cancer due to genetic alterations affecting one of its components resulting in increased PI3K signaling [23]. PI3K activation frequently occurs in response to activating mutation and/or amplification of receptors tyrosine kinase (RTK’s), amplification of PI3K, loss or inactivation of PTEN, overexpression of downstream kinase Akt, mutational activation of the PIK3CA gene encoding the p110a catalytic subunit and activation by mutant forms of the Ras oncogene [10,24].
Activating mutations in PIK3CA gene have been described in several tumor types [10,25,26,27,28], and have been usually identified in two key regions in exons 9 (that encodes the helical domain of p110a) and 20 (that encodes the catalytic domain of p110a) [25,27]. However, such somatic mutations are relatively infrequent in lung cancer and appear only in about 5% of NSCLC cell lines [29] and 23% of SCLC cell lines [30]. Transgenic mice, in which mutant p110a was lung-specific induced, carrying mutations in exon 20, developed lung adenocarcinomas [31]. Additionally, there are studies suggesting that the presence of these mutations may be responsible for resistance to agents targeting RTKs [32,33]. Genomic amplification of PIK3CA was also identified in a large number of NSCLC tumors and pre-invasive lesions [34]. Yamamoto et al. were not able to identify PIK3CA mutations in SCLC cell lines, but reported PIK3CA copy number gains, associated with increased expression of activated Akt, also identified in 33.1% of squamous cell lung cancer and 6.2% of lung adenocarcinomas [29]. Another study reported PIK3CA gene copy number gain in 76% of SCLC tumors and 54% of SCLC cell lines [35].
Overexpression of the downstream kinase Akt may also result in the PI3K pathway activation. Mutations in AKT1, AKT2, AKT3 genes have been identified in various forms of human cancer but only a limited number of NSCLC tumors harbor mutations in the AKT2 gene responsible for oncogenesis [36], leading to the assumption that the deregulation of the pathway is probably located at a post-transcriptional level. Overactivation of Akt has been reported in NSCLC cell lines, and was closely related to chemo and radioresistance [37], and also in pre-malignant and malignant human bronchial epithelial cells, but not in normal tissue [38]. Activated Akt was also traced in primary NSCLC tumors and was suggested to be a poor prognostic factor for patients with early stage NSCLC [39,40]. Mutant AKT1 gene was reported in 64% of SCLC tumors and 39% of SCLC cell lines [35]. In other studies, high levels of activated Akt were detected in SCLC tumor tissue samples, suggesting the key role of PI3K pathway in disease progression [34,41]. The presence of phosphorylated Akt in SCLC cells that initially developed chemoresistance, supported the hypothesis that activated Akt may be involved in mechanisms responsible for increased chemo and radioresistance [42].
The most common genetic alteration of the PI3K pathway observed in human cancer is deletion or down-regulated expression of the tumor suppressor gene PTEN. PTEN acting as a direct antagonist of PI3K, negatively regulates PI3K pathway. Homozygous or hemizygous deletions of PTEN and missense mutations may result in increased activation of the PI3K pathway and are frequently observed in many cancer types [43,44,45], but are not very frequent in NSCLC [46,47,48,49]. However, partial or complete loss of PTEN protein expression is frequently observed in lung cancer [50,51]. Transcriptional repression and epigenetic silencing of PTEN, commonly through promoter lypermethylation has been described as mechanism of PTEN inactivation in several studies [52,53].
Another downstream regulator of the PI3K pathway, the mammalian target of rapamycin (mTOR), was found to be mutated in more than 30% of 188 lung adenocarcinomas [23] and is also frequently activated in lung cancer cell lines, and especially in these harboring genetic mutations [54,55,56]. There are studies correlating the activation of mTOR with tumor progression and metastatic potential in KRAS-mutated NSCLC models [57]. Anagnostou et al. thus reported a better outcome for patients with early stage lung adenocarcinoma that overexpressed mTOR [58].
In many human cancers, RTK’s are often mutated, amplified or overexpressed, resulting in PI3K overactivation. Lung cancers with somatic mutations in epithelial growth factor receptor (EGFR), show EGFR-induced activation of PI3K pathway [31]. When RTK’s-targeted therapies are effective, consequently, PI3K activation is lost and cell death is induced. On the other hand, NSCLC cell lines indicating resistance to tyrosine kinase inhibitor gefitinib, have showed increased levels of PI3K activation [5,59]. Therefore, targeting the PI3K pathway in EGFR-mutant lung cancer showing resistance to TKIs was suggested to be a promising approach [60,61]. Indeed, the class I PI3K-mTOR inhibitor PI-103 was able to induce apoptosis in EGFR-mutant lung cancer cells that initially showed hepatocyte growth factor (HGF)-induced resistance to EGFR-TKIs and in combination with the EGFR inhibitor gefitinib, halt the tumor growth in murine xenograft models [62].
Mutations of the Ras oncogene are also frequently observed in human cancer [63,64]. The GTPase Ras directly bounds p110a subunit resulting in PI3K activation [65]. Mutant p110a subunit, lacking the ability to interact with Ras, inhibited K-Ras-induced lung adenocarcinomas in mice [66]. Moreover, Engelman et al. showed that deletion of Pikr1 and Pikr2 was able to halt K-Ras G12D-induced lung oncogenesis [31]. Even though PI3K pathway activation seems to have a key role in K-Ras-induced carcinogenesis, preclinical data suggest that inhibiting PI3K signaling alone may not be entirely effective against K-Ras mutant cancer cell lines or tumors [31,67].
4. PI3K Pathway Inhibitors in Lung Cancer
Although major progress in the treatment of lung cancer has been achieved over the last few decades, it still remains the cancer type with the highest mortality [1]. Therefore, the need for new therapeutic options with less toxicity, better selectivity and higher effectiveness rises significantly. Small molecule inhibitors (tyrosine kinase inhibitors, TKIs) targeting numerous downstream components of intracellular signal transduction pathways, are prominent therapeutic approaches that have already reached the clinical stage. Targeting PI3K signaling pathway and its downstream mediators is still in early stage, but has already showed promising results and is rapidly processing. In this section, we describe PI3K pathway inhibitors that have reached clinical trials for the treatment of lung cancer, considering four different categories: PI3K inhibitors, dual PI3K- mTOR inhibitors, Akt inhibitors and mTOR inhibitors.
4.1. PI3K Inhibitors
The natural product wortmannin and its derivative LY294002 are pan-Class I inhibitors [68,69]. As aforementioned, Akt-mediated activation of the PI3K pathway has been associated with chemo and radioresistance in NSCLC [37]. Even though both compounds were able to increase chemo- and radiosensitivity in NSCLC and SCLC cell lines [37,70,71], they are considered too toxic for human use and have not reached the clinical stage.
PX-866 is also a pan-Class I inhibitor that has the ability to bind PI3K irreversibly [72]. Ihle et al. reported the ability of PX-866 to demonstrate antitumor activity in vivo against a variety of cancer cell lines [72,73]. Interestingly, cancer cell lines harboring PIK3CA mutations or PTEN loss appeared to be more sensitive to PX-866 [67]. The major toxicity reported was hyperglycemia and decreased glucose tolerance which could be overcome when treated with the antidiabetic agent pioglitazone [74]. In mouse models of oncogenic KRAS-induced lung cancer, PX-866 was able to halt PI3K-induced bronchioalveolar stem cell expansion [75]. The agent is about to enter a Phase I study to determine the maximally tolerated dose (MTD) in combination with docetaxel in patients with solid tumors and a Phase II study to determine the efficacy of PX-866 in combination with docetaxel in patients with NSCLC or Squamous Cell Carcinoma of the Head and Neck (SCCHN) [76] (Table 1).
Table 1.
Ongoing trials with PI3K pathway inhibitors in the treatment of lung cancer.
