Abstract
Purpose
Current treatments for ovarian cancers have limited therapeutic outcomes due to advanced stage of the disease at diagnosis. Among new therapies, conditionally-replicating adenoviruses (CRAds), designed to selectively lyse cancer cells, hold promise. In clinical trials, CRAds exhibited limited efficacy thus far. Second generation CRAds are being developed to express a therapeutic protein to enhance antitumor efficacy. One attractive target in the tumor microenvironment is the matrix metalloproteinases (MMPs) that degrade the extracellular matrix, and are upregulated in ovarian cancer. Tissue inhibitor of metalloproteinase 2 (TIMP2) is an endogenous inhibitor of MMPs. The present study developed and evaluated a novel CRAd (Ad5/3-CXCR4-TIMP2) for ovarian cancer therapy.
Experimental Design
A targeted CRAd, Ad5/3-CXCR4-TIMP2 was developed using the CXCR4 promoter for enhanced replication, expressing the TIMP2 transgene. The efficacy of this armed CRAd was determined in both established human ovarian cancer cell lines and in primary ovarian tumor samples.
Results
Ad5/3-CXCR4-TIMP2 mediated expression of functional TIMP2, as demonstrated by the inhibition of MMP activity. In addition, arming with TIMP2 did not inhibit viral replication nor oncolytic potency, as the TIMP2 armed viruses showed enhanced killing of cancer cells when compared to the unarmed viruses. We also examined viral replication in primary ovarian cancer tissues obtained from patients with stage III and IV ovarian cancer. In four of the five tumor samples, Ad5/3-CXCR4-TIMP2 revealed a 21- to 89- fold increase in replication when compared to the Ad5/3 virus.
Conclusion
Results support the translational potential of Ad5/3-CXCR4-TIMP2 for treatment of patients with advanced ovarian cancer.
Keywords: Conditionally Replicating Adenovirus, CXCR4, TIMP2
Introduction
Ovarian cancer is the leading cause of gynecological cancer death in the U.S., accounting for approximately 14,600 deaths annually. In most cases, diagnosis is made at an advanced stage, where the cancer has disseminated, thus significantly lowering survival. Despite advancements in surgical techniques and chemotherapeutic agents, the overall mortality rate has remained unchanged over the last four decades (1). Therefore, new and effectively targeted therapies are urgently needed to limit ovarian cancer progression.
Among new therapies based on the approaches of molecular medicine, the use of conditionally replicative adenoviruses (CRAds) are highly promising for the treatment of ovarian cancer. Due to their oncolytic potential, CRAds have been rapidly translated into human clinical trials, where their safety has been demonstrated, but with only modest clinical results (2), indicating the need for further improvement of CRAds for successful clinical translation. Towards this goal, we designed a CRAd which combines the property of tumor selective replication with delivery of a therapeutic transgene to target ovarian cancer survival and dissemination.
Many cancer cells, including ovarian cancer cells, exhibit a paucity of the adenovirus serotype 5 (Ad5) receptor, the coxsackie and adenovirus receptor (CAR), thereby limiting CRAds infection and thus therapeutic potential (3, 4). To increase the transduction of ovarian cancer cells, adenoviruses have been genetically manipulated allowing retargeting to an alternative receptor that is more abundantly expressed on target cells. The Ad5/3 virus, which still retains the shaft and tail of Ad5 fiber, but has had the Ad5 knob replaced with the Ad3 fiber knob, exhibits enhanced transduction both in vitro and in vivo as compared to the Ad5 wildtype vector (5, 6). This fiber knob modification was included in the CRAd used in this study. For targeted replication and to minimize deleterious effects on non-target tissues, a tumor selective promoter (TSP) was also used to limit viral replication to cancer cells. The CXCR4 promoter exhibits a “tumor on, liver off” profile, thus making it ideal for the targeting of ovarian cancer cells (7).
As with most solid tumors, ovarian cancer growth, angiogenesis, invasion and metastasis all require the degradation of the extracellular matrix (ECM). Matrix metalloproteinases (MMPs) are capable of degrading all components of the ECM. Physiologically, MMPs play an important role in tissue remodeling. However, their dysregulation has been shown to be central to tumor progression (8, 9). In particular, MMP-2 and MMP-9 have been shown to be consistently upregulated in ovarian cancer and are associated with poor prognosis (10, 11). Activity of MMPs is tightly regulated by a group of endogenous inhibitors, the tissue inhibitors of metalloproteinases (TIMPs). To date, four mammalian TIMPs have been identified; with each TIMP exhibiting differences in structures, biochemical properties and expression profiles (12, 13). Among the members of the TIMP family, TIMP2 has been most extensively studied. In addition to its antitumor activity as an inhibitor of MMPs, TIMP2 can also inhibit tumor growth and angiogenesis by a variety of mechanisms independent of MMP inhibition (14–16).
