Abstract
One of the hallmarks of leukemic cells is their ability to proliferate and survive in the absence of exogenous growth factors (GFs). However, the molecular mechanisms used by myeloid tumor cells to escape apoptosis are not fully understood. Here we report that Myc/Raf-transformed macrophages require the transcription factor C/EBPβ to prevent cell death. In contrast to wild-type cells, C/EBPβ−/− macrophages were completely dependent on macrophage colony-stimulating factor or granulocyte-macrophage colony-stimulating factor for survival and displayed impaired tumorigenicity in vivo. Microarray analysis revealed that C/EBPβ-deficient cells expressed significantly reduced levels of the prosurvival factor insulin-like growth factor I (IGF-I). Overexpression of C/EBPβ stimulated transcription from the IGF-I promoter, indicating that IGF-I is a direct transcriptional target of C/EBPβ. Serological neutralization of IGF-I in C/EBPβ+/+ tumor cell cultures induced apoptosis, showing that IGF-I functions as an autocrine survival factor in these cells. Macrophage tumor cells derived from IGF-I−/− mice were GF dependent, similar to C/EBPβ-deficient cells. Forced expression of either C/EBPβ or IGF-I in C/EBPβ−/− bone marrow cells restored Myc/Raf-induced transformation and permitted neoplastic growth without exogenous GFs. Thus, our findings demonstrate that C/EBPβ is essential for oncogenic transformation of macrophages and functions at least in part by regulating expression of the survival factor IGF-I.
Mammalian cells typically require exogenous growth factors (GFs) to proliferate and/or survive in culture (59). In contrast, most tumor cells are able to undergo rapid cell division and can suppress apoptosis in the absence of external GF signals. The GF independence of transformed cells often correlates with their tumorigenicity in vivo. The intrinsic ability of cancer cells to evade apoptosis can arise from a variety of genetic lesions, including those that inactivate apoptotic pathways (e.g., p53), cause constitutive (ligand-independent) signaling from GF receptors, or up-regulate expression of GFs or prosurvival genes (e.g., Bcl-2). Suppression of programmed cell death is now appreciated to be a key step in tumor development, and inhibition of prosurvival pathways has emerged as a promising strategy for cancer intervention (20). Hence, it is important to develop a detailed understanding of the molecular components and cellular pathways that regulate tumor cell survival.
Most myeloid leukemia cells harbor oncogenic mutations that circumvent the requirement for hematopoietic GFs, such as macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage CSF (GM-CSF), and granulocyte CSF (G-CSF) (38, 45). Myeloid cell transformation can also be induced by oncogenic viruses, which have been particularly useful in defining the genetic pathways that cause these cancers. One such example is the avian MH2 retrovirus, which carries the v-myc and v-raf (v-mil) oncogenes. Infection of chicken hematopoietic cells with the MH2 virus results in the growth of transformed, factor-independent cells that display macrophage-like properties (22). A related mammalian retrovirus, J2, also expresses v-myc and v-raf and transforms murine bone marrow, producing immortalized, GF-independent cells of the monocyte/macrophage lineage (6, 15). Analysis of deletion mutants in the MH2 viral genome showed that both oncogenes are required to induce monocytic and liver tumors in vivo (9, 22). In addition, the presence of either v-myc or v-raf alone in the MH2 and J2 viruses is insufficient to elicit macrophage transformation in vitro (7, 22). Therefore, the myc and raf oncogenes cooperate to induce myeloid leukemogenesis. Transformation is thought to result from the constitutive proliferative stimulus provided by Myc (10, 19, 47), while Raf signaling primarily regulates GF independence and survival (22, 38, 39).
In the present study we have examined the role of the transcription factor CCAAT/enhancer binding protein β (C/EBPβ) in transformation of macrophages by the J2 virus. C/EBPβ is expressed in numerous cell types and is particularly abundant in macrophages and hepatocytes (1, 16, 25, 67). C/EBPβ-deficient mice are viable but show defects in innate immunity that result from impaired bactericidal activity of macrophages (60). C/EBPβ−/− animals demonstrate numerous other phenotypes, including female sterility (57), defective mammary gland development (51, 52), impaired liver regeneration (23), and skin abnormalities (70). Interestingly, C/EBPβ-deficient mice are completely resistant to development of skin tumors when subjected to a multistage carcinogenesis protocol that normally induces papillomas carrying Ras mutations (71). This finding, coupled with observations showing that C/EBPβ can be activated by oncogenic Ras signaling (28, 43, 71), has led to the proposal that C/EBPβ is an essential effector of Ras tumorigenesis in keratinocytes. C/EBPβ−/− animals also displayed a 17-fold-higher frequency of apoptotic keratinocytes in the epidermis after carcinogen treatment compared to wild-type (wt) mice, suggesting that C/EBPβ may be involved in suppressing apoptosis in precancerous skin cells. At present, it is unclear whether C/EBPβ functions to promote survival of early tumor precursors, acts at later stages of tumor development, or both. In addition, the molecular nature of the C/EBPβ-dependent prosurvival activity has not been resolved, nor is it known whether C/EBPβ affects tumorigenesis in other tissues.
Here we have used J2-mediated transformation of C/EBPβ-deficient bone marrow cells to examine the role of C/EBPβ in an experimental model of myeloid leukemogenesis. Our findings reveal that C/EBPβ is essential for Myc/Raf-induced transformation of macrophages and that C/EBPβ-dependent regulation of insulin-like growth factor I (IGF-I) expression plays a critical role in the ability of these myeloid tumor cells to escape apoptosis.
MATERIALS AND METHODS
Reagents.
Fluorescein isothiocyanate (FITC)-labeled immunoglobulin G2a (IgG2a) and MAC-1 (Pharmingen) and F4-80 (Serotec) antibodies were used for fluorescence-activated cell sorter (FACS) analysis of macrophage cell surface markers. Anti-IGF-I neutralizing antibody and IgG1 control antibody were obtained from R&D Systems. Human M-CSF and IGF-I and murine interleukin-3 (IL-3) and stem cell factor (SCF) were purchased from Peprotech. The kinase inhibitors LY294002 and PD98059 were purchased from Calbiochem, and IGF-Ia RNase protection assay (RPA) probe was obtained from BD-Pharmingen.
Animals.
C/EBPβ−/− mice have been described previously (57). C/EBPβ+/− 129/Sv females were mated with C/EBPβ+/− C57BL/6 males to obtain F1 C/EBPβ−/− and C/EBPβ+/+ progeny. Nude mice (athymic NCr-nu BALB/c) were obtained from Charles River Laboratories. IGF-I mutant and heterozygous control animals were generated as described previously (35). All animals were maintained according to National Institutes of Health guidelines.
Cells and cell culture.
φNX retroviral packaging cells and ψCREJ2 cells (a producer cell line for the J2 retrovirus [15] provided by H. Young) were maintained in Dulbecco's modified Eagle's medium (DMEM; GIBCO-Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone), 200 mM l-glutamine, and 100 U of penicillin-streptomycin (Gibco)/ml. Primary bone marrow-derived macrophages (BMDM) and transformed macrophage cell lines were generated from bone marrow of mice lacking C/EBPβ or IGF-I and their control littermates. Bone marrow was collected from the femurs and tibias and placed into phosphate-buffered saline (PBS) containing 2% FBS. The bone marrow plugs were disaggregated and pelleted, and red blood cells were lysed using NH4Cl lysis solution (Sigma). For primary BMDM, the cells were cultured in the presence of 100 ng of M-CSF/ml for 24 h. Nonadherent cells were then collected and cultured for 5 to 7 days in the presence of M-CSF. To generate immortalized macrophage cell lines, 1 × 107 to 5 × 107 BM cells were resuspended in ψCREJ2 cell supernatant supplemented with 8 μg of Polybrene (Sigma)/ml and 100 ng of M-CSF/ml. After 24 h at 37°C, the supernatant was removed and the cells were maintained in the presence of 100 ng of M-CSF/ml. All primary BMDM cultures and cell lines were maintained in DMEM supplemented with 10% heat-inactivated FBS, 200 mM l-glutamine, and 100 U of penicillin-streptomycin (Gibco)/ml.
Transactivation assays.
The rat IGF-I promoter-1-luciferase construct (IGF1711b-Luc) and artificial promoter constructs containing four tandem copies of the 19-bp HS3D C/EBP site (4xHS3D-Luc) or a mutated HS3D element (4xHS3Dmut-Luc) (37, 61) were provided by T. McCarthy. HepG2 hepatoma cells (ATCC HB-8065) were transfected with 0.5 μg of the specified IGF-I reporter construct and 150 ng of either pcDNA3.1 or pcDNA3.1-C/EBPβ. A total of 5 × 105 cells were seeded into six-well plates and transfected using FuGENE reagent (Roche Molecular Biochemicals). After 24 h, the cells were washed with PBS and fed with serum-free DMEM. Forty hours after transfection, the cells were lysed (cell lysis reagent; Promega Corp.), and luciferase activity was analyzed in the supernatant using the luciferase assay system (Promega). Luciferase expression was normalized to total protein concentration.
Retroviral expression constructs and transduction of primary bone marrow cells.