| Title | Phase | Protocol ID | Cancer type | Compounds | Mechanism |
|---|---|---|---|---|---|
| Study of PX-866 and Docetaxel in Solid Tumors [76] | Phase I + Phase II | NCT01204099 | Solid tumors(NSCLC) | PX-866 + Docetaxel | PI3K inhibitor |
| A Study of the Safety and Pharmacology Of PI3-Kinase Inhibitor GDC-0941 In Combination With Either Paclitaxel And Carboplatin (With or Without Bevacizumab) or Pemetrexed, Cisplatin, And Bevacizumab in Patients With Advanced Non Small Cell Lung Cancer [80] | Phase I | NCT00974584 | NSCLC | GDC-0941 + Paclitaxel + Carboplatin(with or without Bevacizumab) or Pemetrexed + Cisplatin + Bevacizumab | PI3K inhibitor |
| A Study of the Safety and Pharmacology of GDC-0941 in Combination With Erlotinib in Patients With Advanced Solid Tumors [81] | Phase I | NCT00975182 | Solid tumors | GDC-0941 + Erlotinib | PI3K inhibitor |
| Study Evaluating the Safety and Efficacy Of Carboplatin/Paclitaxel And Carboplatin/Paclitaxel/Bevacizumab With and Without GDC-0941 in Patients With Previously Untreated Advanced Or Recurrent Non-small Cell Lung [82] | Phase II | NCT01493843 | NSCLC | Carboplatin + Paclitaxel or Carboplatin + Paclitaxel + Bevacizumab with and without GDC-0941 | PI3K inhibitor |
| Safety Study of XL147 (SAR245408), in Combination With Paclitaxel and Carboplatin in Adults With Solid Tumors [84] | Phase I | NCT00756847 | Solid tumors | XL-147 + Paclitaxel + Carboplatin | PI3K inhibitor |
| A Trial of Gefitinib in Combination With BKM120 in Patients With Advanced Non-Small Cell Lung Cancer, With Enrichment for Patients Whose Tumors Harbour Molecular Alterations of PI3K Pathway and Known to Overexpress EGFR [88] | Phase I | NCT01570296 | NSCLC | BKM120 + Gefitinib | PI3K inhibitor |
| Trial of Erlotinib and BKM120 in Patients With Advanced Non Small Cell Lung Cancer Previously Sensitive to Erlotinib [89] | Phase I+Phase II | NCT01487265 | NSCLC | BKM120 + Erlotinib | PI3K inhibitor |
| Safety and Efficacy of BKM120 in Patients With Metastatic Non-small Cell Lung Cancer [90] | Phase II | NCT01297491 | NSCLC | BKM120 + Docetaxel orDocetaxel + Pemetrexed | PI3K inhibitor |
| A Phase I Study of BKM120 and Everolimus in Advanced Solid Malignancies [91] | Phase I | NCT01470209 | Solid tumors | BKM120 + Everolimus | PI3K inhibitor |
| BKM120 in Cancers With PIK3CA Activating Mutations [92] | Phase II | NCT01501604 | Solid tumors with PIK3CA mutations | BKM120 | PI3K inhibitor |
| Dose Defining Study For MK-2206 Combined With Gefitinib In Non Small Cell Lung Cancer (NSCLC) [113] | Phase I | NCT01147211 | NSCLC | MK-2206 + Gefitinib | Akt inhibitor |
| MK2206 and Erlotinib Hydrochloride in Treating Patients With Advanced Non-Small Cell Lung Cancer Who Have Progressed After Previous Response to Erlotinib Hydrochloride Therapy [114] | Phase II | NCT01294306 | NSCLC | MK-2206 + Erlotinib | Akt inhibitor |
| Temsirolimus and Pemetrexed for Recurrent or Refractory Non-Small Cell Lung Cancer [136] | Phase I + Phase II | NCT00921310 | NSCLC | Temsirolimus + Pemetrexed | mTOR inhibitor |
| Temsirolimus and Vinorelbine Ditartrate in Treating Patients With Unresectable or Metastatic Solid Tumors [137] | Phase I | NCT01155258 | Solid tumors | Temsirolimu + Vinorelbine | mTOR inhibitor |
| Phase I Study of Docetaxel and Temsirolimus in Resistant Solid Malignancies [138] | Phase I | NCT00703625 | Solid tumors | Temsirolimus + Docetaxel | mTOR inhibitor |
| Phase 1b Trial of RAD001 in Patients With Operable Non-Small Cell Lung Cancer (NSCLC) [154] | Phase I | NCT00401778 | NSCLC | Everolimus | mTOR inhibitor |
| Combination of RAD001 With Carboplatin, Paclitaxel and Bevacizumab in Non-small-cell Lung Cancer (NSCLC) Patients [155] | Phase I | NCT00457119 | NSCLC | Everolimus + Carboplatin + Paclitaxel + Bevacizumab | mTOR inhibitor |
| RAD001 With Paclitaxel and Carboplatin in First Line Treatment of Patients With Advanced Large Cell Lung Cancer With Neuroendocrine Differentiation [160] | Phase II | NCT01317615 | LCLC | Everolimus + Paclitaxel + Carboplatin | mTOR inhibitor |
| Combination Anticancer Therapy of Paclitaxel and Everolimus for Relapsed or Refractory Small Cell Lung Cancer [161] | Phase I | NCT01079481 | SCLC | Everolimus + Paclitaxel | mTOR inhibitor |
| Everolimus, Carboplatin, and Etoposide in Treating Patients With Small Cell Lung Cancer or Other Advanced Solid Tumors [162] | Phase I | NCT00807755 | SCLC(Solid tumors) | Everolimus + Carboplatin + Etoposide | mTOR inhibitor |
| Safety of RAD001 in Combination With Cisplatin and Etoposide in Lung Cancer Patients [163] | Phase I | NCT00466466 | SCLC | Everolimus + Cisplatin + Etoposide | mTOR inhibitor |
| A Study of Ridaforolimus in Non-Small Cell Lung Cancer (NSCLC) Patients With Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) Mutations (MK-8669-021 AM1) [170] | Phase II | NCT00818675 | NSCLC | Ridaforolimus | mTOR inhibitor |
| Ridaforolimus With Cetuximab for Patients With Advanced Head and Neck Cancer, Non-Small Cell Lung Cancer and Colon Cancer [171] | Phase I | NCT01212627 | Solid tumors | Ridaforolim + Cetuximab | mTOR inhibitor |
GDC-0941 is a pan-Class I inhibitor that derives from the pyridofuropyrimidine scaffold and shows high oral availability [77]. It is quickly absorbed and has moderate to long half-life as was shown in a Phase I study [78]. It has already entered clinical trials in patients with solid tumors, with three out of 19 patients showing some level of antitumor activity, and grade 3 headache and pleural effusion to be the dose limiting toxicities (DLTs) observed [79]. The compound will soon enter a Phase I study in combination with paclitaxel and carboplatin (with or without bevacizumab) or pemetrexed, cisplatin, and bevacizumab in patients with advanced NSCLC [80] and in combination with the EGFR inhibitor erlotinib in patients with advanced solid tumors [81]. A Phase II study has already been designed to evaluate the safety and efficacy of GDC-0941 in combination with carboplatin-paclitaxel or carboplatin-paclitaxel and bevacizumab in patients with previously untreated advanced or recurrent NSCLC [82]. (Table 1)
XL-147 (SAR245408) is a pan-Class I inhibitor with long half-life and prolonged absorption. In a Phase I study in patients with solid tumors treated orally once a day with XL-147, thirteen patients remained on trial for more than 16 weeks and one patient with NSCLC showed partial response (PR) by RECIST criteria. The compound demonstrated reduction in PI3K and MEK/ERK pathway signaling and prolonged stable disease. Grade 3 rash and grade 4 arterial thrombosis were the serious adverse events (SAEs) observed in the trial, with skin rash to be the most common drug-related toxicity [83]. Currently, the entity is in a Phase I study in combination with paclitaxel and carboplatin in adults with solid tumors including NSCLC patients [84] (Table 1) and has already completed a Phase I study in combination with the EGFR inhibitor erlotinib in patients with solid tumors in which 8 patients with NSCLC were enrolled. The combination of XL-147 and erlotinib was well tolerated (rash, nausea, diarrhea, fatigue and vomiting were the most frequently observed toxicities) in dose levels up to 400 mg XL147 and 150 mg erlotinib daily and showed parallel inhibition of PI3K and EGFR signaling [85].
NVP-BKM120 is a highly specific, orally available pan-Class I PI3K inhibitor that has already completed a Phase I dose-escalation study in patients with advanced malignancies. In this study, thirty-five patients were enrolled and orally treated with NVP-BKM120 daily. The MTD was set at 100 mg/day. The most frequent drug-related adverse events were rash, hyperglycemia, diarrhea, anorexia, and mood alteration. Seven of 35 patients remained in the study for over 8 months and the entity was able to present preliminary antiproliferative activity [86]. The combination of NVP-BKM120 with the mTOR inhibitor rapamycin resulted in synergistic growth inhibition in NSCLC cell lines. Moreover, NVP-BKM120 when combined with the mTOR inhibitor RAD001 (Everolimus), managed to inhibit the growth of lung cancer cells in vitro and also in murine lung cancer xenograft models [87]. Currently, NVP-BKM120 is in a Phase I study with the EGFR inhibitor gefitinib in patients with advanced NSCLC particularly enriched with patients harboring alterations of the PI3K pathway and overexpress EGFR [88] and also in a Phase I/II trial with the EGFR inhibitor erlotinib in patients previously sensitive to erlotinib [89]. NVP-BKM120 is undergoing a Phase II study with docetaxel or docetaxel and pemetrexed in patients with metastatic NSCLC [90] and a Phase I study in combination with the mTOR inhibitor everolimus in patients with advanced solid tumors [91]. Lastly, a Phase II study of NVP-BKM120 is currently being conducted in patients with PIK3CA activating mutations [92] (Table 1).
4.2. Dual PI3K-mTOR Inhibitors
Chemical compounds that have the ability to inhibit both mTOR and the p110 catalytic subunits are termed dual PI3K- mTOR inhibitors. These inhibitors have the possible advantage of multi-blocking the PI3K pathway, even though it is still unclear if they can effectively inhibit all p110 isoforms and mTORC1- mTORC2 in doses tolerable for clinical use.
NVP-BEZ235 is an imidazo-quinoline derivative, orally available, that belongs to the family of dual PI3K-mTOR inhibitors [93,94]. It was the first entity of this class to enter Phase I studies in patients with advanced solid tumors (many patients with breast cancer were enrolled) in which NVP-BEZ235 showed efficacy and anti-tumor activity [95]. NVP-BEZ235 was able to achieve a decrease in cell proliferation and G1 cell cycle arrest in a variety of cancer cell lines and halt further tumor growth in xenograft models of these cancer types [31,93,96,97]. Compared to mTORC1 inhibitor rapamycin, NVP-BEZ235 was more efficient in blocking tumor cell growth [98]. Moreover, NVP-BEZ235 was able to show anti-tumor efficacy in vitro and in vivo and also increase radiosensitivity in KRAS-mutant NSCLC cell lines [96]. Another study with genetically engineered mice demonstrated that even though the compound, as single-agent, failed to inhibit murine KRAS-mutant lung tumors, when combined with a MEK inhibitor (ARRY-142886) resulted in tumor shrinkage [31]. In the same study, NVP-BEZ235 was highly effective at shrinking a murine lung adenocarcinoma with a somatic mutation in the p110α kinase domain (H1047R) [31]. These results led to the assumption that lung cancer tumors harboring PIK3CA mutations could benefit from the inhibition of PI3K signaling and the combination of both PI3K and MEK inhibitors might show efficacy in KRAS-mutant lung cancers [31]. Sos ML et al. using a panel of NSCLC cell lines, confirmed that tumors with activating mutations in RTKs present high dependence on PI3K signaling and mutations in the RAS/RAF pathway is strongly correlated with MAPK signaling [99]. In another study, EGFR-mutant NSCLC models did not respond to single-agent NVP-BEZ235, but when combined with a MEK inhibitor (AZD6244), tumor regression could be observed suggesting the key role of simultaneous PI3K and MEK inhibition in lung cancers with EGFR mutations [100].
XL-765 (SAR245409) is another dual PI3K-mTOR inhibitor that recently completed a Phase I dose-escalation study in patients with advanced solid tumors. The compound was orally administered and well tolerated with elevated hepatic enzymes, with nausea and diarrhea being the most frequent drug-related adverse events (AEs). No partial responses were observed, but five of 36 patients presented stable disease, one of them with NSCLC. Evidence of 60% to 90% pathway inhibition was found in hair and skin [101]. The combination of XL-765 with the EGFR inhibitor erlotinib was tested in a Phase I study in 21 patients with advanced solid tumors, including 14 patients with NSCLC. The combination was well tolerated in doses up to 50 mg XL-765 and 100 mg erlotinib with skin and subcutaneous tissue disorders (including rash) and diarrhea to be the most commonly observed treatment-related adverse events (AEs) and showed satisfactory dual PI3K and EGFR signaling inhibition [102].
Another dual PI3K-mTOR inhibitor PI-103, a pyridinylfuranopyrimidine compound, was able to induce apoptosis in NSCLC cell lines with resistance to EGFR inhibitor gefitinib [103]. Cell lines harboring activating mutations of the PIK3CA gene were more sensitive than wild-type PIK3CA cancer cell lines [103]. Moreover, PI-103 increased sensitivity to radiation in tumor cells in vitro [104] and caused vascular normalization in murine xenograft models in vivo [105]. The entity is still under clinical evaluation.