The present study developed and determined the therapeutic potential of an armed CRAd (Ad5/3-CXCR4-TIMP2) for ovarian cancer therapy. Transductional selectivity of the CRAd was achieved by genetically replacing the Ad5 knob with the Ad3 knob. The CXCR4 promoter was used to mediate tumor selective replication of the vector. The TIMP2 gene was incorporated to increase the antitumor effects. We hypothesized that the production of TIMP2 from virally infected cells should bind and inhibit the excess extracellular MMPs and thereby inhibit tumor progression through both MMP-dependent and MMP-independent pathways. In addition to validating the virus with ovarian cancer cell lines, the present study also evaluated the effects of the armed-CRAd in tumor tissues obtained from stage III and IV ovarian cancer patients, identifying the superior potential of this vector for patients presenting with an advanced stage of ovarian cancer.
Materials and Methods
Cell lines
The A549 human lung carcinoma cell line and THLE-3 normal human liver epithelial cell line were purchased from the American Type Culture Collection (ATCC; Manassas, VA). Ovarian adenocarcinoma cell lines SKOV3.ip1 and OV-4 were kind gifts from Drs. Janet Price and Judy Wolf, respectively (MD Anderson Cancer Center, Houston, TX). Cells were cultured in a 1:1 mixture of Dulbecco’s modified Eagle medium (DMEM) and Ham’s F-12 medium, supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), L-glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100 μg/ml). All cell lines were cultured at 37 °C in a humidified atmosphere and at 5% CO2. Media and supplements were purchased from Mediatech (Herndon, VA).
Human ovarian cancer tissue and culture
Human primary ovarian tumor samples were obtained from the Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of Alabama at Birmingham, with institutional review board approval at the time of initial debulking from patients with histologically confirmed ovarian adenocarcinoma. Samples were kept on ice in University of Wisconsin solution (Barr Laboratories, Inc. Pomona, NY) until slicing. Time from harvest to slicing was kept at an absolute minimum (≤ 2 hours). The precision tissue cut technique was performed using the Krumdieck Tissue Slicer as previously described (17). Briefly, tissue slices (250 μm -thick) were placed into 24-well plates (1 slice per well) containing 1 mL of complete culture media (RPMI with 1% antibiotics, 1% L-glutamine, and 10% FCS). The plates were then incubated at 37°C and 5% CO2 in a humidified environment under normal oxygen concentrations for up to 4 days. A plate rocker set at 60 rpm was used to agitate slices to ensure adequate oxygenation and viability (18).
Construction and production of the viruses
The wild-type human adenovirus serotype 5, (Adwt300), was purchased from ATCC. The tropism-modified control virus, Ad5/3, containing the Ad5 fiber shaft and tail has had the knob domain replaced with that of the Ad3 virus (19). The control, Ad5/3-CXCR4, is a CRAd with the CXCR4 promoter inserted into the E1A position (20).
The TIMP2 armed viruses were constructed as follows: A full-length human TIMP2 cDNA was isolated from Ad-TIMP2, an E1- and E3-deleted replication deficient Ad5 vector, which expresses TIMP2 under the control of the CMV promoter (21). The TIMP2 gene was subcloned in place of the E3B region into pZErO-2 E3 6.9, a transfer vector containing a 6.9 kb fragment of the Ad5 genome including the E3 region (kind gift of Dr. Nik Korokhov, VectorLogics, Inc., Birmingham, AL). The resultant plasmid was linearized with BamHI and cotransformed into E. coli BJ5183 (Stratagene, La Jolla, CA) with a SwaI-linearized plasmid, pVK500CΔE3, containing the Ad5 genome deleted for both the E3 region and the fiber gene (22). The resulting plasmid was cotransformed into E. coli BJ5183 with an EcoRI-linearized plasmid, pKAN.F5/3, containing the Ad5 fiber shaft and tail and an Ad3 fiber knob (23). The resultant plasmid, pVK500C-TIMP2-F5/3 was linearized with PacI and used to transfect A549 cells to generate the tropism-modified armed replicating adenovirus, Ad5/3-TIMP2. To produce the tropism-modified armed CRAd, Ad5/3-CXCR4-TIMP2, pVK500C-TIMP2-F5/3, was subjected to a final round of recombination by linearization with SwaI and cotransformation into E. coli BJ5183 with a PmeI-linearized pCXCR4, a plasmid containing the CXCR4 promoter (7). This final plasmid, pVK500C-CXCR4-TIMP2-F5/3, was linearized with PacI and used to transfect A549 cells to generate Ad5/3-CXCR4-TIMP2. Viruses were amplified in the A549 cell line and purified by two rounds of cesium chloride density centrifugation. The titers of viral particles and infectious units were determined (24).