Recombinant MSCV-IRES-GFP viruses (14, 24) expressing C/EBPα, C/EBPδ, C/EBPβ, C/EBPβ-T217A, or LIP were generated by inserting the appropriate C/EBP coding regions between the BglII and XhoI sites of MSCV-IRES-GFP. IGF-I-MSCV-IRES-GFP contains the rat IGF-I cDNA EcoRI fragment excised from IGF-I-pAT154 (provided by T. McCarthy and L. Murphy) and inserted into the EcoRI site of MSCV-IRES-GFP. Viral DNAs were transfected into the φNX viral packaging line using a standard CaPO4 method to produce infectious ecotropic retroviruses. Transfection efficiency (usually >80%) was monitored by green fluorescent protein (GFP) expression. Viral supernatants were collected 24, 36, and 48 h after transfection and filtered. For infections, bone marrow from C/EBPβ−/− mice was resuspended in viral supernatant supplemented with 8 μg of Polybrene/ml, 100 ng of M-CSF/ml, 30 ng of IL-3/ml, and 100 ng of SCF/ml. A total of three mouse stem cell virus (MSCV) infections were performed over a 36-h period, after which the cells were infected once with J2 virus supernatant or control medium supplemented with 8 μg of Polybrene/ml and 100 ng of M-CSF/ml and cultured for 3 days. The cells were washed to remove M-CSF, counted, and plated for colony (CFU) assays without exogenous GFs.
CFU assays.
A total of 104 J2-transformed macrophages or 105 primary bone marrow cells were mixed with 2 ml of 0.35% agar (SeaPlaque, low-melting-temperature agarose; FMC) in DMEM-10% FBS, with or without 400 ng of M-CSF/ml or the indicated concentration of IGF-I. The cells were plated into gridded 35-mm dishes and incubated at 37°C for 7 days, and colonies (>50 cells) were scored.
Neutral red viability assay.
Neutral red dye uptake was used to measure viable cells after GF withdrawal. wt and C/EBPβ−/− BMDM or the J2-transformed cell lines were seeded at 6 × 104 to 8 × 104 cells/well in six-well dishes and cultured overnight, at which point M-CSF was either removed or maintained in the medium. At the indicated time points, 12 μl of 0.33% neutral red (Sigma) was added to the wells and incubated for 1.5 h at 37°C. The medium was removed, and the cells were washed three times with PBS. Dye was extracted from the cells using 1.0 ml of 50% ethanol-50 mM sodium citrate, pH 4.2, and the absorbance was measured at 540 nm.
Apoptosis detection by annexin V staining.
J2-WT1 and J2-KO1 cells were seeded at 5 × 105 cells in 10-cm dishes. After 24 h, the cells were given fresh medium with or without M-CSF, and 24 h later the medium was collected and adherent cells were dislodged with 1 ml of trypsin-EDTA (GIBCO). Adherent and nonadherent populations were pooled and centrifuged for 5 min at 350 × g. A total of 2 × 105 cells were resuspended in 50 μl of 1× annexin V binding buffer (10 mM HEPES [pH 7.4], 140 mM NaCl, and 2.5 mM CaCl2) supplemented with 3 μl of annexinV-FITC (Pharmingen) and 3 μl of propidium iodide (PI) solution (50 μg/ml). The cells were gently mixed and incubated for 5 min at room temperature in the dark, and then 350 μl of 1× annexin V binding buffer was added to each sample. Cells were collected ungated (10,000 events) and analyzed by two-color flow cytometry using a FACScan instrument (Becton Dickinson). The data were further analyzed using CellQuest (Becton Dickinson) to discriminate apoptotic from necrotic cells. Both annexin V-positive and annexin V-PI-double-positive cells were considered to be apoptotic.
DNA fragmentation assay.
The apoptotic DNA ladder kit (Roche) was used to analyze DNA fragmentation in apoptotic cells according to the manufacturer's instructions. A total of 2 × 106 cells were seeded into 10-cm dishes and cultured overnight in the presence of M-CSF. M-CSF was withdrawn, the cells were harvested, and nuclear DNA was prepared at 0, 4, and 8 h. DNA was analyzed on a 1% agarose gel stained with ethidium bromide.
Immunoblotting.
Proteins (50 μg of nuclear extract) were electrophoresed on 12% polyacrylamide Tris-glycine gels (Pharmingen) and transferred to Immobilon-P membranes (Millipore). Membranes were blocked with 5% dry milk in Tris-buffered saline (pH 7.6), probed with antibodies to C/EBPα, -β, or -δ (Santa Cruz), and developed using horseradish peroxidase-conjugated secondary antibody (Roche) and the ECL detection system (Pierce).
Expression arrays.
A murine apoptosis and survival expression array (R&D Systems) was used to identify differentially expressed proapoptotic and prosurvival genes. Briefly, the RNeasy maxi kit (Qiagen) was used to purify total RNA from J2-WT1 and J2-KO1 cells. Specific primers were used to synthesize 32P-labeled cDNAs, and the radiolabeled cDNA probes were then hybridized to duplicate nylon membranes containing the gene array. Following a series of high-stringency washes, the membranes were exposed to phosphorimager screens and the signals were quantitated using a Storm 860 PhosphorImager (Molecular Dynamics). The transcript levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
RPA and RT-PCR.
Total RNA was extracted from BMDM and the J2-transformed cell lines using TRIzol reagent (Invitrogen). For RPA, a probe set containing murine IGF-I, GAPDH, and L32 probes (BD-Pharmingen) was labeled with [α-32P]UTP (Amersham) using the Riboquant in vitro assay kit (BD-Pharmingen), and protection assays were performed on 10 μg of each RNA using the Riboquant RPA kit (BD-Pharmingen) as recommended by the supplier. Products were analyzed on 5% denaturing polyacrylamide gels. IGF-I transcripts were quantitated using the Storm 860 PhosphorImager (Molecular Dynamics) and normalized to GAPDH. To analyze IGF-I expression by reverse transcriptase PCR (RT-PCR), 2 μg of total RNA was reverse transcribed using an oligo(dT) primer and the reverse transcription kit (Promega) as described by the manufacturer. A fraction of each cDNA reaction mixture was amplified with primers specific for IGF-I (sense primer, 5′-ATGTCGTCTTCACATCTCTTC; antisense primer, 5′-CATTCTGTAGGTCTTGTTTCC) or GAPDH (18) for 25 and 15 cycles, respectively. The products (IGF-I, 384-bp doublet; GAPDH, 190 bp) were analyzed on 2% agarose gels stained with ethidium bromide. The IGF-I product was verified by sequencing.
Tumor assays and tumor-derived cell lines.
A total of 5 × 104 J2-WT1 or J2-KO1 cells were injected into the peritoneal cavities of nude mice. The animals were monitored for tumors, as evidenced by peritoneal swelling and morbidity, and mice were sacrificed upon onset of severe disease. To generate tumor-derived cell lines, peritoneal fluid was collected from animals showing tumor development. The cells were cultured in M-CSF for several weeks and analyzed for survival following M-CSF withdrawal, as well as for IGF-I expression.
RESULTS
C/EBPβ−/− bone marrow cells are refractory to J2-induced transformation.
Infection of murine bone marrow cultures with the J2 (myc/raf) retrovirus leads to the eventual outgrowth of transformed, macrophage-like cells that display GF independence (6, 15). To determine whether C/EBPβ is required for transformation of these cells, we infected bone marrow from C/EBPβ-deficient mice or their wt littermates with J2 and cultured the cells in the presence of GM-CSF for 7 days. GM-CSF was then removed, and viable cells were monitored over an 18-day period. As shown in Fig. 1A, wt cells subjected to this protocol became immortalized and their numbers increased over time. In contrast, C/EBPβ−/− cells failed to proliferate and no viable cells were obtained. In four independent experiments, we were unable to isolate immortalized macrophages from C/EBPβ-deficient bone marrow, showing that these cells are blocked at a critical step in transformation.
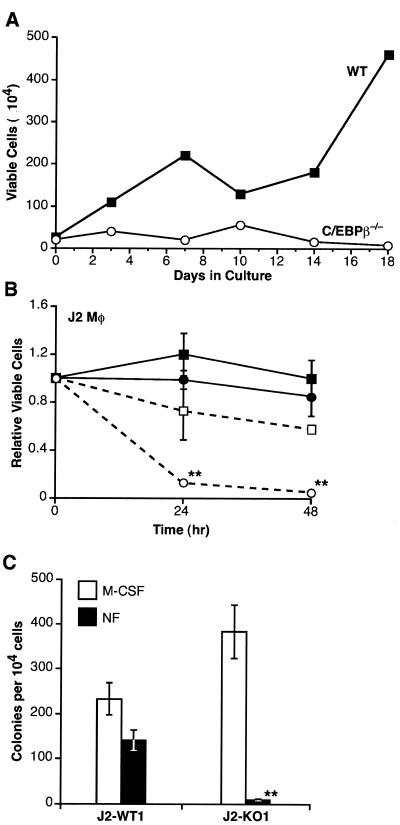
FIG. 1.