4.3. Akt Inhibitors
Akt inhibitors are chemical agents based on staurosporine and derivatives that have the ability to block the serine/threonine kinase Akt, crucial component of the PI3K pathway [106,107]. However, preclinical data suggest that parallel inhibition of both Akt1 and Akt2 could result in peripheral insulin resistance and drug-induced, dose-dependent hyperglycemia and hyperinsulinemia [108]. In several studies using mouse models, Akt inhibitors have been implicated for causing hyperglycemia [109,110], supporting the hypothesis of having a key role in insulin signaling and glucose homeostasis. Therefore, concerns have been raised for possible limitation in the therapeutic applications of Akt inhibitors due to metabolic toxicities.
MK-2206 is an orally administered pan-AKT kinase inhibitor presenting high selectivity for Akt. Preclinical data demonstrated the ability of MK-2206 to inhibit cancer cell proliferation when combined with cytotoxic agents (such as docetaxel, doxorubicin, gemcitabine, 5-FU and carboplatin) or targeted therapeutic agents (such as erlotinib or lapatinib) [111]. In a Phase I study, thirty-three patients with solid tumors were treated with MK-2206, establishing the MTD at 60 mg. One patient with pancreatic adenocarcinoma presented 60% reduction in cancer antigen 19-9 levels and 23% tumor shrinkage. Skin rash, nausea, pruritus, hyperglycemia and diarrhea were the most commonly observed adverse events [112]. Currently, a dose-escalation Phase I study of MK-2206 combined with the EGFR-TKI gefitinib is being conducted in patients with NSCLC, particularly enriched with patients harboring EGFR mutations [113]. Also, a Phase II study of MK-2206 and the EGFR inhibitor erlotinib is currently recruiting patients with NSCLC who have progressed after previous response to erlotinib, in order to assess the safety of the drug combination [114] (Table 1). Other Akt inhibitors such as A-443654 and GSK690693 are currently under clinical evaluation [109,115].
4.4. mTOR Inhibitors
Compounds targeting the mTOR pathway can be grouped into two main subtypes: the allosteric mTOR inhibitors (like rapamycin and its derivatives) and the ATP-competitive mTOR inhibitors.
Rapamycin (sirolimus, Rapamune®), a macrolide isolated from Streptomyces hygroscopicus, is an allosteric inhibitor of the mTORC1 complex (but not mTORC2) with antifungal, immunosuppressive and antiproliferative activity [116]. Even though the anti-tumor efficacy of rapamycin is well documented both in vitro and in vivo, it is still not entirely understood. By reducing the levels of cyclins (especially cyclin D) and increase the levels of cyclin-dependent kinase inhibitors p21cip1 and p27kip1, rapamycin blocks G1 cell cycle progression [117,118,119,120]. The compound presents also anti-angiogenic properties by inhibiting endothelial cell proliferation, reducing the levels of produced vascular endothelial growth factor (VEGF) and reducing the response of endothelial cells to VEGF via mTOR inhibition [121,122,123]. There are preclinical data suggesting compounds’ ability to block the growth of human NSCLC cells and inhibit the growth of a Ras-induced NSCLC tumor and alveolar epithelial neoplasia [124,125,126]. The combination of rapamycin and docetaxel was found to synergistically inhibit the growth of lung cancer cells [127] and also the addition of rapamycin to PI3K inhibitors LY294002 and NVP-BKM120 resulted in synergistic act against NSCLC specimens [128], suggesting the possible efficacy of using rapamycin in combination with chemotherapy or other targeted agents in the treatment of lung cancer. However, the unfavorable pharmacological properties of rapamycin have promoted the discovery and development of rapamycin analogues suitable for clinical use, such as everolimus (RAD001), temsirolimus (CCI-779) and deforolimus (AP23573).
CCI-779 (temsirolimus) is an orally available rapamycin analogue with significant antiproliferative activity against a variety of human cancer types including SCLC [129]. Ohara et al. recently reported the ability of temsirolimus to inhibit tumor cell proliferation in NSCLC cell lines in a dose-dependent manner [130]. In a Phase I dose-escalation study, CCI-779 was i.v. administered in 63 patients with solid tumors. One patient with NSCLC had a confirmed partial response (PR) maintained for 12.7 months, three out of 63 patients had unconfirmed PRs and in two patients stable disease was observed for over 24 weeks. The most frequent drug-related toxicities were fatigue, mucositis and nausea and the maximally tolerated dose was 15 mg/m2 for heavily pretreated patients and 19 mg/m2 for patients with minimal prior treatment [131]. In another Phase I trial, patients with advanced solid tumors were orally treated with CCI-779 and the MTD was set at 75 mg. Elevated liver enzymes and rash were the dose-limiting toxicities observed in the study, and mucositis, rash and asthenia the most commonly identified drug-related adverse events [132]. Temsirolimus was also administered in 87 patients with extensive-stage SCLC in remission after induction chemotherapy. The overall median progression-free survival (PFS) time was 2.2 months and the median overall survival (OS) time was 8 months. Only one patient experienced a PR and six patients achieved disease stabilization, thus temsirolimus failed to prolong PFS in stable or responding patients with extensive-stage SCLC after induction chemotherapy [133]. Fifty-five patients with advanced NSCLC received CCI-779 i.v. at a dose of 25 mg/week in a Phase II study. Four patients had confirmed PR and SD maintained for 8 weeks or more was observed in fourteen patients. Dyspnea, fatigue, hyperglycemia, hypoxia, nausea and rash were the most frequent drug-related adverse events reported [134]. In a Phase I dose-escalation study, CCI-779 was tested in combination with the EGFR inhibitor EKB-569 in 48 patients with advanced solid tumors. The MTD was established at 30 mg on days 1–3 and 15–17 in a 28-day cycle for temsirolimus and 35 mg daily for EKB-569. The most common grade 3/4 toxicities observed were diarrhea, dehydration, and nausea-vomiting. Four out of 48 patients had a partial response and 15 patients showed stable disease [135]. Currently, CCI-779 is undergoing numerous clinical studies either as a single agent or in combination with cytotoxic agents (such as pemetrexed, vinorelbine and docetaxel) in patients with lung cancer [136,137,138] (Table 1).
RAD001 (everolimus) is an orally available rapamycin derivative that has already been approved in Europe as an immunosuppressive agent to prevent rejection in adult cardiac and renal transplant patients [139,140]. RAD001 showed significant antitumor activity in cancer cell lines and xenograft models of various cancer types including lung cancer [141,142,143,144,145]. Everolimus has the ability to bind with high affinity to FKB12, the intracellular receptor for mTOR, form a complex that interacts with mTOR and consequently halt downstream signaling [143].
RAD001 was evaluated in a Phase II nonrandomized study comparing NSCLC patients with two or fewer prior lines of chemotherapy, one platinum-based (arm 1) to those who failed second line chemotherapy in combination with an EGFR inhibitor (arm 2). Eighty-five patients (42 in arm 1 and 43 in arm 2) were enrolled and treated with Everolimus at a dose of 10 mg/day until progression disease or unacceptable toxicity. The overall response rate (ORR) was 4.7% and the overall disease control rate was 47.1%. The median progression-free survival (PFS) was 2.6 months in arm 1 and 2.7 months in arm 2. The most commonly observed toxicities were fatigue, dyspnea, stomatitis, anorexia, anemia and thrombocytopenia [146].
In a Phase I trial, RAD001 was orally administered in combination with the EGFR inhibitor gefitinib in patients with advanced NSCLC. Ten patients were enrolled in this study and the maximum tolerated dose of everolimus was set at 5 mg/day when coadministered with 250 mg gefitinib daily. At this dosage, the combination of RAD001 and gefitinib had only mild to moderate toxicities. When everolimus was administered at a higher dose, two dose-limiting toxicities were observed (grade 5 hypotension and grade 3 stomatitis). Among the eight evaluated patients, only two confirmed partial responses (PRs) were identified [147]. The combination of everolimus and gefitinib was also evaluated in a Phase II study in which 62 NSCLC patients participated. The patients were stratified into two cohorts based on whether they had been previously treated with cisplatin or carboplatin and docetaxel or pemetrexed (arm 2) or if they had received no prior treatment (arm 1). Only in 8 out of 62 patients, PRs were identified, thus the overall response rate was 13%. The median time to progression was 4 months and the median overall survival was 12 months, 27 months in arm 1 and 11 months in arm 2. Even though the combination was well tolerated with only mild to moderate toxicities, the partial response rate observed did not meet the predefined response to justify further investigation [148].
Campone et al. conducted a Phase I study of everolimus and paclitaxel weekly administered in patients with solid tumors. Sixteen patients were enrolled, eleven of whom achieved disease stabilization. The main DLT observed in this study was myelosuppression [149].
Twenty-four patients with advanced NSCLC and progression after platinum-based chemotherapy were enrolled in a Phase I study of RAD001 in combination with docetaxel. The DLTs were fever with grade 3/4 neutropenia, grade 3 fatigue and grade 3 mucositis. Among 21 patients evaluated, one patient with lung adenocarcinoma had a PR and 10 patients achieved disease stabilization. The recommended Phase II doses for the combination are 60 mg/m2 for docetaxel and 5 mg daily for everolimus [150].
The combination of everolimus and pemetrexed was also tested in a Phase I dose-escalation study in patients with NSCLC who progressed after one prior treatment. Forty-three patients were enrolled and in 5 of them, PR was identified. Everolimus 5 mg daily or 50 mg weekly in combination with the standard regimen of pemetrexed, was well tolerated and the most common grade 3/4 adverse events observed were neutropenia, dyspnea and thrombocytopenia [151].
Everolimus and the EGFR inhibitor erlotinib were administered in patients with advanced NSCLC previously treated with chemotherapy in a Phase I trial. Sixty-one patients were enrolled in the study and the drug-combination was well tolerated with mucositis, rash, diarrhea, vomiting and neutropenia to be the DLTs observed. One patient had complete response (CR), three patients had PR and 17 patients presented stable disease [152]. The combination was further tested in a Phase II study in which 133 patients participated. Even though the combination arm of everolimus plus erlotinib had 11% better disease control rate (DCR) at 3 months than the single-agent erlotinib arm, it did not meet the prespecified threshold for a Phase III study [153].
In a Phase I clinical study, RAD001 will be tested in patients with operable NSCLC [154]. In another Phase I study, the combination of everolimus and carboplatin-paclitaxel with or without bevacizumab is being evaluated in patients with NSCLC [155] (Table 1).
In SCLC cell lines RAD001 were able to inhibit cell growth in vitro and also in xenograft models of SCLC [156,157]. Moreover, SCLC cell lines exhibiting overactivation of the Akt/mTOR signaling were shown to be more sensitive to RAD001 treatment, suggesting the possible key role of inhibiting mTOR in patients with SCLC [156]. In a Phase II study, 10 mg everolimus were administered daily to 40 previously treated patients with SCLC after progression. In 35 evaluated patients, one had PR, and in eight patients disease stabilization was identified. The disease control rate at 6 weeks was 26%, the median time to progression was 1.3 months and the median survival was 6.7 months. Thrombocytopenia, neutropenia, infection, pneumonitis, fatigue, elevated transaminases, diarrhea and acute renal failure were the grade 3 adverse events observed. Even though the entity was well tolerated in this study, it had only moderate efficacy in pre-treated patients with relapsed SCLC [158].