Quantitative reverse-transcriptase polymerase chain reaction (QRT-PCR) for expression of TIMP2 and viral genes
SKOV3.ip1 and OV-4 cells were infected with Ad5/3-CXCR4 or Ad5/3-CXCR4-TIMP2 at a multiplicity of infection (MOI) of 10 infectious units (IU) per cell. At 12, 24, and 36 h post-infection, total RNA was isolated from cell lysates using the RNeasy Mini Kit (Qiagen, Valencia, CA) and subjected to QRT-PCR analysis (LightCycler 480 system, Roche Diagnostics, Indianapolis, IN). RNA from cells infected with the armed CRAd was assayed for expression of TIMP2, whereas samples from cells infected with Ad5/3-CXCR4 were assayed for expression of the E3B gene RIDα. All samples were analyzed for expression of ADP and fiber. Expression of human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control. Results were expressed as copy number/ng of total RNA. Primer sequences used in the study are as follows:
TIMP2:
forward primer: 5′-TCTGGATGGACTGGGTCACA-3′
reverse primer: 5′-CTTGATGCAGGCGAAGAACTT-3′
probe: 5′-6FAM -AGAAGAACATCAACGGGCACCAGGC- TAMRA-3′
RIDα:
forward primer: 5′-GCTGGAAACGAATAGATGCCA-3′
reverse primer: 5′-GTTGCAGTGGAAGCATAGCG-3′
probe: 5′-6FAM-ACCACCCAACTTTCCCCGCGC-TAMRA-3′
ADP:
forward primer: 5′-TCTGCTGCCTAAAGCGCAA-3′
reverse primer: 5′-TTTGGGTGTAGCACAATGATGG-3′
probe: 5′-6FAM-CGCGCCCGACCACCCATCTATAGT-TAMRA-3′
Fiber:
forward primer 5′-TGATGTTTGACGCTACAGCCATA-3′
reverse primer: 5′-GATTTGTGTTTGGTGCATTAGGTG-3′
probe: 5′-6FAM-ACCAAATTCAAGCCCATCTCCTGCATTAATG- TAMRA-3′
GAPDH:
forward primer: 5′-GGTTTACATGTTCCAATATGATTCCA-3′
reverse primer: 5′-ATGGGATTTCCATTGATGACAAG-3′
probe: 5′-CGTTCTCAGCCTTGACGGTGCCAT-3′
Expression and bioactivity of the TIMP2 protein from armed viruses
SKOV3.ip1 cells were infected with Ad5/3-CXCR4, Ad5/3- TIMP2 or Ad5/3-CXCR4-TIMP2 at an MOI of 10 IU per cell and overlaid with 1 ml serum-free medium. At 24, 48, and 72 hours post-infection, 1 ml samples of medium were concentrated to 100 μl using a Microcon centrifugal filter device (Millipore, Bedford, MA). The presence of TIMP2 was detected by SDS-PAGE followed by immunoblotting using a mouse anti-human TIMP2 primary antibody (R&D Systems Inc., Minneapolis, MN; diluted 1:250) and an anti-mouse horseradish peroxidase-conjugated secondary antibody (Amersham Biotech, Arlington Heights, IL; diluted 1:5000). Blots were developed with enhanced chemiluminescence Western blotting detection reagents (Amersham Biotech). Recombinant human TIMP2 was used as the positive control (R & D Systems Inc.).