J2-transformed macrophages require C/EBPβ for survival in the absence of exogenous GFs. (A) wt and C/EBPβ−/− bone marrow cells were infected with J2 and maintained in GM-CSF. After 7 days, GF was removed and viable cells were measured over a time course by trypan blue exclusion. The experiment shown is representative of four independent trials. (B) GF dependence of macrophage cell lines. J2-transformed wt (J2-WT1; solid symbols) and C/EBPβ−/− (J2-KO1; open symbols) macrophage (Mφ) cell lines were cultured in the presence (squares) or absence (circles) of 100 ng of M-CSF/ml. Cell viability was measured at 0, 24, and 48 h by neutral red dye uptake. Data are the average of four independent experiments, with standard errors indicated. (C) CFU assays. A total of 104 J2-WT1 or J2-KO1 cells were plated in soft agar in the presence or absence of 200 ng of M-CSF/ml (NF, no factor). After 7 days, colonies (>50 cells) were scored. Data are the average ± standard error of the mean of two independent experiments, each performed in duplicate. Statistical analysis of the data in panels B and C was performed using the unpaired Student's t test (**, P < 0.01).
To test the possibility that transformed C/EBPβ−/− cells do not survive because they remain dependent on exogenous GFs, J2-infected cells were cultured continuously in the presence of myeloid GFs. In these experiments we used M-CSF, since it is a potent growth and survival factor for macrophages (63). By maintaining cells in M-CSF continuously, we could readily establish cell lines from both wt and C/EBPβ−/− bone marrow (J2-WT1 and J2-KO1, respectively). We were also able to obtain J2-transformed C/EBPβ−/− cell lines by using GM-CSF (data not shown). Analysis of cell surface markers confirmed that J2-WT1 and J2-KO1 cells belong to the monocyte/macrophage lineage (data not shown). Mock-infected cells eventually ceased to proliferate, while J2-infected cells of both genotypes showed unabated growth in culture, demonstrating that the cells were virally transformed.
Transformed C/EBPβ−/− macrophages are GF dependent.
Having established viable C/EBPβ-deficient cell lines, we next asked whether these cells could proliferate and/or survive after M-CSF withdrawal. M-CSF was removed from J2-WT1 and J2-KO1 cells, and cell viability was monitored over time using the neutral red dye uptake assay (Fig. 1B). Within 24 h, nearly all of the C/EBPβ−/− cells had died, whereas the wt cells remained viable. Similar results were obtained using clonal isolates of these cell lines (J2-WT1.CL14 and J2-KO1.CL10), as well as with an independent pair of wt and C/EBPβ−/− polyclonal cell lines (data not shown). We also examined the GF dependence of wt and C/EBPβ null cell lines using colony formation (CFU) assays. Cells were plated in soft agar with or without M-CSF, and colonies were scored after 7 days (Fig. 1C). J2-WT1 cells formed colonies efficiently in both the presence and absence of M-CSF, while J2-KO1 cells were dependent on M-CSF for colony growth. Thus, in contrast to J2-WT1 cells, transformed C/EBPβ−/− cells showed complete dependence on exogenous GFs for proliferation and/or survival in both liquid culture and colony assays.
We next examined whether J2-KO1 cells undergo apoptosis upon removal of M-CSF. DNA extracted from J2-KO1 cells following M-CSF withdrawal showed the 200-bp laddering pattern indicative of cells undergoing apoptosis (Fig. 2A). To confirm that M-CSF protects J2-KO1 cells from apoptosis, we analyzed programmed cell death in wt and C/EBPβ-deficient macrophages using annexin V staining. As shown in Fig. 2B, approximately 20% of J2-WT1 and J2-KO1 cells maintained in M-CSF were apoptotic by this assay. However, GF withdrawal for 24 h caused death in 80% of the J2-KO1 cells, while only 30% of the J2-WT1 cells were apoptotic. Collectively, these data demonstrate that J2-KO1 macrophages require an exogenous GF to inhibit cell death.
FIG. 2.
Apoptosis is induced in transformed C/EBPβ-deficient macrophages following M-CSF withdrawal. (A) J2-KO1 cells (2 × 106) were plated and cultured with or without M-CSF (NF, no factor). DNA was isolated at 0, 4, and 8 h after M-CSF withdrawal. Three micrograms of each DNA sample, or positive control DNA from apoptotic U937 cells (lane 7), was analyzed by agarose gel electrophoresis. (B) J2-WT1 or J2-KO1 macrophages (106) were incubated in the presence or absence of M-CSF for 24 h. Adherent and nonadherent cells were collected and stained with annexin V-FITC and PI to assess apoptosis. Dot plots of flow cytometry data from a representative experiment are shown, with PI staining on the y axis and annexin V staining on the x axis. The percentages of each subset are indicated. Cells in the lower right quadrant (annexin V positive) and the upper right quadrant (annexin V-PI double positive) are considered apoptotic. In the right panel, the results are graphed as the mean percentage of apoptotic cells (± standard error of the mean) from three to five independent experiments. (C) GF dependence of primary macrophages. Untransformed BMDM from wt and C/EBPβ−/− mice were cultured in the presence or absence of 100 ng of M-CSF/ml. Cell viability was measured at 0, 24, and 48 h by neutral red dye uptake. Data are the average of three independent experiments, with standard errors indicated. Statistical analysis of the data in panels B and C was performed using the unpaired Student's t test (*, P < 0.05; **, P < 0.01).
To determine whether C/EBPβ also influences the survival of nontransformed cells, we examined the GF dependence of BMDM from wt and C/EBPβ−/− mice. BMDM were generated by culturing bone marrow in M-CSF for 7 days, after which M-CSF was removed and cell survival was assessed. Cells of both genotypes underwent apoptosis within 24 h following GF withdrawal, exhibiting nearly identical death curves (Fig. 2C). These results confirmed the strict GF dependence of primary hematopoietic cells (44, 63) and, more importantly, demonstrated that C/EBPβ does not affect apoptosis of nontransformed macrophages. Thus, C/EBPβ is critical for GF independence of transformed macrophages but does not influence survival of normal cells.
Transformed macrophages require the MEK1/2 and phosphatidylinositol 3-kinase (PI3K) signaling pathways to suppress apoptosis.
To begin to elucidate the signaling pathway(s) in which C/EBPβ functions to promote cell survival and transformation, we examined the effects of specific kinase inhibitors on cell viability. J2-WT1 and J2-KO1 cells were treated with inhibitors of MEK1/2 (PD98059) or PI3K (LY294002) in the presence or absence of M-CSF, and viability was monitored. Blocking MEK1/2 signaling induced death of wt cells, but only in the absence of M-CSF (Fig. 3A). PD98059 also accelerated apoptosis of J2-KO1 cells when GF was withdrawn, while M-CSF again blocked cell death. LY294002 elicited apoptosis of both wt and mutant cells, irrespective of whether M-CSF was present or absent (Fig. 3B). This result is consistent with previous studies showing that a PI3K-dependent pathway mediates the prosurvival effects of M-CSF (27, 34). Taken together, these experiments indicate that signaling through MEK1/2 is necessary for transformed cells to survive in the absence of exogenous GFs. Furthermore, cells of both genotypes require PI3K activity regardless of whether they are stimulated by M-CSF, suggesting that PI3K functions in both GF-dependent and GF-independent pathways to suppress apoptosis.
FIG. 3.
Signaling through MEK1/2 and PI3K is required for survival of transformed macrophages. (A) J2-WT1 and J2-KO1 macrophages (∼1 × 105 cells) were treated with 20 μM PD98059 (to inhibit MEK1/2) or vehicle (dimethyl sulfoxide), in the presence and absence of M-CSF (NF, no factor). Neutral red uptake was used to measure viable cells at 0, 24, and 48 h after addition of the inhibitor. The mean values and standard errors at each time point were derived from three to four independent experiments. Statistical analysis (unpaired Student's t test) showed that J2-WT1 cells treated with PD98059 had significantly reduced survival (*, P < 0.05) and that J2-KO1 cells cultured in the presence of M-CSF were unaffected by treatment with the inhibitor. (B) The same experiment was performed as in panel A, except that cells were treated with 40 μM LY294002 (to inhibit PI3K). Statistical analysis indicated that both cell lines showed a significant loss in viability upon addition of the inhibitor, with or without M-CSF treatment.
IGF-I is a C/EBPβ target gene in transformed macrophages.
The fact that J2-KO1 cells, but not J2-WT1 cells, require GFs to suppress apoptosis suggests that C/EBPβ may regulate the expression of an antiapoptotic gene(s). To identify possible transcriptional targets of C/EBPβ, we screened a microarray containing genes involved in apoptosis and survival. The array was hybridized with labeled cDNAs derived from J2-WT1 and J2-KO1 RNA samples, and the ratio of normalized expression of each gene in the two cell lines was determined (Table 1). Twenty-three genes showed detectable transcript levels, some of which were decreased or increased moderately in C/EBPβ−/− cells. Notably, the expression of IGF-I was reduced nearly sixfold in J2-KO1 cells. As IGF-I has potent mitogenic and antiapoptotic activities and IGF-I signaling has been linked to the growth and survival of many tumor cells (42, 68), this gene was a plausible candidate to mediate the prooncogenic effects of C/EBPβ.
TABLE 1.