Preclinical data suggest that everolimus and the EGFR inhibitor erlotinib have synergistic effect in atypical bronchial carcinoids (AC) and large cell neuroendocrine lung carcinomas (LCNEC), indicating the clinical importance of EGFR and mTOR as therapeutic targets in bronchial neuroendocrine tumors [159]. A Phase II study of everolimus with paclitaxel and carboplatin as first line treatment in patients with advanced large cell lung cancer with neuroendocrine differentiation is being conducted [160] (Table 1).
Currently, everolimus is in a Phase I study in combination with paclitaxel in patients with relapsed or refractory SCLC [161] and recently a dose-escalation Phase I study of everolimus in combination with carboplatin and etoposide in patients with SCLC or other advanced malignancies was terminated due to increased number of toxicities observed in the trial [162]. The combination of everolimus and cisplatin-etoposide is being evaluated in a Phase I trial in non-previously-treated patients with extensive-stage SCLC [163] (Table 1).
Drug-related pulmonary toxicity has been described for mTOR inhibitors in several studies and has been reported to be as high as 25% to 36% with typical radiographic findings, including lung consolidation and nonspecific areas of ground glass attenuation [164,165]. Patients with mTOR pneumonitis can be asymptomatic or have only mild symptoms, thus careful monitoring is required and treatment with mTOR inhibitors can often be continued.
AP23573 (ridaforolimus) is a rapamycin analogue and a small molecule mTOR inhibitor. The compound has shown significant antiproliferative activity in various human cancer cell lines and murine xenografts, as a single agent or in combination with cytotoxic or targeted agents [166,167]. In a Phase I study, ridaforolimus was administered to 13 Japanese patients with advanced solid tumors, and was well tolerated up to a dose of 40 mg. The most common adverse events identified were stomatitis, hypertriglyceridemia and proteinuria. In this study, one patient with NSCLC experienced PR [168]. In another dose-escalation Phase I study, thirty-two patients with advanced tumors received AP23573 intravenously daily for 5 days every 2 weeks in a 28-day cycle. The entity was well tolerated and the maximum-tolerated dose (MTD) was 18.75 mg/d. One patient with NSCLC had PR [169].
Up to date, a Phase II study of AP23573 in NSCLC patients with KRAS-mutations is ongoing [170] and a Phase I study of AP23573 combined with cetuximab in patients with NSCLC, head and neck cancer and colon cancer [171] (Table 1).
ATP competitive mTOR inhibitors have the ability of blocking both mTORC1 and mTORC2 complex producing a more significant antitumor activity compared to rapamycin derivatives [172].
AZD8055 is an ATP competitive mTOR inhibitor that has already completed a Phase I study in patients with solid tumors and lymphomas. Forty-nine patients were treated with AZD8055 and the most frequently observed drug-related adverse events were increased transaminases and fatigue. The maximum tolerated dose was set at 90 mg. Even though seven patients had stable disease maintained for over 4 months, no responses by RECIST criteria were identified [173]. Preclinical data suggest that AZD8055 and the MEK inhibitor selumetinib have synergistic antitumor efficacy in murine xenograft models of human lung adenocarcinomas [174]. Other ATP competitive mTOR inhibitors such as KU-0063794, WYE-354, WYE-132, OXA-01 are currently under clinical research in patients with solid tumors including lung cancer patients.
5. Conclusions
Even though major progress has been made in the treatment of patients with lung cancer, the survival rates remain poor. The importance of intracellular signal transduction pathways such as PI3K/AKT/mTOR pathway in cell growth, survival and proliferation has been justified over the last few years. The overactivation of such pathways has been identified in many cancer types including lung cancer and is strongly correlated with tumor development and progression, metastasis, chemo and radioresistance. Many downstream regulators of PI3K pathway have become targets for cancer treatment with encouraging results up to date. Indeed, numerous targeted agents directly against the PI3K pathway have already reached the clinical stage either as single agents or in combination with conventional chemotherapy or other targeted therapies, presenting a much better toxicity profile compared to conventional chemotherapy. Many frequently observed side effects, such as peripheral insulin resistance deriving from the use of Akt-inhibitors, are expected and can be justified by the mechanism of action of these agents. Moreover, small molecule agents with the ability to inhibit various signaling pathways in parallel seem to be more effective compared to single-target agents. More clinical trials along with the identification of biomarkers, able to characterize the “PI3K activated” tumors and predict clinical benefit from the use of PI3K pathway inhibitors, are required in order to produce more definite results for this fatal disease.
Conflict of Interest
The authors declare no duality of interest.
References
- 1.Jemal A., Siegel R., Xu J., Ward E. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Robinson C.G., Bradley J.D. The treatment of early-stage disease. Semin. Radiat. Oncol. 2010;20:178–185. doi: 10.1016/j.semradonc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Burdett S., Stephens R., Stewart L., Tierney J., Auperin A., Le Chevalier T., Le Pechoux C., Pignon J.P., Arriagada R., Higgins J., et al. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: A systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J. Clin. Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govindan R., Page N., Morgensztern D., Read W., Tierney R., Vlahiotis A., Spitznagel E.L., Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006;24:4539–44. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 5.Jackman D.M., Johnson B.E. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 6.Demedts I.K., Vermaelen K.Y., van Meerbeeck J.P. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur. Respir. J. 2010;35:202–215. doi: 10.1183/09031936.00105009. [DOI] [PubMed] [Google Scholar]
- 7.Molina J.R., Adjei A.A., Jett J.R. Advances in chemotherapy of non-small cell lung cancer. Chest. 2006;130:1211–1219. doi: 10.1378/chest.130.4.1211. [DOI] [PubMed] [Google Scholar]
- 8.Memmott R.M., Dennis P.A. The role of the Akt/MTOR pathway in tobacco carcinogen-induced lung tumorigenesis. Clin. Cancer Res. 2010;16:4–10. doi: 10.1158/1078-0432.CCR-09-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennessy B.T., Smith D.L., Ram P.T., Lu Y., Mills G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 10.Yuan T.L., Cantley L.C. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman J.A., Luo J., Cantley L.C. The evolution of phosphatidylinositol 3-Kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 12.Katso R., Okkenhaug K., Ahmadi K., White S., Timms J., Waterfield M.D. cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 13.Kurosu H., Maehama T., Okada T., Yamamoto T., Hoshino S., Fukui Y., Ui M., Hazeki O., Katada T. Heterodimeric phosphoinositide 3-kinase consisting of P85 and P110beta is synergistically activated by the betagamma subunits of G proteins and phosphotyrosyl peptide. J. Biol. Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- 14.Roche S., Downward J., Raynal P., Courtneidge S.A. A function for phosphatidylinositol 3-Kinase Beta (P85alpha-P110beta) in fibroblasts during mitogenesis: Requirement for insulin and lysophosphatidic acid-mediated signal transduction. Mol. Cell Biol. 1998;18:7119–7129. doi: 10.1128/mcb.18.12.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanhaesebroeck B., Waterfield M.D. Signaling by distinct classes of phosphoinositide 3-Kinases. Exp. Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 16.Cantley L.C. The phosphoinositide 3-Kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 17.Shaw R.J., Cantley L.C. Ras, PI(3)K and MTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 18.Grant S., Qiao L., Dent P. Roles of ERBB family receptor tyrosine kinases, and downstream signaling pathways, in the control of cell growth and survival. Front. Biosci. 2002;7:d376–d389. doi: 10.2741/grant. [DOI] [PubMed] [Google Scholar]
- 19.Krasilnikov M.A. Phosphatidylinositol-3 kinase dependent pathways: The role in control of cell growth, survival, and malignant transformation. Biochemistry (Mosc.) 2000;65:59–67. [PubMed] [Google Scholar]
- 20.Alessi D.R., James S.R., Downes C.P., Holmes A.B., Gaffney P.R., Reese C.B., Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase balpha. Curr. Biol. 1997;7:261–269. doi: 10.1016/S0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 21.Currie R.A., Walker K.S., Gray A., Deak M., Casamayor A., Downes C.P., Cohen P., Alessi D.R., Lucocq J. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem. J. 1999;337:575–583. doi: 10.1042/0264-6021:3370575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duronio V. The life of a cell: Apoptosis regulation by the PI3K/PKB pathway. Biochem. J. 2008;415:333–344. doi: 10.1042/BJ20081056. [DOI] [PubMed] [Google Scholar]
- 23.Ding L., Getz G., Wheeler D.A., Mardis E.R., McLellan M.D., Cibulskis K., Sougnez C., Greulich H., Muzny D.M., Morgan M.B., et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao L., Vogt P.K. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuels Y., Wang Z., Bardelli A., Silliman N., Ptak J., Szabo S., Yan H., Gazdar A., Powell S.M., Riggins G.J., et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 26.Ikenoue T., Kanai F., Hikiba Y., Obata T., Tanaka Y., Imamura J., Ohta M., Jazag A., Guleng B., Tateishi K., et al. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 2005;65:4562–4567. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 27.Samuels Y., Velculescu V.E. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004;3:1221–1224. doi: 10.4161/cc.3.10.1164. [DOI] [PubMed] [Google Scholar]
- 28.Shayesteh L., Lu Y., Kuo W.L., Baldocchi R., Godfrey T., Collins C., Pinkel D., Powell B., Mills G.B., Gray J.W. PIK3CA Is implicated as an oncogene in ovarian cancer. Nat. Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto H., Shigematsu H., Nomura M., Lockwood W.W., Sato M., Okumura N., Soh J., Suzuki M., Wistuba I.I., Fong K.M., et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer Res. 2008;68:6913–6921. doi: 10.1158/0008-5472.CAN-07-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibata T., Kokubu A., Tsuta K., Hirohashi S. Oncogenic Mutation of PIK3CA in small cell lung carcinoma: A potential therapeutic target pathway for chemotherapy-resistant lung cancer. Cancer Lett. 2009;283:203–211. doi: 10.1016/j.canlet.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 31.Engelman J.A., Chen L., Tan X., Crosby K., Guimaraes A.R., Upadhyay R., Maira M., McNamara K., Perera S.A., Song Y., et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat. Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelman J.A., Mukohara T., Zejnullahu K., Lifshits E., Borras A.M., Gale C.M., Naumov G.N., Yeap B.Y., Jarrell E., Sun J., et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J. Clin. Invest. 2006;116:2695–2706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berns K., Horlings H.M., Hennessy B.T., Madiredjo M., Hijmans E.M., Beelen K., Linn S.C., Gonzalez-Angulo A.M., Stemke-Hale K., Hauptmann M., et al. A functional genetic approach identifies the PI3K pathway as a major determinant of Trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 34.Massion P.P., Taflan P.M., Shyr Y., Rahman S.M., Yildiz P., Shakthour B., Edgerton M.E., Ninan M., Andersen J.J., Gonzalez A.L. Early involvement of the phosphatidylinositol 3-kinase/akt pathway in lung cancer progression. Am. J. Respir. Crit Care Med. 2004;170:1088–1094. doi: 10.1164/rccm.200404-487OC. [DOI] [PubMed] [Google Scholar]