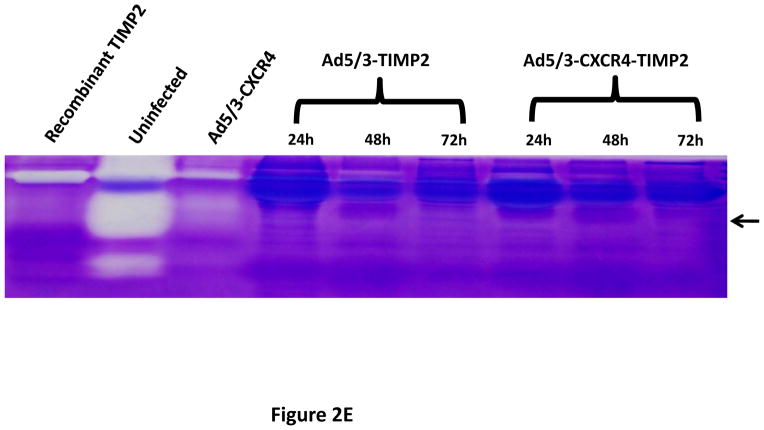
To determine functional activity of TIMP2, reverse zymography was performed as previously described (25). Briefly, the samples were electrophoresed in 0.1% SDS, 12% polyacrylamide gels containing 1 mg/ml gelatin and MMP-2 (Reverse Zymography Kit, School of Biological Sciences University of East Anglia, UK). After electrophoresis the gels were washed overnight with 2.5% Triton X-100, incubated in 50mM Tris/HCl, pH 7.5 and 5mM CaCl2 for 16–18 h at 37°C, then stained and destained to visualize TIMP activity. Dark bands on the gel indicated the inhibition of gelatin degradation by MMP due to the presence of TIMP.
Viral DNA replication in human ovarian cancer cell lines
SKOV3.ip1 and OV-4 cells were infected with Ad5/3-CXCR4-TIMP2 or each of the control viruses at an MOI of 0.1 IU per cell and overlaid with 1 ml medium. Two and 4 days post-infection, DNA was extracted from 200 μl samples of medium (QIAamp DNA Blood Mini Kit, Qiagen) and analyzed by Q-PCR for the Ad5 E4 gene as an indicator of viral replication, using previously described primers and probe (26). Results were expressed as copy number/ng of total DNA.
Viral DNA replication in human ovarian tumor tissues
Tumors were sliced as described above. These slices were approximately 15-cell layers thick and contained about 1 × 106 cells per slice. Tumor slices were placed in 24-well plates and infected with 5 × 106 IU of Ad5/3-CXCR4-TIMP2 or each of the control viruses, diluted in 1 ml RPMI medium with 2% (v/v) FBS. The following day, infection mixtures were replaced with 1 ml RPMI medium containing 10% (v/v) FBS and all supplements. At 1 and 3 days post-infection, 200 μl samples of medium were harvested, DNA was purified as described above, and samples were analyzed for the presence of the Ad5 E4 gene by Q-PCR, using previously described primers and probe (26). Results were expressed as copy number/ng of total DNA.
Flow cytometry
CXCR4 expression was determined as a measure of CXCR4 promoter activity by flow cytometry. Cells were harvested and washed twice with FACS buffer (phosphate-buffered saline containing 2% FBS, 0.1% sodium azide). Cell lines were then stained with PE-labeled CD184 (eBiosciences) and analyzed by FACS Aria flow cytometer (BD Biosciences).
Oncolytic potency of CRAds
To determine the oncolytic potency of the viruses, cell viability was measured quantitatively with a Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) according to the manufacturer’s instructions. Briefly, SKOV3.ip1, OV-4, fibroblasts and THLE-3 cells were plated in 96 well plates and infected in triplicate with the replicating viruses at a range of MOIs: 1–10−5 IU per cell. Eight days post-infection, 20 μl of the MTS reagent 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] was added to each well that contained 100 μl of medium. Cells were then incubated for 3 h at 37°C and the resultant absorbance was record at 490 nm. All cell viability results were expressed as a percentage of viable cells compared to uninfected control.
Immunohistochemistry
Briefly, paraffin sections of human primary ovarian tumors were deparaffinized in xylene and hydrated through graded alcohol. Antigen retrieval was performed in citrate buffer (pH 6.0), under steam for 20 min. Sections were cooled to room temperature, and endogenous peroxidase was removed using 0.3% H2O2 in methanol for 30 min and blocked with 5% BSA for 30 min. Tissue sections were then incubated with primary antibodies overnight at 4°C. Sections were washed in Phosphate-buffered saline (PBST) containing 0.05% Tween-20 and again incubated at room temperature with biotin-conjugated goat anti-goat/anti-mouse secondary antibody for 2 h. After washing, sections were incubated with streptavidin-conjugated horseradish peroxidase for 1 h at room temperature. After another wash with PBST, immunodetection was performed using 3,3′-diaminobenzidine-H2O2 (Vector Labs) and counterstained with hematoxylin.
Statistical analysis
Data were analyzed by one-way ANOVA. A Tukey test was also applied for multiple comparisons wherever applicable. Values provided are the mean ± SE, and the differences were considered significant if P < 0.05.