Analysis of putative C/EBPβ target genesa
| Gene | Description | KO/WTb |
|---|---|---|
| MCSFR | CSF receptor | 0.96 |
| GMCSFR-α | CSF 2 receptor α (low affinity) | 0.62 |
| IL-1Rγ | IL-1 receptor, γ chain | 0.93 |
| TGF-β | TGF β-1 | 0.75 |
| IGF-I | Insulin-like growth factor I | 0.17 |
| TNFSF13B | B-cell activating factor | 3.11 |
| BAK | BCL2 homologous antagonist | 0.56 |
| Bax-a | BCL2-associated X protein | 0.76 |
| Bag-1 | BCL2-associated athanogene 1 | 1.00 |
| A1 | B cell leukemia/lymphoma 2-related protein | 3.86 |
| MCL-1 | Myeloid cell leukemia sequence 1 | 1.36 |
| Cyt C | Cytochrome c | 1.07 |
| RBP1 | RBP I isoform III | 1.14 |
| RP105 | Lymphocyte antigen 78 | 1.36 |
| PAK | P21-activated kinase 1 | 2.14 |
| MDM-2 | Mouse 3T3 cell double minute 2 | 0.60 |
| TRP53 | Transformation-related p53 | 0.86 |
| CBP | CREB binding protein | 1.40 |
| DP1 | Transcription factor DP1 | 0.37 |
| CDC2 | Cell division cycle 2 homologue A | 1.10 |
| ODC | Ornithine decarboxylase | 1.11 |
| Apex/Ref-1 | Apurinic/apyrimidinic endonuclease | 1.01 |
| Ctsd | Cathepsin D | 1.20 |
Total RNA from J2-WT1 and J2-KO1 cell lines was used to prepare radiolabeled cDNA probes that were hybridized to a mouse apoptosis-survival gene array (R&D Systems). The hybridization signals were quantitated by phosphorimaging and normalized to GAPDH.
Normalized values from J2-KO1 cells were divided by J2-WT1 values to calculate relative expression in the two cell lines (KO/WT). This experiment was performed twice with similar results. Note that IGF-I expression decreased nearly sixfold in J2-KO1 cells.
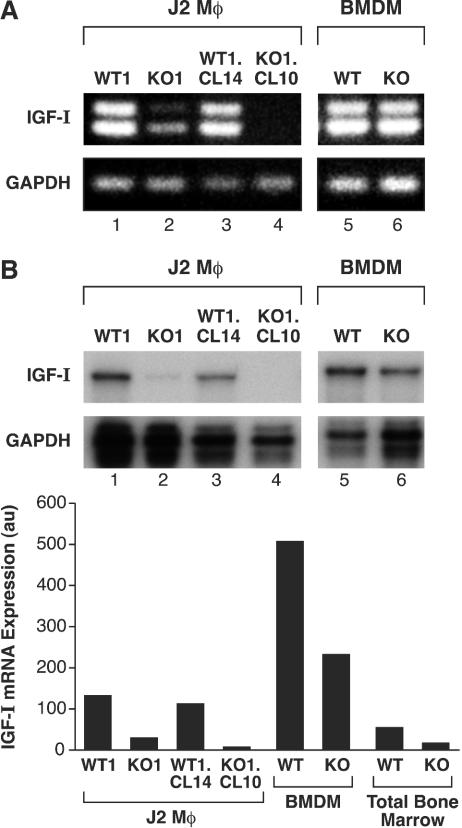
To confirm that IGF-I transcripts are decreased in C/EBPβ−/− cells, we performed RT-PCR and RPA. RT-PCR analysis (Fig. 4A) showed that IGF-I mRNA levels were markedly diminished in J2-KO1 cells (lane 2) compared to J2-WT1 cells (lane 1). This decrease was even more significant in the clonal cell lines J2-WT1.CL14 and J2-KO1.CL10 (lanes 3 and 4). RPA analysis gave similar results (Fig. 4B). Quantitation of the RPA signals showed that IGF-I levels in J2-KO1 and J2-KO1.CL10 cells were approximately 5- and 10-fold lower than in J2-WT1 and J2-WT1.CL14 cells, respectively. We also examined IGF-I transcripts in nontransformed BMDM prepared from wt and C/EBPβ−/− animals (Fig. 4). IGF-I transcripts were relatively abundant in both wt and C/EBPβ-deficient BMDM. However, IGF-I levels in C/EBPβ−/− BMDM were approximately twofold lower than in wt cells, indicating that C/EBPβ makes some contribution to IGF-I expression in nontransformed cells. This decrease was also observed in freshly isolated total bone marrow from wt and C/EBPβ−/− mice. In summary, C/EBPβ has a moderate effect on IGF-I expression in primary macrophages but is critical for maintaining IGF-I gene transcription in J2-transformed cells.
FIG. 4.
Diminished IGF-I mRNA expression in transformed macrophages and bone marrow from C/EBPβ−/− mice. (A) RT-PCR analysis was performed on total RNA from J2-WT1 and J2-KO1 cells, their clonal derivatives (J2-WT1.CL14 and J2-KO1.CL10), and primary macrophages (BMDM). Total RNA was reverse transcribed with an oligo(dt) primer, and the cDNA was amplified with IGF-I- and GAPDH-specific primers, generating 384-bp (doublet) and 190-bp products, respectively. The IGF-I product was verified by sequencing. (B) RPAs were used to analyze the RNA samples shown in panel A and total bone marrow RNA isolated from wt and C/EBPβ−/− female mice. RNAs were hybridized to IGF-I and GAPDH probes and digested with RNase. Protected fragments corresponding to IGF-I transcripts were quantitated by phosphorimaging and normalized to GAPDH (au, arbitrary units). The experiment was performed twice with similar results.
Promoter 1 of the IGF-I gene contains a C/EBP site (termed HS3D) in the first exon that mediates transcriptional activation by C/EBPδ in osteoblasts (61, 64, 65). Since IGF-I levels are substantially diminished in J2-KO1 macrophages, we examined whether IGF-I promoter 1 can be activated by C/EBPβ. Due to the poor transfection efficiency of macrophages, we used the human hepatocellular carcinoma cell line HepG2 for these transactivation experiments. As illustrated in Fig. 5, overexpression of C/EBPβ strongly increased expression from a reporter construct driven by IGF-I promoter 1 (IGF1711b-Luc) and, to a lesser extent, activated an artificial promoter containing four copies of the putative C/EBP site (4xHS3D-Luc). An analogous construct containing a mutated C/EBP element (4xHS3Dmut-Luc) showed much lower activity both with and without C/EBPβ overexpression. Thus, C/EBPβ can directly activate transcription of the IGF-I gene.
FIG. 5.
C/EBPβ transactivates the IGF-I promoter 1. The IGF-I promoter-reporter constructs IGF1711b-Luc, 4xHS3D-Luc, and 4xHS3Dmut-Luc were cotransfected with either empty pcDNA3.1 or pcDNA3.1-C/EBPβ into HepG2 cells. The cells were serum starved for 24 h, lysates were prepared, and luciferase activity was analyzed. The luciferase data were normalized to total protein and are the average of two independent experiments, each performed in triplicate with standard errors indicated. Statistical analysis of the data was performed using Student's unpaired t test (**, P < 0.01).
Autocrine IGF-I signaling is required for survival of J2-WT1 cells.
The preceding experiments showed that IGF-I expression in transformed macrophages requires C/EBPβ, raising the possibility that IGF-I provides an autocrine signal that inhibits apoptosis. To test this hypothesis, we treated J2-WT1 cells with a neutralizing antibody against IGF-I. As shown in Fig. 6A, the anti-IGF-I antibody had no effect on cell survival when M-CSF was present. However, in the absence of M-CSF, neutralization of soluble IGF-I significantly reduced cell viability, such that only ∼30% of the cells remained alive after 48 h. In contrast, the same cells cultured with a control antibody survived and continued to proliferate. Thus, J2-WT1 cells treated with the IGF-I antibody require an exogenous GF to suppress apoptosis. We also used CFU assays to determine whether purified IGF-I can substitute for M-CSF to promote colony formation of J2-KO1 cells (Fig. 6B). A significant increase in colony number was observed when the cells were plated in the presence of 10 ng of IGF-I/ml. CFU activity was further enhanced with increasing doses of IGF-I, up to a maximum of 110 colonies with 200 ng of IGF-I/ml.
FIG. 6.
IGF-I is required for survival of transformed macrophages. (A) J2-WT1 cells were plated (105 cells per dish) and treated for the indicated times with 10 μg of IGF-I neutralizing antibody or nonimmune IgG control/ml, in the presence or absence of M-CSF (NF, no factor). Neutral red dye uptake was used to measure cell viability at 0, 24, and 48 h after antibody treatment. The data were normalized to the number of viable cells at 0 h and are the average ± standard error of the mean of three independent experiments. (B) J2-KO1 cells (104 cells per dish) were plated in soft agar with increasing concentrations of human IGF-I. After 7 days, colonies (>50 cells) were scored. Each assay was performed in duplicate with standard errors indicated. (C) Transformed IGF-I−/− macrophages require M-CSF for survival. IGF-I−/− and IGF-I+/− bone marrow cells were infected with J2 and maintained in M-CSF for several weeks. Cells (5 × 104) were plated in soft agar in the presence or absence of M-CSF, and after 7 days colonies (>50 cells) were scored. The data are means ± standard errors of the means from two independent experiments, each performed in duplicate. Statistical significance was evaluated using Student's unpaired t test (*, P < 0.05; **, P < 0.01).