- 35.Voortman J., Lee J.H., Killian J.K., Suuriniemi M., Wang Y., Lucchi M., Smith W.I., Jr., Meltzer P., Wang Y., Giaccone G. Array comparative genomic hybridization-based characterization of genetic alterations in pulmonary neuroendocrine tumors. Proc. Natl. Acad. Sci. USA. 2010;107:13040–13045. doi: 10.1073/pnas.1008132107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soung Y.H., Lee J.W., Nam S.W., Lee J.Y., Yoo N.J., Lee S.H. Mutational analysis of AKT1, AKT2 and AKT3 genes in common human carcinomas. Oncology. 2006;70:285–289. doi: 10.1159/000096289. [DOI] [PubMed] [Google Scholar]
- 37.Brognard J., Clark A.S., Ni Y., Dennis P.A. Akt/Protein Kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 38.Chun K.H., Kosmeder J.W., Sun S., Pezzuto J.M., Lotan R., Hong W.K., Lee H.Y. Effects of deguelin on the phosphatidylinositol 3-Kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J. Natl. Cancer Inst. 2003;95:291–302. doi: 10.1093/jnci/95.4.291. [DOI] [PubMed] [Google Scholar]
- 39.David O., Jett J., LeBeau H., Dy G., Hughes J., Friedman M., Brody A.R. Phospho-Akt overexpression in non-small cell lung cancer confers significant stage-independent survival disadvantage. Clin. Cancer Res. 2004;10:6865–6871. doi: 10.1158/1078-0432.CCR-04-0174. [DOI] [PubMed] [Google Scholar]
- 40.Tsurutani J., Fukuoka J., Tsurutani H., Shih J.H., Hewitt S.M., Travis W.D., Jen J., Dennis P.A. Evaluation of two phosphorylation sites improves the prognostic significance of akt activation in non-small-cell lung cancer tumors. J. Clin. Oncol. 2006;24:306–314. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- 41.Blackhall F.H., Pintilie M., Michael M., Leighl N., Feld R., Tsao M.S., Shepherd F.A. Expression and prognostic significance of kit, protein kinase b, and mitogen-activated protein kinase in patients with small cell lung cancer. Clin. Cancer Res. 2003;9:2241–2247. [PubMed] [Google Scholar]
- 42.Kraus A.C., Ferber I., Bachmann S.O., Specht H., Wimmel A., Gross M.W., Schlegel J., Suske G., Schuermann M. In vitro chemo- and radio-resistance in small cell lung cancer correlates with cell adhesion and constitutive activation of AKT and MAP kinase pathways. Oncogene. 2002;21:8683–8695. doi: 10.1038/sj.onc.1205939. [DOI] [PubMed] [Google Scholar]
- 43.Han S.Y., Kato H., Kato S., Suzuki T., Shibata H., Ishii S., Shiiba K., Matsuno S., Kanamaru R., Ishioka C. Functional evaluation of pten missense mutations using in vitro phosphoinositide phosphatase assay. Cancer Res. 2000;60:3147–3151. [PubMed] [Google Scholar]
- 44.Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S.I., Puc J., Miliaresis C., Rodgers L., McCombie R., et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 45.Sun X., Huang J., Homma T., Kita D., Klocker H., Schafer G., Boyle P., Ohgaki H. Genetic alterations in the PI3K pathway in prostate cancer. Anticancer Res. 2009;29:1739–1743. [PubMed] [Google Scholar]
- 46.Teng D.H., Hu R., Lin H., Davis T., Iliev D., Frye C., Swedlund B., Hansen K.L., Vinson V.L., Gumpper K.L., et al. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997;57:5221–5225. [PubMed] [Google Scholar]
- 47.Forgacs E., Biesterveld E.J., Sekido Y., Fong K., Muneer S., Wistuba I.I., Milchgrub S., Brezinschek R., Virmani A., Gazdar A.F., et al. Mutation analysis of the PTEN/MMAC1 gene in lung cancer. Oncogene. 1998;17:1557–1565. doi: 10.1038/sj.onc.1202070. [DOI] [PubMed] [Google Scholar]
- 48.Forgacs E., Zöchbauer-Müller S., Oláh E., Minna J.D. Molecular genetic abnormalities in the pathogenesis of human lung cancer. Pathol Oncol Res. 2001;7:6–13. doi: 10.1007/BF03032598. [DOI] [PubMed] [Google Scholar]
- 49.Yokomizo A., Tindall D.J., Drabkin H., Gemmill R., Franklin W., Yang P., Sugio K., Smith D.I., Liu W. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene. 1998;17:475–479. doi: 10.1038/sj.onc.1201956. [DOI] [PubMed] [Google Scholar]
- 50.Marsit C.J., Zheng S., Aldape K., Hinds P.W., Nelson H.H., Wiencke J.K., Kelsey K.T. PTEN Expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum. Pathol. 2005;36:768–776. doi: 10.1016/j.humpath.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Soria J.C., Lee H.Y., Lee J.I., Wang L., Issa J.P., Kemp B.L., Liu D.D., Kurie J.M., Mao L., Khuri F.R. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin. Cancer Res. 2002;8:1178–1184. [PubMed] [Google Scholar]
- 52.García J.M., Silva J., Peña C., Garcia V., Rodríguez R., Cruz M.A., Cantos B., Provencio M., España P., Bonilla F. Promoter methylation of the pten gene is a common molecular change in breast cancer. Genes Chromosomes Cancer. 2004;41:117–124. doi: 10.1002/gcc.20062. [DOI] [PubMed] [Google Scholar]
- 53.Goel A., Arnold C.N., Niedzwiecki D., Carethers J.M., Dowell J.M., Wasserman L., Compton C., Mayer R.J., Bertagnolli M.M., Boland C.R. Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res. 2004;64:3014–3021. doi: 10.1158/0008-5472.CAN-2401-2. [DOI] [PubMed] [Google Scholar]
- 54.Balsara B.R., Pei J., Mitsuuchi Y., Page R., Klein-Szanto A., Wang H., Unger M., Testa J.R. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–2059. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 55.Han S., Khuri F.R., Roman J. Fibronectin stimulates non-small cell lung carcinoma cell growth through activation of akt/mammalian target of rapamycin/s6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways. Cancer Res. 2006;66:315–323. doi: 10.1158/0008-5472.CAN-05-2367. [DOI] [PubMed] [Google Scholar]
- 56.Conde E., Angulo B., Tang M., Morente M., Torres-Lanzas J., Lopez-Encuentra A., Lopez-Rios F., Sanchez-Cespedes M. Molecular context of the EGFR mutations: evidence for the activation of MTOR/S6K signaling. Clin. Cancer Res. 2006;12:710–717. doi: 10.1158/1078-0432.CCR-05-1362. [DOI] [PubMed] [Google Scholar]
- 57.Wislez M., Spencer M.L., Izzo J.G., Juroske D.M., Balhara K., Cody D.D., Price R.E., Hittelman W.N., Wistuba I.I., Kurie J.M. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-Ras. Cancer Res. 2005;65:3226–3235. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- 58.Anagnostou V.K., Bepler G., Syrigos K.N., Tanoue L., Gettinger S., Homer R.J., Boffa D., Detterbeck F., Rimm D.L. High expression of mammalian target of rapamycin is associated with better outcome for patients with early stage lung adenocarcinoma. Clin. Cancer Res. 2009;15:4157–4164. doi: 10.1158/1078-0432.CCR-09-0099. [DOI] [PubMed] [Google Scholar]
- 59.Kokubo Y., Gemma A., Noro R., Seike M., Kataoka K., Matsuda K., Okano T., Minegishi Y., Yoshimura A., Shibuya M., et al. Reduction of PTEN protein and loss of epidermal growth factor receptor gene mutation in lung cancer with natural resistance to gefitinib (IRESSA) Br. J. Cancer. 2005;92:1711–1719. doi: 10.1038/sj.bjc.6602559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.She Q.B., Solit D., Basso A., Moasser M.M. Resistance to gefitinib in PTEN-Null HER-Overexpressing Tumor Cells Can Be Overcome Through Restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3'-Kinase/Akt pathway signaling. Clin. Cancer Res. 2003;9:4340–4346. [PubMed] [Google Scholar]
- 61.La Monica S., Galetti M., Alfieri R.R., Cavazzoni A., Ardizzoni A., Tiseo M., Capelletti M., Goldoni M., Tagliaferri S., Mutti A., et al. Everolimus restores gefitinib sensitivity in resistant non-small cell lung cancer cell lines. Biochem. Pharmacol. 2009;78:460–468. doi: 10.1016/j.bcp.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 62.Donev I.S., Wang W., Yamada T., Li Q., Takeuchi S., Matsumoto K., Yamori T., Nishioka Y., Sone S., Yano S. Transient PI3K inhibition induces apoptosis and overcomes hgf-mediated resistance to egfr-tkis in egfr mutant lung cancer. Clin. Cancer Res. 2011;17:2260–2269. doi: 10.1158/1078-0432.CCR-10-1993. [DOI] [PubMed] [Google Scholar]
- 63.Land H., Parada L.F., Weinberg R.A. Cellular oncogenes and multistep carcinogenesis. Science. 1983;222:771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- 64.Bos J.L. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 65.Pacold M.E., Suire S., Perisic O., Lara-Gonzalez S., Davis C.T., Walker E.H., Hawkins P.T., Stephens L., Eccleston J.F., Williams R.L. Crystal structure and functional analysis of ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103:931–943. doi: 10.1016/S0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 66.Gupta S., Ramjaun A.R., Haiko P., Wang Y., Warne P.H., Nicke B., Nye E., Stamp G., Alitalo K., Downward J. Binding of Ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 67.Ihle N.T., Lemos R., Jr., Wipf P., Yacoub A., Mitchell C., Siwak D., Mills G.B., Dent P., Kirkpatrick D.L., Powis G. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic ras is a dominant predictor for resistance. Cancer Res. 2009;69:143–150. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powis G., Bonjouklian R., Berggren M.M., Gallegos A., Abraham R., Ashendel C., Zalkow L., Matter W.F., Dodge J., Grindey G. Wortmannin, A potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 69.Vlahos C.J., Matter W.F., Hui K.Y., Brown R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-Morpholinyl)-8-Phenyl-4H-1-Benzopyran-4-One (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 70.Sordella R., Bell D.W., Haber D.A., Settleman J. Gefitinib-Sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 71.Gupta A.K., Soto D.E., Feldman M.D., Goldsmith J.D., Mick R., Hahn S.M., Machtay M., Muschel R.J., McKenna W.G. Signaling pathways in NSCLC as a predictor of outcome and response to therapy. Lung. 2004;182:151–162. doi: 10.1007/s00408-004-0310-8. [DOI] [PubMed] [Google Scholar]
- 72.Ihle N.T., Williams R., Chow S., Chew W., Berggren M.I., Paine-Murrieta G., Minion D.J., Halter R.J., Wipf P., Abraham R., et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol. Cancer Ther. 2004;3:763–772. [PubMed] [Google Scholar]
- 73.Ihle N.T., Paine-Murrieta G., Berggren M.I., Baker A., Tate W.R., Wipf P., Abraham R.T., Kirkpatrick D.L., Powis G. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in a-549 human non-small cell lung cancer xenografts. Mol. Cancer Ther. 2005;4:1349–1357. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ihle N.T., Lemos R., Schwartz D., Oh J., Halter R.J., Wipf P., Kirkpatrick L., Powis G. Peroxisome proliferator-activated receptor gamma agonist pioglitazone prevents the hyperglycemia caused by phosphatidylinositol 3-kinase pathway inhibition by PX-866 without affecting antitumor activity. Mol. Cancer Ther. 2009;8:94–100. doi: 10.1158/1535-7163.MCT-08-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y., Iwanaga K., Raso M.G., Wislez M., Hanna A.E., Wieder E.D., Molldrem J.J., Wistuba I.I., Powis G., Demayo F.J., et al. Phosphatidylinositol 3-kinase mediates bronchioalveolar stem cell expansion in mouse models of oncogenic K-Ras-induced lung cancer. PLoS. One. 2008;3:e2220. doi: 10.1371/journal.pone.0002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oncothyreon Inc. Study of PX-866 and Docetaxel in Solid Tumors. [(accessed on 14 Novermber 2012)]. Available online: http://clinicaltrials.gov/show/NCT01204099.