Results
Construction and characterization of a tropism-modified, TIMP2 armed CRAd
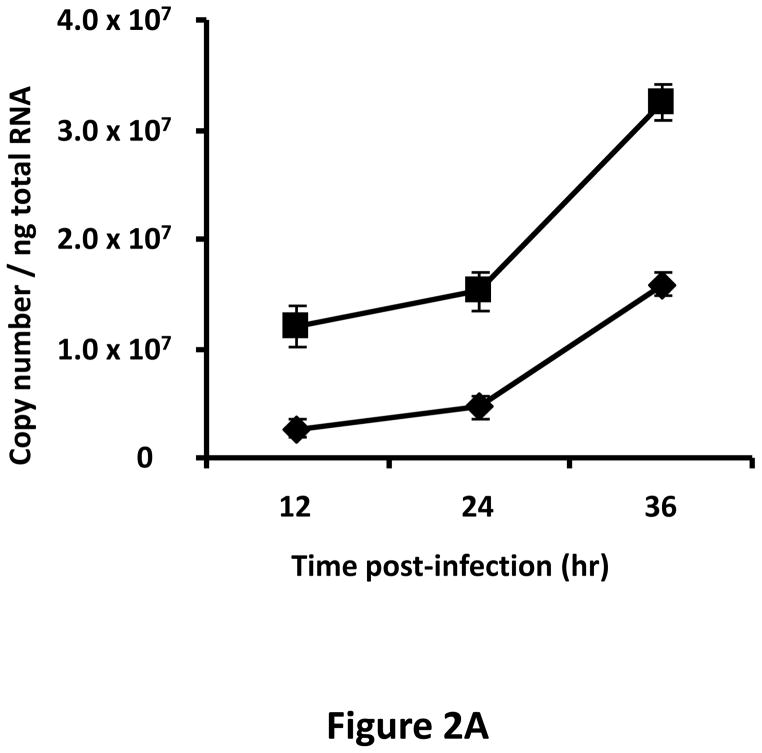
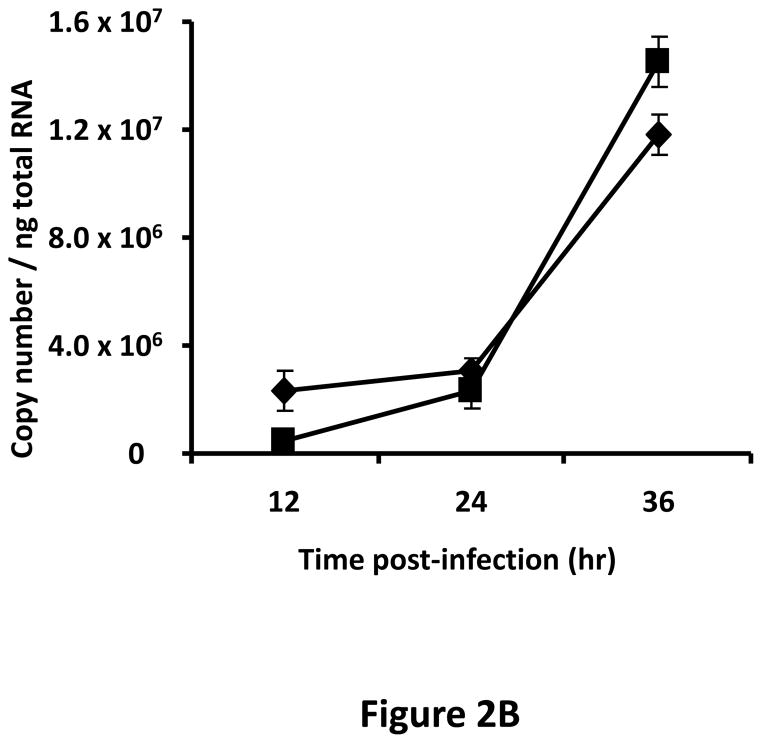
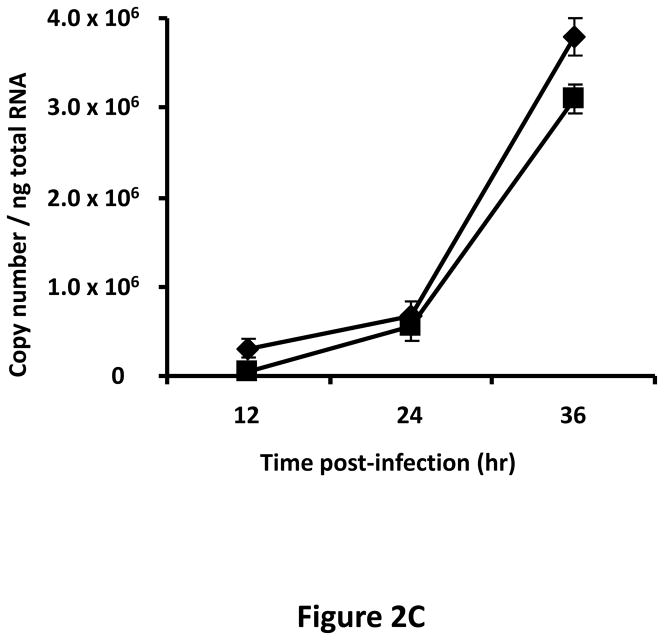
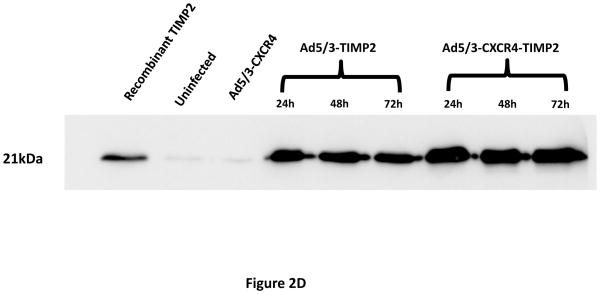
A tropism-modified CRAd, armed with TIMP2, was constructed for the treatment of ovarian cancer. The genomes of this CRAd (Ad5/3-CXCR4-TIMP2), along with control viruses are shown schematically in Fig. 1. The TIMP2 transgene was engineered into the E3B region of the genome under the control of the native promoter, splicing and polyadenylation signals. The gene encoding the adenovirus death protein (ADP) was retained to mediate efficient lysis and viral progeny release from infected cells. This armed CRAd was validated first for transgene expression in the human ovarian cancer cell line, SKOV3.ip1. Quantitative RT-PCR confirmed the expression of TIMP2 from the E3B region of Ad5/3-CXCR4-TIMP2. More importantly, TIMP2 expression in the armed CRAd correlated with the expression profile of the native E3B gene, RIDα, from the unarmed CRAd, Ad5/3-CXCR4 (Fig. 2A). In addition, expression of TIMP2 by the armed CRAd, did not inhibit upstream ADP (Fig. 2B) or downstream fiber gene expressions (Fig. 2C). Secreted TIMP2 was detectable in the medium at 24 