To test genetically whether IGF-I has a prosurvival function in macrophage tumor cells, we infected bone marrow cells isolated from IGF-I−/− mice or their heterozygous littermates with J2 and established cell lines by growth in M-CSF. We then assessed transformation by analyzing CFU activity in the presence or absence of M-CSF (Fig. 6C). Both cell lines formed colonies efficiently in the presence of GF (>600 colonies/105 cells). However, J2-transformed IGF-I+/− cells maintained high CFU activity in the absence of M-CSF (190 colonies/105 cells), whereas only 14 colonies were obtained with IGF-I−/− cells. Thus, as was observed for C/EBPβ−/− cells, transformed IGF-I−/− macrophages showed nearly complete dependence on exogenous GFs. The data of Fig. 6 suggest that IGF-I is a critical effector of the C/EBPβ-dependent survival pathway in transformed macrophages.
Ectopic expression of C/EBPβ or IGF-I in C/EBPβ−/− cells restores J2-mediated transformation.
To demonstrate that C/EBPβ deficiency is responsible for the impaired survival of C/EBPβ−/− macrophages, we sought to reinstate GF independence in these cells by ectopically expressing C/EBPβ. For technical reasons, we were unable to transduce J2-transformed macrophages using viral or plasmid vectors. Therefore, we used a protocol in which bone marrow from C/EBPβ−/− mice was sequentially infected with a retrovirus expressing C/EBPβ and then with J2. After maintaining the doubly infected cells for several days in culture, the cells were plated in soft agar without GFs to assess CFU activity. As a test of this approach, we infected wt and C/EBPβ−/− bone marrow with J2 alone and plated the cells in soft agar without M-CSF (Fig. 7A). Colonies were obtained with J2-infected but not uninfected wt bone marrow, while no colonies were observed with C/EBPβ-deficient cells. These data confirm the absolute requirement for C/EBPβ in J2-transformed monocytic cells and show that C/EBPβ function can be assessed using the J2 infection and CFU assay.
FIG.7.
Expression of C/EBPβ or IGF-I promotes survival of J2-transformed C/EBPβ−/− bone marrow cells. (A) Bone marrow was collected from wt and C/EBPβ−/− mice, mock infected (−) or infected with J2 virus (+), cultured in M-CSF for 3 days, and assayed for colony formation in soft agar without M-CSF. Colonies were scored after 7 days. Each assay was performed in duplicate, and the data were averaged. (B) Using the protocol depicted, C/EBPβ−/− bone marrow cells were infected with either empty MSCV vector (Ctrl or C) or MSCV containing murine C/EBPβ, LIP (a truncated form of C/EBPβ lacking the transactivation domain), C/EBPβT217A (containing a disrupted Rsk phosphorylation site), C/EBPα, C/EBPδ, or rat IGF-I cDNA, followed by infection with the J2 virus (J2) or medium alone (Mock). Forty-eight hours after J2 infection, M-CSF was removed and 105 cells were plated in soft agar. After 7 days colonies (>50 cells) were counted. The data are means of two independent experiments, each performed in duplicate, with the indicated standard errors. Statistical analysis of the data in panels A and B was performed using the unpaired Student's t test (*, P < 0.05; **, P < 0.01). Lower panel: C/EBPβ−/− cells infected with the indicated C/EBP vectors and transformed with J2 were grown in liquid culture with M-CSF. After 10 days, nuclear extracts were prepared and analyzed by Western blotting to assess C/EBP protein expression. Proteins of interest are indicated with arrows; nonspecific, cross-reacting bands are indicated with asterisks. (C) Morphology of colonies from C/EBPβ−/− bone marrow infected with MSCV-IGF-I (IGF-I) or MSCV-C/EBPβ (C/EBPβ) and transformed with J2. Photographs were taken at 4× magnification and are representative of two to three experiments. IGF-I-expressing colonies were similar in size to those from C/EBPβ+/+ J2-infected cells (data not shown).
We next asked whether introducing C/EBPβ into C/EBPβ−/− bone marrow cells using a viral vector (MSCV-C/EBPβ) renders them susceptible to J2 transformation. C/EBPβ restored CFU activity in C/EBPβ−/− cells that were infected with J2 (Fig. 7B); however, expression of C/EBPβ without J2 infection (mock) did not promote colony formation. Two other C/EBP family members, C/EBPα and C/EBPδ, failed to complement C/EBPβ deficiency in this assay. The expression of each C/EBP protein was confirmed by Western blotting (Fig. 7B, lower panel). Thus, C/EBPβ is unique in its ability to support J2-induced transformation.
A previous study suggested that phosphorylation of Thr217 in murine C/EBPβ by p90 Rsk is required to suppress apoptosis in CCl4-treated hepatic stellate cells (12). To determine if a similar Thr217-dependent mechanism is operative in transformed macrophages, we tested a C/EBPβ alanine substitution mutant (T217A) in the CFU assay. T217A was only slightly less effective than wt C/EBPβ in restoring transformation to C/EBPβ-deficient cells. Moreover, a truncated form of C/EBPβ known as LIP (17), which lacks the N-terminal transactivation domain but retains the Thr217 phospho-acceptor site, did not exhibit CFU-promoting activity. The results indicated that phosphorylation of Thr217 is dispensable for J2 transformation and suggested that the transcriptional activity of C/EBPβ is required for its prooncogenic function.
We also used the CFU assay to test whether enforced expression of IGF-I can substitute for C/EBPβ in neoplastic transformation. Infection of C/EBPβ−/− cells with a virus encoding the rat IGF-I cDNA (MSCV-IGF-I) caused partial restoration of J2-induced colony formation. Interestingly, IGF-I was considerably less active than C/EBPβ in facilitating transformation as assessed by CFU number (Fig. 7B). In addition, colonies from the C/EBPβ-transduced cells were very large, while those expressing IGF-I were of normal size (Fig. 7C). These observations indicate that C/EBPβ is more effective than IGF-I in promoting growth and survival of transformed cells. Although there are several potential explanations for this result, it is possible that C/EBPβ regulates the expression of at least one other growth and/or survival factor besides IGF-I and that the combined effects of these autocrine factors are required for efficient neoplastic transformation.
Impaired tumorigenicity of C/EBPβ-deficient cells in nude mice.
J2-transformed macrophages are highly tumorigenic when injected into nude mice, producing histiocytic tumors and mortality within approximately 3 weeks (7, 8). To determine whether C/EBPβ deficiency affects the growth of J2 tumor cells in vivo, we analyzed the tumorigenicity of J2-WT1 and J2-KO1 cells in athymic nude mice. Cells were injected into the peritoneal cavities of recipient mice, and the animals were monitored for signs of disease. Mice receiving J2-WT1 cells began to show abdominal swelling and morbidity after ∼20 days, and by 40 days only ∼10% of the animals remained viable (Fig. 8A). By contrast, ∼80% of the animals containing J2-KO1 cell xenografts showed viability after 40 days; however, the majority of these mice eventually succumbed to disease. J2-KO1 cells thus exhibit reduced tumorigenic potential.
FIG. 8.
J2-KO1 cells display delayed tumorigenicity in nude mice. (A) Athymic nude mice were injected intraperitoneally with 5 × 104 J2-WT1 or J2-KO1 cells. The animals were monitored for histiocytic tumor development (indicated by peritoneal swelling and morbidity) and were sacrificed upon onset of severe disease symptoms. The data (percent surviving animals) are combined from two independent experiments, each containing 10 to 13 animals. (B) Peritoneal tumor cells were collected from mice showing signs of disease and placed in culture with M-CSF. One wt (J2-WT1.T20) and two mutant (J2-KO1.T32 and J2-KO1.T37) tumor-derived cell lines were established and then assayed for survival in the presence or absence of M-CSF (NF, no factor). Viable cells were determined at 0, 24, and 48 h by neutral red dye uptake. The data are the average of three independent experiments. (C) IGF-I mRNA expression was measured by RPA in the parental J2-WT1 and J2-KO1 cells and in tumor-derived cell lines (J2-WT1.T20, J2-KO1.T32, and J2-KO1.T37). IGF-I transcripts were quantitated by phosphorimaging and normalized to GAPDH and are expressed as arbitrary units (au).
The delayed tumorigenicity of C/EBPβ−/− cells could be due to their impaired growth and/or survival rates, which are nonetheless sufficient to cause disease. Alternatively, the cells may be nontumorigenic initially but undergo a mutation or selection process in vivo such that variants with an enhanced capacity to survive eventually predominate. To distinguish between these possibilities, we recovered tumor cells from recipient mice and reestablished the cells in culture by growth in M-CSF. One wt and two mutant tumor-derived cell lines were obtained (J2-WT1.T20, J2-KO1.T32, and J2-KO1.T37) and tested for GF dependence (Fig. 8B). J2-WT1.T20 cells continued to proliferate in the absence of M-CSF. Withdrawal of M-CSF from the two tumor-derived C/EBPβ−/− cell lines caused a reduced rate of proliferation but no cell death was observed, in distinct contrast to the original J2-KO1 cell line. Thus, C/EBPβ−/− tumor-derived cells display increased resistance to apoptosis. Notably, IGF-I mRNA levels in J2-KO1.T32 and J2-KO1.T37 cells were elevated approximately fourfold compared to the parental J2-KO1 cell line (Fig. 8C). Collectively, these results suggest that C/EBPβ-deficient cells undergo selection in vivo that leads to the establishment of GF-independent tumor cells with increased IGF-I expression.