- 77.Folkes A.J., Ahmadi K., Alderton W.K., Alix S., Baker S.J., Box G., Chuckowree I.S., Clarke P.A., Depledge P., Eccles S.A., et al. The identification of 2-(1H-Indazol-4-Yl)-6-(4-Methanesulfonyl-Piperazin-1-Ylmethyl)-4-Morpholin-4-Yl-t Hieno[3,2-d]Pyrimidine (GDC-0941) As a potent, selective, orally bioavailable inhibitor of class I PI3 Kinase for the treatment of cancer. J. Med. Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 78.Sarker D., Kristeleit R., Mazina K.E., Ware J.A., Yan Y., Dresser M., Derynck M.K., De-Bono J. A phase I study evaluating the pharmacokinetics (PK) and pharmacodynamics (PD) of the oral Pan-Phosphoinositide-3 kinase (PI3K) inhibitor GDC-0941. J. Clin. Oncol. 2009;27 abstr 3538. [Google Scholar]
- 79.Wagner A.J., Von Hoff D.H., LoRusso P.M., Tibes R., Mazina K.E., Ware J.A., Yan Y., Derynck M.K., Demetri G.D. A first-in-human phase I study to evaluate the Pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J. Clin. Oncol. 2009;27 abstr 3501. [Google Scholar]
- 80.Genentech. A study of the safety and pharmacology of pi3-kinase inhibitor gdc-0941 in combination with either paclitaxel and carboplatin (with or without bevacizumab) or pemetrexed, cisplatin, and bevacizumab in patients with advanced non small cell lung cancer. [(accessed on 14 Novermber 2012)]. Available online: http://clinicaltrials.gov/show/NCT00974584.
- 81.Genentech. A study of the safety and pharmacology of GDC-0941 in combination with erlotinib in patients with advanced solid tumors. [(accessed on 14 Novermber 2012)]. Available online: http://clinicaltrials.gov/show/NCT00975182.
- 82.Genentech. Study evaluating the safety and efficacy of carboplatin/paclitaxel and carboplatin/paclitaxel/bevacizumab with and without GDC-0941 in patients with previously untreated advanced or recurrent non-small cell lung cancer. [(accessed on 14 Novermber 2012)]. Available online: http://clinicaltrials.gov/show/NCT01493843,
- 83.Edelman G., Bedell C., Shapiro G., Pandya S.S., Kwak E.L., Scheffold C., Nguyen L.T., Laird A., Baselga J., Rodon J. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts) with advanced malignancies. J. Clin. Oncol. (Meeting Abstracts) 2010;28 abstr 3004. [Google Scholar]
- 84.Sanofi-Aventis. Safety study of XL147 (SAR245408), in combination with paclitaxel and carboplatin in adults with solid tumors. [(accessed on 14 Novermber 2012)]. Available online: http://clinicaltrials.gov/show/NCT00756847.
- 85.Moldovan C., Soria J., LoRusso P., Guthrie T., Song C., Nguyen L.T., Martini J., Infante J.R., Burris H.A. A phase I safety and pharmacokinetic (PK) study of the PI3K inhibitor XL147 (SAR245408) in combination with erlotinib in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2010;28 abstr 3070. [Google Scholar]
- 86.Bendell J.C., Rodon J., Burris H.A., de Jonge M., Verweij J., Birle D., Demanse D., De Buck S.S., Ru Q.C., Peters M., et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 87.Ren H., Chen M., Yue P., Tao H., Owonikoko T.K., Ramalingam S.S., Khuri F.R., Sun S.Y. The combination of RAD001 and NVP-BKM120 synergistically inhibits the growth of lung cancer in vitro and in vivo. Cancer Lett. 2012;325:139–146. doi: 10.1016/j.canlet.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.National Cancer Centre; Singapore. A Trial of gefitinib in combination with BKM120 in patients with advanced non-small cell lung cancer, with enrichment for patients whose tumours harbour molecular alterations of PI3K pathway and known to overexpress EGFR. [(accessed on 14 Novermber 2012)]. Available online: http://clinicaltrials.gov/show/NCT01570296.
- 89.Sarah Cannon Research Institute; Novartis. Trial of erlotinib and BKM120 in patients with advanced non small cell lung cancer previously sensitive to erlotinib. [(accessed on 14 Novermber 2012)]. Available online: http://clinicaltrials.gov/show/NCT01487265.
- 90.Novartis Pharmaceuticals. Safety and Efficacy of BKM120 in Patients With Metastatic Non-small Cell Lung Cancer. [(accessed on 14 Novermber 2012)]. Available online: http://clinicaltrials.gov/show/NCT01297491.
- 91.Emory University; Novartis Pharmaceuticals. A Phase I Study of BKM120 and Everolimus in Advanced Solid Malignancies. [(accessed on 14 Novermber 2012)]. Available online: http://clinicaltrials.gov/show/NCT01470209.
- 92.Massachusetts General Hospital; Novartis Pharmaceuticals. BKM120 in Cancers With PIK3CA Activating Mutations. [(accessed on 14 Novermber 2012)]. Available online: http://clinicaltrials.gov/show/NCT01501604.
- 93.Maira S.M., Stauffer F., Brueggen J., Furet P., Schnell C., Fritsch C., Brachmann S., Chene P., De P.A., Schoemaker K., et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 94.Stauffer F., Maira S.M., Furet P., Garcia-Echeverria C. Imidazo[4,5-c]Quinolines as inhibitors of the PI3K/PKB-pathway. Bioorg. Med. Chem. Lett. 2008;18:1027–1030. doi: 10.1016/j.bmcl.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 95.Burris H., Rodon J., Sharma S., Herbst R.S., Tabernero J., Infante J.R., Silva A., Demanse D., Hackl W., Baselga J. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J. Clin. Oncol. (Meeting Abstracts) 2010;28 abstr 3005. [Google Scholar]
- 96.Konstantinidou G., Bey E.A., Rabellino A., Schuster K., Maira M.S., Gazdar A.F., Amici A., Boothman D.A., Scaglioni P.P. Dual phosphoinositide 3-Kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69:7644–7652. doi: 10.1158/0008-5472.CAN-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Serra V., Markman B., Scaltriti M., Eichhorn P.J., Valero V., Guzman M., Botero M.L., Llonch E., Atzori F., Di Cosimo S., et al. NVP-BEZ235, a dual PI3K/MTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 98.Cho D.C., Cohen M.B., Panka D.J., Collins M., Ghebremichael M., Atkins M.B., Signoretti S., Mier J.W. The efficacy of the novel dual PI3-kinase/MTOR inhibitor NVP-BEZ235 compared with rapamycin in renal cell carcinoma. Clin. Cancer Res. 2010;16:3628–3638. doi: 10.1158/1078-0432.CCR-09-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sos M.L., Fischer S., Ullrich R., Peifer M., Heuckmann J.M., Koker M., Heynck S., Stuckrath I., Weiss J., Fischer F., et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc. Natl. Acad. Sci. USA. 2009;106:18351–18356. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Faber A.C., Li D., Song Y., Liang M.C., Yeap B.Y., Bronson R.T., Lifshits E., Chen Z., Maira S.M., Garcia-Echeverria C., et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc. Natl. Acad. Sci. USA. 2009;106:19503–19508. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.LoRusso P., Markman B., Tabernero J., Shazer R., Nguyen L., Heath E., Patnaik A., Papadopoulos K. A Phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765, a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced solid tumors. J. Clin. Oncol. (Meeting Abstracts) 2009;27 abstr 3502. [Google Scholar]
- 102.Cohen R.B., Janne P.A., Engelman J.A., Martínez P., Nishida Y., Gendreau S., Wu B., Felip E. A phase I safety and pharmacokinetic (PK) study of PI3K/TORC1/TORC2 inhibitor XL765 (SAR245409) in combination with erlotinib (E) in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2010;28:3015. [Google Scholar]
- 103.Zou Z.Q., Zhang X.H., Wang F., Shen Q.J., Xu J., Zhang L.N., Xing W.H., Zhuo R.J., Li D. A novel dual PI3Kalpha/MTOR inhibitor PI-103 with high antitumor activity in non-small cell lung cancer cells. Int. J. Mol. Med. 2009;24:97–101. doi: 10.3892/ijmm_00000212. [DOI] [PubMed] [Google Scholar]
- 104.Prevo R., Deutsch E., Sampson O., Diplexcito J., Cengel K., Harper J., O'Neill P., McKenna W.G., Patel S., Bernhard E.J. Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine inhibitor pi-103 enhances tumor radiosensitivity. Cancer Res. 2008;68:5915–5923. doi: 10.1158/0008-5472.CAN-08-0757. [DOI] [PubMed] [Google Scholar]
- 105.Qayum N., Muschel R.J., Im J.H., Balathasan L., Koch C.J., Patel S., McKenna W.G., Bernhard E.J. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Res. 2009;69:6347–6354. doi: 10.1158/0008-5472.CAN-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Q., Zhu G.D. Targeting serine/threonine protein kinase B/Akt and Cell-Cycle checkpoint kinases for treating cancer. Curr. Top. Med. Chem. 2002;2:939–971. doi: 10.2174/1568026023393318. [DOI] [PubMed] [Google Scholar]
- 107.Kumar C.C., Madison V. Drugs Targeted Against Protein Kinases. Expert. Opin. Emerg. Drugs. 2001;6:303–315. doi: 10.1517/14728214.6.2.303. [DOI] [PubMed] [Google Scholar]
- 108.Cherrin C., Haskell K., Howell B., Jones R., Leander K., Robinson R., Watkins A., Bilodeau M., Hoffman J., Sanderson P., et al. An Allosteric Akt inhibitor effectively blocks akt signaling and tumor growth with only transient effects on glucose and insulin levels in vivo. Cancer Biol. Ther. 2010;9:493–503. doi: 10.4161/cbt.9.7.11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luo Y., Shoemaker A.R., Liu X., Woods K.W., Thomas S.A., de Jong R., Han E.K., Li T., Stoll V.S., Powlas J.A., et al. Potent and selective inhibitors of akt kinases slow the progress of tumors in vivo. Mol. Cancer Ther. 2005;4:977–986. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 110.Crouthamel M.C., Kahana J.A., Korenchuk S., Zhang S.Y., Sundaresan G., Eberwein D.J., Brown K.K., Kumar R. Mechanism and management of AKT inhibitor-induced hyperglycemia. Clin. Cancer Res. 2009;15:217–225. doi: 10.1158/1078-0432.CCR-08-1253. [DOI] [PubMed] [Google Scholar]
- 111.Hirai H., Sootome H., Nakatsuru Y., Miyama K., Taguchi S., Tsujioka K., Ueno Y., Hatch H., Majumder P.K., Pan B.S., et al. MK-2206, an allosteric akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 112.Yap T.A., Yan L., Patnaik A., Fearen I., Olmos D., Papadopoulos K., Baird R.D., Delgado L., Taylor A., Lupinacci L., et al. First-in-man clinical trial of the oral Pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J. Clin. Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 113.National Taiwan University Hospital. Dose defining study for MK-2206 combined with gefitinib in non small cell lung cancer (NSCLC) [(acesseed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT01147211.