hours postinfection from ovarian cancer cells infected with the armed viruses (Fig. 2D). Moreover, the amount of TIMP2 produced was higher in cells infected with Ad5/3-CXCR4-TIMP2 than cells infected with Ad5/3-TIMP2, suggesting that the usage of the CXCR4 promoter enhanced viral replication and thereby increased transgene expression. Furthermore, TIMP2 produced by the armed viruses was biologically active, which was indicated by the inhibition of gelatin cleavage by the MMPs in the reverse zymography analysis (Fig. 2E). Similar results were obtained when OV-4 ovarian cancer cells were infected (not shown).
Figure 1. Genomic organization of armed CRAd and control viruses.
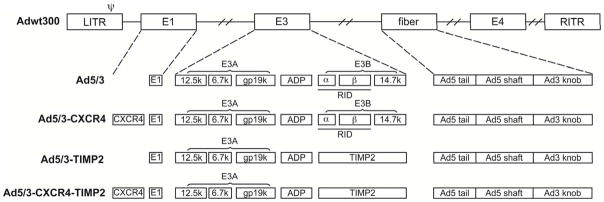
Adwt300, is the wild- type Ad5 virus. Ad5/3 is a replicating Ad5 virus with a modified fiber that contains the shaft and tail of Ad5 fiber and the Ad3 fiber knob. Ad5/3-CXCR4 is a CRAd with a CXCR4 promoter in E1 to limit viral replication to the cancer cells in conjunction with the aforementioned fiber modification. Ad5/3-TIMP2 is a replicating virus armed with TIMP2 in E3B region and with the F5/3 modified fiber. Ad5/3-CXCR4-TIMP2 is a CRAd with the CXCR4 promoter in E1, armed with TIMP2 in the E3B region and the F5/3 modified fiber.
Figure 2. Characterization of armed CRAd.