DISCUSSION
In this study we demonstrate that the transcription factor C/EBPβ is essential for oncogenic transformation of macrophage-like cells by myc and raf. Transformed C/EBPβ−/− macrophages undergo programmed cell death in the absence of exogenous hematopoietic GFs, whereas C/EBPβ+/+ tumor cells are intrinsically resistant to apoptosis. The survival of these transformed macrophages requires autocrine signaling by IGF-I, whose expression is regulated by C/EBPβ. These findings represent the first direct evidence that C/EBPβ and IGF-I are essential for oncogenic transformation of mammalian myeloid cells and provide a molecular mechanism to explain the self-sufficiency (GF independence) of macrophage tumor cells. Previous work using an avian system showed that v-Raf induces an IL-6-like cytokine called chicken myelomonocytic growth factor (cMGF) that is necessary for transformation of myeloid cells (22, 40) and that the avian homologue of C/EBPβ, NF-M, regulates the cMGF promoter (55, 56). Thus, in both the avian and mammalian systems C/EBPβ appears to activate an autocrine loop that allows transformed cells to become GF independent.
How do C/EBPβ, IGF-I, and M-CSF suppress apoptosis in transformed macrophages? The survival defect in C/EBPβ-deficient tumor cells can be overcome by administration of M-CSF, indicating that the antiapoptotic pathway activated by this cytokine does not require C/EBPβ as a downstream target. Previous studies have shown that M-CSF signaling in myeloid cells activates the Ras and PI3K cascades and that inhibition of PI3K blocks the prosurvival activity of M-CSF (27, 31, 34). The importance of PI3K and its effector, Akt, in IGF-I signal transduction has been widely documented (2, 29, 46). Therefore, we suggest that the PI3K/Akt signaling pathway suppresses apoptosis in macrophage tumor cells by a C/EBPβ-independent mechanism. The fact that transformed C/EBPβ+/+ cells cultured without M-CSF also require PI3K activity to survive (Fig. 3B) indicates that this pathway is engaged even in the absence of exogenous GFs and suggests that autocrine IGF-I activates PI3K signaling in transformed macrophages. C/EBPβ-deficient cells, lacking IGF-I expression, are unable to activate the PI3K cascade and thus require external GF signals to stimulate this critical pathway.
Inhibitor studies demonstrated that MEK1/2 signaling also plays an essential role in suppressing apoptosis in J2-transformed cells (Fig. 3A). We anticipated that the MEK1/2 requirement might be due to posttranslational activation of C/EBPβ by v-Raf signaling, resulting in transactivation of the IGF-I promoter. In this case, IGF-I expression should be blocked by PD98059. However, we observed no reduction in IGF-I mRNA levels when J2-WT1 cells were treated with the MEK1/2 inhibitor for up to 36 h (data not shown). These results imply the existence of a MEK1/2 downstream target that promotes cell survival but is distinct from IGF-I. The MEK1/2-dependent survival pathway could be either C/EBPβ dependent or independent.
Although the IGF-I gene is an important transcriptional target of C/EBPβ in transformed macrophages, several observations suggest that other C/EBPβ-regulated genes affect neoplastic growth and/or survival of these cells. First, overexpression of IGF-I by retroviral transduction was not as effective as C/EBPβ in restoring J2-induced CFU activity to C/EBPβ−/− BM cells. Second, C/EBPβ-transduced cells formed very large colonies in soft agar, while IGF-I overexpression caused minimal increase in colony size. Notably, a few of these large colonies were also observed in J2-transformed IGF-I−/− cells overexpressing C/EBPβ (data not shown). Third, relatively high concentrations of IGF-I were required to promote survival of C/EBPβ−/− cells in CFU assays. Taken together, these results imply that C/EBPβ may control the expression of a second factor, in addition to IGF-I, that is a potent mitogen in macrophages. Preliminary analyses indicate that this factor is distinct from M-CSF, GM-CSF, G-CSF, tumor necrosis factor alpha, gamma interferon, IL-6, IL-3, SCF, and members of the transforming growth factor β family (J. Wessells and P. F. Johnson, unpublished results). A recent study suggests that the oncogenic activity of cyclin D1 is mediated in part by its association with C/EBPβ, resulting in expression of a novel set of growth-promoting genes (30). Therefore, it will be of interest to determine whether these cyclin D1 target genes are regulated by C/EBPβ in transformed macrophages and are important for neoplastic growth of these cells.
The involvement of C/EBPβ in tumorigenesis was first demonstrated by the observation that C/EBPβ knockout mice fail to develop Ras-dependent skin papillomas in response to carcinogens such as dimethylbenzanthracene (71). A large increase in apoptotic epidermal cells was observed in carcinogen-treated C/EBPβ−/− mice compared to wt animals, indicating that C/EBPβ might promote survival of transformed keratinocytes and that the block to tumorigenesis in the C/EBPβ−/− epidermis may result from defective survival of precancerous cells. Although additional studies are needed to prove this model, it is possible that C/EBPβ plays a similar prosurvival role in macrophage and keratinocyte tumor cells. In this regard, IGF-I could also be an effector of the C/EBPβ-dependent survival pathway in skin tumors.
Although C/EBPβ has not been implicated as an oncogene per se, it was recently reported that enforced expression of C/EBPβ in MCF10A mammary epithelial cells induces a neoplastic phenotype, including focal growth, anchorage independence, and increased invasiveness in vitro (13). It is possible that overexpression of C/EBPβ in mammary epithelial cells activates expression of GFs such as IGF-I, causing sustained autocrine signaling that induces a neoplastic phenotype. Increased C/EBPβ expression has been observed in several kinds of tumors, including breast (26, 69), ovary (58), and colon (48). The functional significance of elevated C/EBPβ levels in these tumor cells has not been demonstrated; therefore, it will be important to determine whether C/EBPβ contributes to the transformed state of these and other cancers.
The prosurvival effects of C/EBPβ are not restricted to tumors, having also been observed in nontransformed liver-derived cells. Experimental liver injury elicited by CCl4 stimulates proliferation of hepatic stellate cells derived from wt mice, whereas CCl4 induces apoptosis in C/EBPβ-deficient stellate cells (11). The ability of C/EBPβ to prevent apoptosis apparently requires phosphorylation of Thr217 by the p90 Rsk kinase (11), generating an XEXD-like motif that serves as a caspase inhibitor site. The T217A substitution mutant was defective in suppressing apoptosis of stellate cells, lending credence to this model. In contrast, we found that the C/EBPβ T217A mutant was nearly as effective as wt C/EBPβ in restoring J2-induced colony formation to C/EBPβ−/− BM cells (Fig. 7B). Thus, phosphorylation of Thr217 by Rsk is clearly dispensable for the ability of C/EBPβ to inhibit apoptosis in transformed macrophages. C/EBPβ may function in distinct ways to promote cell survival in transformed myeloid cells and hepatic stellate cells, using transcriptional and nontranscriptional mechanisms, respectively.
A large body of evidence associates IGF signaling with tumorigenesis and cancer progression (32, 33). Increased levels of IGF-I or its receptor have been observed in many tumor cells (5), and IGF-I can promote the growth or survival of cancer cells in vitro (21, 42, 68). In addition, antisense inhibition of IGF-I or IGF-I receptor (IGF-IR) expression in melanoma and glioblastoma cells diminishes their proliferation and tumorigenicity in animals (3, 49, 50, 62). The fact that IGF-I null mice have poor viability (only 5 to 10% of mutant animals survive to adulthood) (4, 36) has impeded the investigation of tumorigenesis in these animals. To date, IGF-IR−/− mice have been more widely studied with respect to transformation and cancer (41, 53, 54, 66). Our results using IGF-I−/− and C/EBPβ−/− cells now provide direct evidence that IGF-I can function as an autocrine survival factor in cellular transformation.
This study identifies C/EBPβ as a physiological activator of the IGF-I gene in some cell types, particularly myeloid tumor cells and to a lesser extent in normal bone marrow. IGF-I mRNA expression is relatively high in both wt and C/EBPβ−/− BMDM but is significantly diminished in transformed cells that lack C/EBPβ, indicating that C/EBPβ is required to maintain high IGF-I levels in tumor cells. At present we do not know the mechanism that underlies the much-more-significant role of C/EBPβ in regulating IGF-I expression in transformed cells. However, a preliminary analysis of female mouse tissues showed that IGF-I mRNA levels in nonhematopoietic cells are largely unaffected by the absence of C/EBPβ, which suggests that additional transcription factors—perhaps C/EBP family members such as C/EBPδ—can control IGF-I gene expression. Further evidence for C/EBPβ-independent regulation is apparent from the tumor-derived C/EBPβ−/− macrophage cell lines, which display elevated IGF-I mRNA levels relative to the parental J2-KO1 cell line (Fig. 8C). We did not observe increased levels of C/EBPα or C/EBPδ in these cells (data not shown), consistent with our observation that neither of these C/EBP family members promotes J2-induced transformation of C/EBPβ−/− bone marrow cells. It remains to be determined whether the IGF-I gene can be activated in a completely C/EBP-independent manner.