- 114.Beckman Research Institute; National Cancer Institute (NCI) MK2206 and erlotinib hydrochloride in treating patients with advanced non-small cell lung cancer who have progressed after previous response to erlotinib hydrochloride therapy. [(acesseed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT01294306.
- 115.Rhodes N., Heerding D.A., Duckett D.R., Eberwein D.J., Knick V.B., Lansing T.J., McConnell R.T., Gilmer T.M., Zhang S.Y., Robell K., et al. Characterization of an Akt Kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68:2366–2374. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 116.Douros J., Suffness M. New Antitumor Substances of Natural Origin. Cancer Treat. Rev. 1981;8:63–87. doi: 10.1016/S0305-7372(81)80006-0. [DOI] [PubMed] [Google Scholar]
- 117.Decker T., Hipp S., Ringshausen I., Bogner C., Oelsner M., Schneller F., Peschel C. Rapamycin-induced G1 arrest in cycling B-CLL cells is associated with reduced expression of Cyclin D3, Cyclin E, Cyclin A, and Survivin. Blood. 2003;101:278–285. doi: 10.1182/blood-2002-01-0189. [DOI] [PubMed] [Google Scholar]
- 118.Albers M.W., Williams R.T., Brown E.J., Tanaka A., Hall F.L., Schreiber S.L. FKBP-Rapamycin inhibits a Cyclin-dependent kinase activity and a Cyclin D1-Cdk association in early G1 of an osteosarcoma cell line. J. Biol. Chem. 1993;268:22825–22829. [PubMed] [Google Scholar]
- 119.Luo Y., Marx S.O., Kiyokawa H., Koff A., Massague J., Marks A.R. Rapamycin resistance tied to defective regulation of P27Kip1. Mol. Cell Biol. 1996;16:6744–6751. doi: 10.1128/mcb.16.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zezula J., Sexl V., Hutter C., Karel A., Schutz W., Freissmuth M. The cyclin-dependent Kinase inhibitor P21cip1 mediates the growth inhibitory effect of phorbol esters in human venous endothelial cells. J. Biol. Chem. 1997;272:29967–29974. doi: 10.1074/jbc.272.47.29967. [DOI] [PubMed] [Google Scholar]
- 121.Humar R., Kiefer F.N., Berns H., Resink T.J., Battegay E.J. Hypoxia enhances vascular cell proliferation and angiogenesis in Vitro via rapamycin (MTOR)-dependent signaling. FASEB J. 2002;16:771–780. doi: 10.1096/fj.01-0658com. [DOI] [PubMed] [Google Scholar]
- 122.Hudson C.C., Liu M., Chiang G.G., Otterness D.M., Loomis D.C., Kaper F., Giaccia A.J., Abraham R.T. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guba M., von Breitenbuch P., Steinbauer M., Koehl G., Flegel S., Hornung M., Bruns C.J., Zuelke C., Farkas S., Anthuber M., et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat. Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 124.Sun S.Y., Rosenberg L.M., Wang X., Zhou Z., Yue P., Fu H., Khuri F.R. Activation of Akt and EIF4E survival pathways by Rapamycin-mediated mammalian target of tapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 125.Boffa D.J., Luan F., Thomas D., Yang H., Sharma V.K., Lagman M., Suthanthiran M. Rapamycin inhibits the growth and metastatic progression of non-small cell lung cancer. Clin. Cancer Res. 2004;10:293–300. doi: 10.1158/1078-0432.CCR-0629-3. [DOI] [PubMed] [Google Scholar]
- 126.Wislez M., Spencer M.L., Izzo J.G., Juroske D.M., Balhara K., Cody D.D., Price R.E., Hittelman W.N., Wistuba I.I., Kurie J.M. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-Ras. Cancer Res. 2005;65:3226–3235. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- 127.Sun S.Y., Fu H., Khuri F.R. Targeting MTOR signaling for lung cancer therapy. J. Thorac. Oncol. 2006;1:109–111. doi: 10.1097/01243894-200602000-00002. [DOI] [PubMed] [Google Scholar]
- 128.Zito C.R., Jilaveanu L.B., Anagnostou V., Rimm D., Bepler G., Maira S.M., Hackl W., Camp R., Kluger H.M., Chao H.H. Multi-level targeting of the phosphatidylinositol-3-kinase pathway in non-small cell lung cancer cells. PLoS. One. 2012;7:e31331. doi: 10.1371/journal.pone.0031331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dudkin L., Dilling M.B., Cheshire P.J., Harwood F.C., Hollingshead M., Arbuck S.G., Travis R., Sausville E.A., Houghton P.J. Biochemical correlates of MTOR inhibition by the rapamycin Ester CCI-779 and tumor growth inhibition. Clin. Cancer Res. 2001;7:1758–1764. [PubMed] [Google Scholar]
- 130.Ohara T., Takaoka M., Toyooka S., Tomono Y., Nishikawa T., Shirakawa Y., Yamatsuji T., Tanaka N., Fujiwara T., Naomoto Y. Inhibition of MTOR by temsirolimus contributes to prolonged survival of mice with pleural dissemination of non-small-cell lung cancer cells. Cancer Sci. 2011;102:1344–1349. doi: 10.1111/j.1349-7006.2011.01967.x. [DOI] [PubMed] [Google Scholar]
- 131.Hidalgo M., Buckner J.C., Erlichman C., Pollack M.S., Boni J.P., Dukart G., Marshall B., Speicher L., Moore L., Rowinsky E.K. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin. Cancer Res. 2006;12:5755–5763. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 132.Buckner J.C., Forouzesh B., Erlichman C., Hidalgo M., Boni J.P., Dukart G., Berkenblit A., Rowinsky E.K. Phase I, Pharmacokinetic study of temsirolimus administered orally to patients with advanced cancer. Invest. New Drugs. 2010;28:334–342. doi: 10.1007/s10637-009-9257-1. [DOI] [PubMed] [Google Scholar]
- 133.Pandya K.J., Dahlberg S., Hidalgo M., Cohen R.B., Lee M.W., Schiller J.H., Johnson D.H. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the eastern cooperative oncology group (E1500) J. Thorac. Oncol. 2007;2:1036–1041. doi: 10.1097/JTO.0b013e318155a439. [DOI] [PubMed] [Google Scholar]
- 134.Reungwetwattana T., Molina J.R., Mandrekar S.J., Allen-Ziegler K., Rowland K.M., Reuter N.F., Luyun R.F., Dy G.K., Marks R.S., Schild S.E., et al. Brief report: a phase II "window-of-opportunity" frontline study of the MTOR inhibitor, temsirolimus given as a single agent in patients with advanced NSCLC, an NCCTG study. J. Thorac. Oncol. 2012;7:919–922. doi: 10.1097/JTO.0b013e31824de0d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bryce A.H., Rao R., Sarkaria J., Reid J.M., Qi Y., Qin R., James C.D., Jenkins R.B., Boni J., Erlichman C., et al. Phase I study of temsirolimus in combination With EKB-569 in patients with advanced solid tumors. Invest. New Drugs. 2012;30:1934–1941. doi: 10.1007/s10637-011-9742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Washington University School of Medicine. Temsirolimus and pemetrexed for recurrent or refractory non-small cell lung cancer. [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT00921310.
- 137.USC/Norris Comprehensive Cancer Center; Wyeth. Temsirolimus and vinorelbine ditartrate in treating patients with unresectable or metastatic solid tumors. [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT01155258.
- 138.Washington University School of Medicine. Phase I study of docetaxel and temsirolimus in resistant solid malignancies. [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT00703625.