SKOV3.ip1 ovarian cancer cells were infected with Ad5/3-CXCR4-TIMP2 (■), or Ad5/3-CXCR4 (◆) at a MOI of 10 IU per cell. At the indicated times postinfection, cellular RNA was extracted and subjected to QRT-PCR to detect expression of: A, the TIMP2 gene in cells infected with Ad5/3-CXCR4-TIMP2 or the RIDα gene in cells infected with Ad5/3-CXCR4. B, the ADP gene. C, the fiber gene. All samples were tested in triplicate and graphed as the mean ± SD. D, Secretion of TIMP2 by infected SKOV3.ip1 cells. At the indicated times postinfection, conditioned medium was harvested and subjected to immunoblot analysis using an anti-TIMP2 antibody. E, Secreted TIMP2 binds active MMPs and inhibits the degradation of gelatin. Conditioned medium was harvested from SKOV3.ip1 cells at indicated times postinfection and subjected to reverse zymography.
Expression of TIMP2 does not inhibit viral replication of a replicating adenovirus in vitro
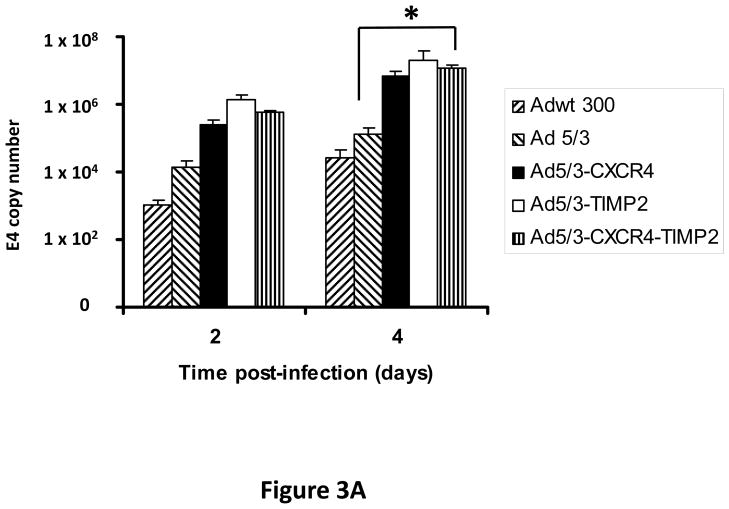
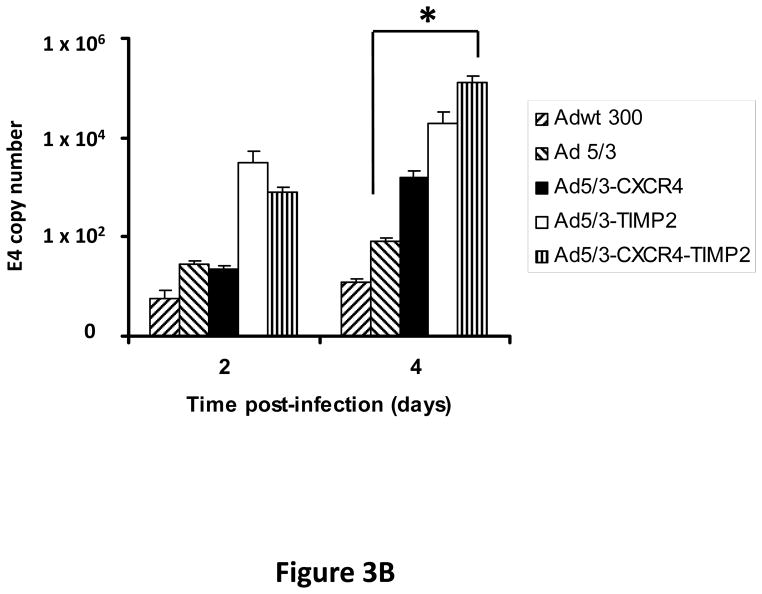
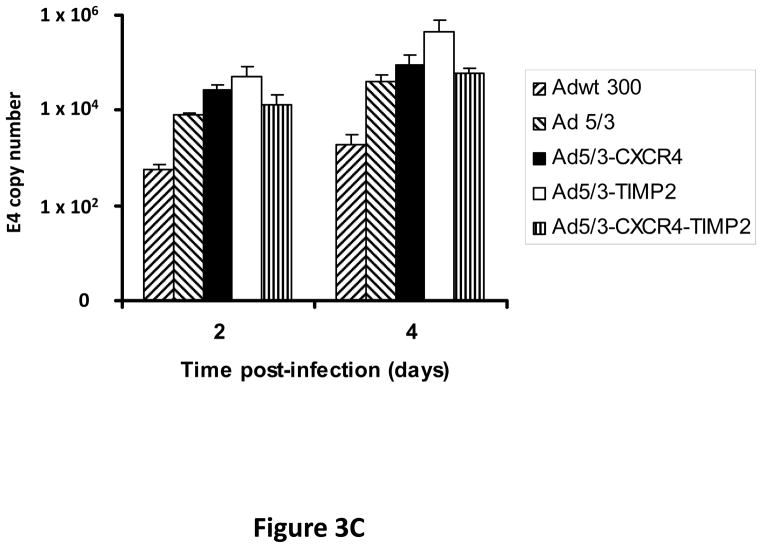
Monolayers of SKOV3.ip1 and OV-4 cells were infected with either Ad5/3-CXCR4-TIMP2 or each of the control viruses at an MOI of 0.1 infectious units per cell (IU/cell). On days 2 and 4 postinfection, DNA was extracted from the media and subjected to quantitative PCR (Q-PCR) analysis to determine the copy number of adenoviral E4 region as a measure of viral DNA replication. The tropism modified TIMP2 armed virus, Ad5/3-TIMP2 and the armed CRAd, Ad5/3-CXCR4-TIMP2, replicated on day 4 at levels similar or higher to their respective unarmed controls in both SKOV3.ip1 (Fig. 3A) and OV-4 cells (Fig. 3B). This indicated that arming with TIMP2 does not inhibit viral replication. Moreover on day 4, viral replication was higher in cells infected with Ad5/3-CXCR4 