In summary, our work establishes that C/EBPβ and IGF-I are critical components of an autocrine survival pathway in myeloid tumor cells. These findings, together with further studies to elucidate C/EBPβ-dependent and -independent mechanisms of IGF-I gene regulation, may facilitate the development of anticancer strategies based on inhibiting IGF-I production and thereby disrupting an essential prosurvival signal.
Acknowledgments
We thank T. McCarthy for the IGF-I cDNA and reporter constructs, H. Young for the J2-CRE cell line, J. Keller for assistance with CFU assays and FACS analysis, A. Gamero for assistance with the Annexin V apoptosis assays, R. Van Etten for the MSCV vector, R. Smart for stimulating discussions, and J. Shuman, C. McCauslin, T. Sebastian, and R. Schwartz for critical comments on the manuscript.
REFERENCES
- 1.Akira, S., H. Isshiki, T. Sugita, O. Tanabe, S. Kinoshita, Y. Nishio, T. Nakajima, T. Hirano, and T. Kishimoto. 1990. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 9:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, D. W., M. Resnicoff, A. E. Flanders, L. Kenyon, M. Curtis, G. Merli, R. Baserga, G. Iliakis, and R. D. Aiken. 2001. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J. Clin. Oncol. 19:2189-2200. [DOI] [PubMed] [Google Scholar]
- 4.Baker, J., J. P. Liu, E. J. Robertson, and A. Efstratiadis. 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75:73-82. [PubMed] [Google Scholar]
- 5.Baserga, R. 1995. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 55:249-252. [PubMed] [Google Scholar]
- 6.Blasi, E., B. J. Mathieson, L. Varesio, J. L. Cleveland, P. A. Borchert, and U. R. Rapp. 1985. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature 318:667-670. [DOI] [PubMed] [Google Scholar]
- 7.Blasi, E., D. Radzioch, L. Merletti, and L. Varesio. 1989. Generation of macrophage cell line from fresh bone marrow cells with a myc/raf recombinant retrovirus. Cancer Biochem. Biophys. 10:303-317. [PubMed] [Google Scholar]
- 8.Blasi, E., D. Radzioch, and L. Varesio. 1988. Inhibition of retroviral mRNA expression in the murine macrophage cell line GG2EE by biologic response modifiers. J. Immunol. 141:2153-2157. [PubMed] [Google Scholar]
- 9.Blasi, E., L. Varesio, and R. H. Wiltrout. 1988. Tumor formation by a murine macrophage cell line immortalized in vitro by v-raf and v-myc oncogenes. Cancer Immunol. Immunother. 27:109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchard, C., P. Staller, and M. Eilers. 1998. Control of cell proliferation by Myc. Trends Cell Biol. 8:202-206. [DOI] [PubMed] [Google Scholar]
- 11.Buck, M., V. Poli, T. Hunter, and M. Chojkier. 2001. C/EBPβ phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol. Cell 8:807-816. [DOI] [PubMed] [Google Scholar]
- 12.Buck, M., V. Poli, P. van der Geer, M. Chojkier, and T. Hunter. 1999. Phosphorylation of rat serine 105 or mouse threonine 217 in C/EBP beta is required for hepatocyte proliferation induced by TGF alpha. Mol. Cell 4:1087-1092. [DOI] [PubMed] [Google Scholar]
- 13.Bundy, L. M., and L. Sealy. 2003. CCAAT/enhancer binding protein beta (C/EBPβ)-2 transforms normal mammary epithelial cells and induces epithelial to mesenchymal transition in culture. Oncogene 22:869-883. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, L., C. Du, D. Murray, X. Tong, Y. A. Zhang, B. P. Chen, and R. G. Hawley. 1997. A GFP reporter system to assess gene transfer and expression in human hematopoietic progenitor cells. Gene Ther. 4:1013-1022. [DOI] [PubMed] [Google Scholar]
- 15.Cox, G. W., B. J. Mathieson, L. Gandino, E. Blasi, D. Radzioch, and L. Varesio. 1989. Heterogeneity of hematopoietic cells immortalized by v-myc/v-raf recombinant retrovirus infection of bone marrow or fetal liver. J. Natl. Cancer Inst. 81:1492-1496. [DOI] [PubMed] [Google Scholar]
- 16.Descombes, P., M. Chojkier, S. Lichtsteiner, E. Falvey, and U. Schibler. 1990. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 4:1541-1551. [DOI] [PubMed] [Google Scholar]
- 17.Descombes, P., and U. Schibler. 1991. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67:569-579. [DOI] [PubMed] [Google Scholar]
- 18.Dveksler, G. S., A. A. Basile, and C. W. Dieffenbach. 1992. Analysis of gene expression: use of oligonucleotide primers for glyceraldehyde-3-phosphate dehydrogenase. PCR Methods Appl. 1:283-285. [DOI] [PubMed] [Google Scholar]
- 19.Evan, G., E. Harrington, A. Fanidi, H. Land, B. Amati, and M. Bennett. 1994. Integrated control of cell proliferation and cell death by the c-myc oncogene. Philos. Trans. R. Soc. London B 345:269-275. [DOI] [PubMed] [Google Scholar]
- 20.Evan, G. I., and K. H. Vousden. 2001. Proliferation, cell cycle and apoptosis in cancer. Nature 411:342-348. [DOI] [PubMed] [Google Scholar]
- 21.Furstenberger, G., and H. J. Senn. 2002. Insulin-like growth factors and cancer. Lancet Oncol. 3:298-302. [DOI] [PubMed] [Google Scholar]
- 22.Graf, T., F. von Weizsaecker, S. Grieser, J. Coll, D. Stehelin, T. Patschinsky, K. Bister, C. Bechade, G. Calothy, and A. Leutz. 1986. v-mil induces autocrine growth and enhanced tumorigenicity in v-myc-transformed avian macrophages. Cell 45:357-364. [DOI] [PubMed] [Google Scholar]
- 23.Greenbaum, L. E., W. Li, D. E. Cressman, Y. Peng, G. Ciliberto, V. Poli, and R. Taub. 1998. CCAAT enhancer-binding protein beta is required for normal hepatocyte proliferation in mice after partial hepatectomy. J. Clin. Investig. 102:996-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley, R. G., F. H. Lieu, A. Z. Fong, and T. S. Hawley. 1994. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1:136-138. [PubMed] [Google Scholar]
- 25.Hu, H. M., M. Baer, S. C. Williams, P. F. Johnson, and R. C. Schwartz. 1998. Redundancy of C/EBP alpha, -beta, and -delta in supporting the lipopolysaccharide-induced transcription of IL-6 and monocyte chemoattractant protein-1. J. Immunol. 160:2334-2342. [PubMed] [Google Scholar]
- 26.Kagan, B. L., R. T. Henke, R. Cabal-Manzano, G. E. Stoica, Q. Nguyen, A. Wellstein, and A. T. Riegel. 2003. Complex regulation of the fibroblast growth factor-binding protein in MDA-MB-468 breast cancer cells by CCAAT/enhancer-binding protein beta. Cancer Res. 63:1696-1705. [PubMed] [Google Scholar]
- 27.Kelley, T. W., M. M. Graham, A. I. Doseff, R. W. Pomerantz, S. M. Lau, M. C. Ostrowski, T. F. Franke, and C. B. Marsh. 1999. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J. Biol. Chem. 274:26393-26398. [DOI] [PubMed] [Google Scholar]
- 28.Kowenz-Leutz, E., G. Twamley, S. Ansieau, and A. Leutz. 1994. Novel mechanism of C/EBP β (NF-M) transcriptional control: activation through derepression. Genes Dev. 8:2781-2791. [DOI] [PubMed] [Google Scholar]
- 29.Kulik, G., A. Klippel, and M. J. Weber. 1997. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol. Cell. Biol. 17:1595-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamb, J., S. Ramaswamy, H. L. Ford, B. Contreras, R. V. Martinez, F. S. Kittrell, C. A. Zahnow, N. Patterson, T. R. Golub, and M. E. Ewen. 2003. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell 114:323-334. [DOI] [PubMed] [Google Scholar]
- 31.Lee, A. W., and D. J. States. 2000. Both src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol. Cell. Biol. 20:6779-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeRoith, D., R. Baserga, L. Helman, and C. T. Roberts, Jr. 1995. Insulin-like growth factors and cancer. Ann. Intern. Med. 122:54-59. [DOI] [PubMed] [Google Scholar]
- 33.LeRoith, D., and C. T. Roberts, Jr. 2003. The insulin-like growth factor system and cancer. Cancer Lett. 195:127-137. [DOI] [PubMed] [Google Scholar]
- 34.Liu, H., H. Perlman, L. J. Pagliari, and R. M. Pope. 2001. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-κB, Bad, or caspase activation. J. Exp. Med. 194:113-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, J. L., A. Grinberg, H. Westphal, B. Sauer, D. Accili, M. Karas, and D. LeRoith. 1998. Insulin-like growth factor-I affects perinatal lethality and postnatal development in a gene dosage-dependent manner: manipulation using the Cre/loxP system in transgenic mice. Mol. Endocrinol. 12:1452-1462. [DOI] [PubMed] [Google Scholar]
- 36.Liu, J. P., J. Baker, A. S. Perkins, E. J. Robertson, and A. Efstratiadis. 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (IGF-1) and type 1 IGF receptor (IGF1r). Cell 75:59-72. [PubMed] [Google Scholar]