- 139.Eisen H.J., Tuzcu E.M., Dorent R., Kobashigawa J., Mancini D., Valantine-von Kaeppler H.A., Starling R.C., Sorensen K., Hummel M., Lind J.M., et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N. Engl. J. Med. 2003;349:847–858. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 140.Lorber M.I., Mulgaonkar S., Butt K.M., Elkhammas E., Mendez R., Rajagopalan P.R., Kahan B., Sollinger H., Li Y., Cretin N., et al. Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: a 3-year randomized, multicenter, phase III study. Transplantation. 2005;80:244–252. doi: 10.1097/01.TP.0000164352.65613.24. [DOI] [PubMed] [Google Scholar]
- 141.Beuvink I., Boulay A., Fumagalli S., Zilbermann F., Ruetz S., O'Reilly T., Natt F., Hall J., Lane H.A., Thomas G. The MTOR Inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of P21 translation. Cell. 2005;120:747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 142.Mabuchi S., Altomare D.A., Cheung M., Zhang L., Poulikakos P.I., Hensley H.H., Schilder R.J., Ozols R.F., Testa J.R. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin. Cancer Res. 2007;13:4261–4270. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 143.Boulay A., Zumstein-Mecker S., Stephan C., Beuvink I., Zilbermann F., Haller R., Tobler S., Heusser C., O'Reilly T., Stolz B., et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 144.Buck E., Eyzaguirre A., Brown E., Petti F., McCormack S., Haley J.D., Iwata K.K., Gibson N.W., Griffin G. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol. Cancer Ther. 2006;5:2676–2684. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- 145.Majumder P.K., Febbo P.G., Bikoff R., Berger R., Xue Q., McMahon L.M., Manola J., Brugarolas J., McDonnell T.J., Golub T.R., et al. MTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 146.Soria J.C., Shepherd F.A., Douillard J.Y., Wolf J., Giaccone G., Crino L., Cappuzzo F., Sharma S., Gross S.H., Dimitrijevic S., et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann. Oncol. 2009;20:1674–1681. doi: 10.1093/annonc/mdp060. [DOI] [PubMed] [Google Scholar]
- 147.Milton D.T., Riely G.J., Azzoli C.G., Gomez J.E., Heelan R.T., Kris M.G., Krug L.M., Pao W., Pizzo B., Rizvi N.A., et al. Phase 1 trial of everolimus and gefitinib in patients with advanced nonsmall-cell lung cancer. Cancer. 2007;110:599–605. doi: 10.1002/cncr.22816. [DOI] [PubMed] [Google Scholar]
- 148.Price K.A., Azzoli C.G., Krug L.M., Pietanza M.C., Rizvi N.A., Pao W., Kris M.G., Riely G.J., Heelan R.T., Arcila M.E., et al. Phase II trial of gefitinib and everolimus in advanced non-small cell lung cancer. J. Thorac. Oncol. 2010;5:1623–1629. doi: 10.1097/JTO.0b013e3181ec1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Campone M., Levy V., Bourbouloux E., Berton R.D., Bootle D., Dutreix C., Zoellner U., Shand N., Calvo F., Raymond E. Safety and pharmacokinetics of paclitaxel and the oral MTOR inhibitor everolimus in advanced solid tumours. Br. J. Cancer. 2009;100:315–321. doi: 10.1038/sj.bjc.6604851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ramalingam S.S., Harvey R.D., Saba N., Owonikoko T.K., Kauh J., Shin D.M., Sun S.Y., Strychor S., Tighiouart M., Egorin M.J., et al. Phase 1 and pharmacokinetic study of everolimus, a mammalian target of rapamycin inhibitor, in combination with docetaxel for recurrent/refractory nonsmall cell lung cancer. Cancer. 2010;116:3903–3909. doi: 10.1002/cncr.25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vansteenkiste J., Solomon B., Boyer M., Wolf J., Miller N., Di Scala L., Pylvaenaeinen I., Petrovic K., Dimitrijevic S., Anrys B., et al. Everolimus in combination with pemetrexed in patients with advanced non-small cell lung cancer previously treated with chemotherapy: a phase i study using a novel, adaptive bayesian dose-escalation model. J. Thorac. Oncol. 2011;6:2120–2129. doi: 10.1097/JTO.0b013e3182307ede. [DOI] [PubMed] [Google Scholar]
- 152.Papadimitrakopoulou V., Blumenschein G.R., Leighl N.B., Bennouna J., Soria J.C., Burris H.A., Dimitrijevic S., Kunz T., Di Scala L., Johnson B.E. a phase 1/2 study investigating the combination of RAD001 (R) (Everolimus) and Erlotinib (E) as 2nd and 3rd line therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC) Previously treated with chemotherapy (C): Phase 1 Results. J. Clin. Oncol. 2008;26 abstr 8051. [Google Scholar]
- 153.Leighl N.B., Soria J., Bennouna J., Blais N., Traynor A.M., Papadimitrakopoulou V., Klimovsky J., Jappe A., Jehl V., Johnson B.E. Phase II study of everolimus plus erlotinib in previously treated patients with advanced non-small cell lung cancer (NSCLC) J. Clin. Oncol. 2010;28 doi: 10.1093/annonc/mdt536. abstr 7524. [DOI] [PubMed] [Google Scholar]
- 154.Emory University. Phase 1b trial of RAD001 in patients with operable non-small cell lung cancer (NSCLC) [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT00401778.
- 155.Novartis Pharmaceuticals. combination of RAD001 with carboplatin, paclitaxel and bevacizumab in non-small-cell lung cancer (NSCLC) patients. [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT00457119.
- 156.Marinov M., Ziogas A., Pardo O.E., Tan L.T., Dhillon T., Mauri F.A., Lane H.A., Lemoine N.R., Zangemeister-Wittke U., Seckl M.J., et al. AKT/MTOR pathway activation and BCL-2 family proteins modulate the sensitivity of human small cell lung cancer cells to RAD001. Clin. Cancer Res. 2009;15:1277–1287. doi: 10.1158/1078-0432.CCR-08-2166. [DOI] [PubMed] [Google Scholar]
- 157.Stracke S., Ramudo L., Keller F., Henne-Bruns D., Mayer J.M. Antiproliferative and overadditive effects of everolimus and mycophenolate mofetil in pancreas and lung cancer cells in vitro. Transplant. Proc. 2006;38:766–770. doi: 10.1016/j.transproceed.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 158.Tarhini A., Kotsakis A., Gooding W., Shuai Y., Petro D., Friedland D., Belani C.P., Dacic S., Argiris A. Phase II Study of everolimus (RAD001) in previously treated small cell lung cancer. Clin. Cancer Res. 2010;16:5900–5907. doi: 10.1158/1078-0432.CCR-10-0802. [DOI] [PubMed] [Google Scholar]
- 159.Bago-Horvath Z., Sieghart W., Grusch M., Lackner A., Hayden H., Pirker C., Komina O., Wesierska-Gadek J., Haitel A., Filipits M., et al. Synergistic effects of erlotinib and everolimus on bronchial carcinoids and large-cell neuroendocrine carcinomas with activated EGFR/AKT/MTOR pathway. Neuroendocrinology. 2012;96:228–237. doi: 10.1159/000337257. [DOI] [PubMed] [Google Scholar]
- 160.Novartis Pharmaceuticals. RAD001 with paclitaxel and carboplatin in first line treatment of patients with advanced large cell lung cancer with neuroendocrine differentiation. [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT01317615.
- 161.Samsung Medical Center. Combination anticancer therapy of paclitaxel and everolimus for relapsed or refractory small cell lung cancer. [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT01079481.
- 162.University of California; Davis; National Cancer Institute (NCI) Everolimus, carboplatin, and etoposide in treating patients with small cell lung cancer or other advanced solid tumors. [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT00807755.
- 163.Novartis Pharmaceuticals. Safety of RAD001 in combination with cisplatin and etoposide in lung cancer patients. [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT00466466.
- 164.White D.A., Schwartz L.H., Dimitrijevic S., Scala L.D., Hayes W., Gross S.H. Characterization of pneumonitis in patients with advanced non-small cell lung cancer treated with everolimus (RAD001) J. Thorac. Oncol. 2009;4:1357–1363. doi: 10.1097/JTO.0b013e3181ba20b1. [DOI] [PubMed] [Google Scholar]
- 165.Duran I., Siu L.L., Oza A.M., Chung T.B., Sturgeon J., Townsley C.A., Pond G.R., Seymour L., Niroumand M. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur. J. Cancer. 2006;42:1875–1880. doi: 10.1016/j.ejca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 166.Rivera M.V., Tang H., Metcalf A.C., Keenan P.T., Sundaramoorthi R., Liu S., Clackson T. Anti-proliferative activity of the mTOR inhibitor AP23573 in combination with cytotoxic and targeted agents. Proc. Am. Assoc. Cancer. Res. 2004;45 abstr 3887. [Google Scholar]
- 167.Clackson T., Metcalf A.C., Rozamus W.L., Knowles L.H., Wardwell D.S., Roses B.J., Burns D.K., Graytock C., Pradeepan S., Notari D.S., et al. Regression of tumor xenografts in mice after oral administration of AP23573, a novel mTOR inhibitor that induces tumor starvation. Proc. Am. Assoc. Cancer. Res. 2002;43 abstr LB95. [Google Scholar]
- 168.Seki Y., Yamamoto N., Tamura Y., Goto Y., Shibata T., Tanioka M., Asahina H., Nokihara H., Yamada Y., Shimamoto T., et al. Phase I Study for ridaforolimus, an oral MTOR inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012;69:1099–1105. doi: 10.1007/s00280-011-1788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mita M.M., Mita A.C., Chu Q.S., Rowinsky E.K., Fetterly G.J., Goldston M., Patnaik A., Mathews L., Ricart A.D., Mays T., et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J. Clin. Oncol. 2008;26:361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 170.Merck. A study of ridaforolimus in non-small cell lung cancer (NSCLC) Patients with kirsten rat sarcoma viral oncogene homolog (KRAS) mutations (MK-8669–021 AM1) [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT00818675/
- 171.Isdale D.G. Ridaforolimus with cetuximab for patients with advanced head and neck cancer, non-small cell lung cancer and colon cancer. [(accessed on 14 November 2012)]. Available online: http://clinicaltrials.gov/show/NCT01212627/
- 172.Feldman M.E., Apsel B., Uotila A., Loewith R., Knight Z.A., Ruggero D., Shokat K.M. Active-site inhibitors of MTOR target rapamycin-resistant outputs of MTORC1 and MTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Naing A., Aghajanian C., Raymond E., Olmos D., Schwartz G., Oelmann E., Grinsted L., Burke W., Taylor R., Kaye S., et al. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of AZD8055 in Advanced Solid Tumours and Lymphoma. Br. J. Cancer. 2012;107:1093–1099. doi: 10.1038/bjc.2012.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Holt S.V., Logie A., Davies B.R., Alferez D., Runswick S., Fenton S., Chresta C.M., Gu Y., Zhang J., Wu Y.L., et al. Enhanced apoptosis and tumor growth suppression elicited by combination of mek (Selumetinib) and MTOR kinase inhibitors (AZD8055) Cancer Res. 2012;72:1804–1813. doi: 10.1158/0008-5472.CAN-11-1780. [DOI] [PubMed] [Google Scholar]