- 37.McCarthy, T. L., M. J. Thomas, M. Centrella, and P. Rotwein. 1995. Regulation of insulin-like growth factor I transcription by cyclic adenosine 3′,5′-monophosphate (cAMP) in fetal rat bone cells through an element within exon 1: protein kinase A-dependent control without a consensus AMP response element. Endocrinology 136:3901-3908. [DOI] [PubMed] [Google Scholar]
- 38.McCubrey, J. A., L. S. Steelman, P. E. Hoyle, W. L. Blalock, C. Weinstein-Oppenheimer, R. A. Franklin, H. Cherwinski, E. Bosch, and M. McMahon. 1998. Differential abilities of activated Raf oncoproteins to abrogate cytokine dependency, prevent apoptosis and induce autocrine growth factor synthesis in human hematopoietic cells. Leukemia 12:1903-1929. [DOI] [PubMed] [Google Scholar]
- 39.McCubrey, J. A., L. S. Steelman, P. W. Moye, P. E. Hoyle, C. Weinstein-Oppenheimer, F. Chang, M. Pearce, M. K. White, R. Franklin, and W. L. Blalock. 2000. Effects of deregulated RAF and MEK1 expression on the cytokine-dependency of hematopoietic cells. Adv. Enzyme Regul. 40:305-337. [DOI] [PubMed] [Google Scholar]
- 40.Metz, T., T. Graf, and A. Leutz. 1991. Activation of cMGF expression is a critical step in avian myeloid leukemogenesis. EMBO J. 10:837-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrione, A., T. DeAngelis, and R. Baserga. 1995. Failure of the bovine papillomavirus to transform mouse embryo fibroblasts with a targeted disruption of the insulin-like growth factor I receptor genes. J. Virol. 69:5300-5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moschos, S. J., and C. S. Mantzoros. 2002. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology 63:317-332. [DOI] [PubMed] [Google Scholar]
- 43.Nakajima, T., S. Kinoshita, T. Sasagawa, K. Sasaki, M. Naruto, T. Kishimoto, and S. Akira. 1993. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc. Natl. Acad. Sci. USA 90:2207-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neumann, C., and C. Sorg. 1980. Sequential expression of functions during macrophage differentiation in murine bone marrow liquid cultures. Eur. J. Immunol. 10:834-840. [DOI] [PubMed] [Google Scholar]
- 45.Okuda, K., U. Matulonis, R. Salgia, Y. Kanakura, B. Druker, and J. D. Griffin. 1994. Factor independence of human myeloid leukemia cell lines is associated with increased phosphorylation of the proto-oncogene Raf-1. Exp. Hematol. 22:1111-1117. [PubMed] [Google Scholar]
- 46.Parrizas, M., A. R. Saltiel, and D. LeRoith. 1997. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J. Biol. Chem. 272:154-161. [DOI] [PubMed] [Google Scholar]
- 47.Pelengaris, S., B. Rudolph, and T. Littlewood. 2000. Action of Myc in vivo—proliferation and apoptosis. Curr. Opin. Genet. Dev. 10:100-105. [DOI] [PubMed] [Google Scholar]
- 48.Rask, K., M. Thorn, F. Ponten, W. Kraaz, K. Sundfeldt, L. Hedin, and S. Enerback. 2000. Increased expression of the transcription factors CCAAT-enhancer binding protein-beta (C/EBβ) and C/EBζ (CHOP) correlate with invasiveness of human colorectal cancer. Int. J. Cancer 86:337-343. [DOI] [PubMed] [Google Scholar]
- 49.Resnicoff, M., D. Coppola, C. Sell, R. Rubin, S. Ferrone, and R. Baserga. 1994. Growth inhibition of human melanoma cells in nude mice by antisense strategies to the type 1 insulin-like growth factor receptor. Cancer Res. 54:4848-4850. [PubMed] [Google Scholar]
- 50.Resnicoff, M., C. Sell, M. Rubini, D. Coppola, D. Ambrose, R. Baserga, and R. Rubin. 1994. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 54:2218-2222. [PubMed] [Google Scholar]
- 51.Robinson, G. W., P. F. Johnson, L. Hennighausen, and E. Sterneck. 1998. The C/EBPβ transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 12:1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seagroves, T. N., S. Krnacik, B. Raught, J. Gay, B. Burgess-Beusse, G. J. Darlington, and J. M. Rosen. 1998. C/EBPβ, but not C/EBPα, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 12:1917-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sell, C., G. Dumenil, C. Deveaud, M. Miura, D. Coppola, T. DeAngelis, R. Rubin, A. Efstratiadis, and R. Baserga. 1994. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol. Cell. Biol. 14:3604-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sell, C., M. Rubini, R. Rubin, J. P. Liu, A. Efstratiadis, and R. Baserga. 1993. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc. Natl. Acad. Sci. USA 90:11217-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sterneck, E., C. Blattner, T. Graf, and A. Leutz. 1992. Structure of the chicken myelomonocytic growth factor gene and specific activation of its promoter in avian myelomonocytic cells by protein kinases. Mol. Cell. Biol. 12:1728-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sterneck, E., C. Muller, S. Katz, and A. Leutz. 1992. Autocrine growth induced by kinase type oncogenes in myeloid cells requires AP-1 and NF-M, a myeloid specific, C/EBP-like factor. EMBO J. 11:115-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sterneck, E., L. Tessarollo, and P. F. Johnson. 1997. An essential role for C/EBPβ in female reproduction. Genes Dev. 11:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sundfeldt, K., K. Ivarsson, M. Carlsson, S. Enerback, P. O. Janson, M. Brannstrom, and L. Hedin. 1999. The expression of CCAAT/enhancer binding protein (C/EBP) in the human ovary in vivo: specific increase in C/EBPβ during epithelial tumour progression. Br. J. Cancer 79:1240-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talapatra, S., and C. B. Thompson. 2001. Growth factor signaling in cell survival: implications for cancer treatment. J. Pharmacol. Exp. Ther. 298:873-878. [PubMed] [Google Scholar]
- 60.Tanaka, T., S. Akira, K. Yoshida, M. Umemoto, Y. Yoneda, N. Shirafuji, H. Fujiwara, S. Suematsu, N. Yoshida, and T. Kishimoto. 1995. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell 80:353-361. [DOI] [PubMed] [Google Scholar]
- 61.Thomas, M. J., Y. Umayahara, H. Shu, M. Centrella, P. Rotwein, and T. L. McCarthy. 1996. Identification of the cAMP response element that controls transcriptional activation of the insulin-like growth factor-I gene by prostaglandin E2 in osteoblasts. J. Biol. Chem. 271:21835-21841. [DOI] [PubMed] [Google Scholar]
- 62.Trojan, J., B. K. Blossey, T. R. Johnson, S. D. Rudin, M. Tykocinski, and J. Ilan. 1992. Loss of tumorigenicity of rat glioblastoma directed by episome-based antisense cDNA transcription of insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 89:4874-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tushinski, R. J., I. T. Oliver, L. J. Guilbert, P. W. Tynan, J. R. Warner, and E. R. Stanley. 1982. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 28:71-81. [DOI] [PubMed] [Google Scholar]
- 64.Umayahara, Y., J. Billiard, C. Ji, M. Centrella, T. L. McCarthy, and P. Rotwein. 1999. CCAAT/enhancer-binding protein delta is a critical regulator of insulin-like growth factor-I gene transcription in osteoblasts. J. Biol. Chem. 274:10609-10617. [DOI] [PubMed] [Google Scholar]
- 65.Umayahara, Y., C. Ji, M. Centrella, P. Rotwein, and T. L. McCarthy. 1997. CCAAT/enhancer-binding protein delta activates insulin-like growth factor-I gene transcription in osteoblasts. Identification of a novel cyclic AMP signaling pathway in bone. J. Biol. Chem. 272:31793-31800. [DOI] [PubMed] [Google Scholar]
- 66.Valentinis, B., and R. Baserga. 2001. IGF-I receptor signalling in transformation and differentiation. Mol. Pathol. 54:133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams, S. C., C. A. Cantwell, and P. F. Johnson. 1991. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 5:1553-1567. [DOI] [PubMed] [Google Scholar]
- 68.Yee, D. 2001. Are the insulin-like growth factors relevant to cancer? Growth Horm. IGF Res. 11:339-345. [DOI] [PubMed] [Google Scholar]
- 69.Zahnow, C. A. 2002. CCAAT/enhancer binding proteins in normal mammary development and breast cancer. Breast Cancer Res. 4:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu, S., H.-S. Oh, M. Shim, E. Sterneck, P. F. Johnson, and R. C. Smart. 1999. C/EBPβ modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol. Cell. Biol. 19:7181-7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu, S., K. Yoon, E. Sterneck, P. F. Johnson, and R. C. Smart. 2002. CCAAT/enhancer binding protein-beta is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling. Proc. Natl. Acad. Sci. USA 99:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]