than cells infected with Ad5/3. This suggested that the usage of the CXCR4 promoter enhanced viral replication in both ovarian cancer cell lines. The selectivity of viral replication was also examined in control fibroblast cells. As shown in Fig. 3C, Ad5/3-CXCR4-TIMP2 replicated less efficiently than Ad5/3-TIMP2 in control fibroblasts. Collectively, the viral replication data suggested that the usage of the CXCR4 promoter was selective, as it enhanced viral replication in ovarian cancer cells but not in control fibroblasts.
Figure 3. Viral replication of the armed CRAds.
SKOV3.ip1 (A), OV-4 (B) ovarian cancer cells and control fibroblasts (C) were infected with the indicated viruses. The conditioned culture medium was harvested on 2 and 4 days postinfection. DNA was extracted and subjected to Q-PCR to detect the E4 gene copy number as a measure of viral DNA replication. All samples were performed in triplicate. *p< 0.05.
Viral replication correlated with CXCR4 promoter activity
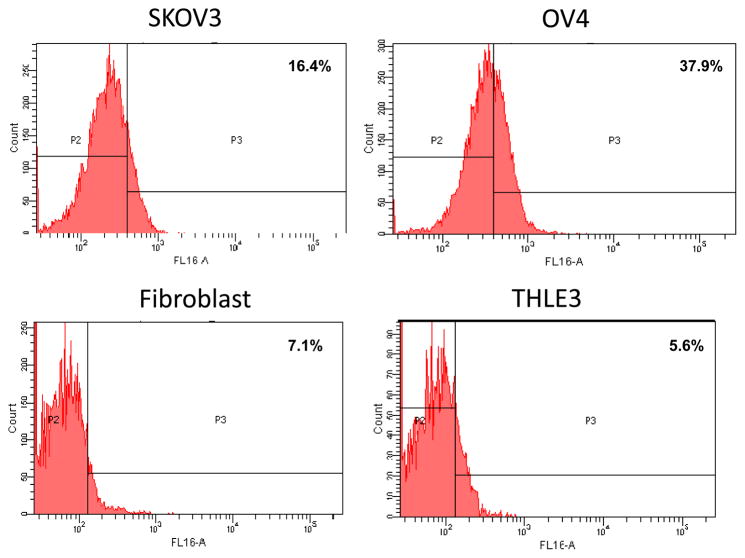
We want to further correlate our viral replication data with the activity of the CXCR4 promoter in the cell lines used. Flow cytometry was performed to assess CXCR4 receptor expression in the cell lines as an indirect, but quantitative measure of the promoter activity, as promoter activity should correlate with protein expression. As seen in Figure 4, SKOV3.ip1 and OV-4 had respectively 16.4% and 37.9% cells positive for CXCR4 expression. In contrast, the control cells, fibroblasts and THLE-3 normal hepatocytes had respectively 7.1% and 5.6% cells positive for CXCR4 expression (Fig. 4). This data coincides with the viral replication data, and confirms that selectivity with the usage of the CXCR4 promoter, where it enhances viral replication in target ovarian cancer cells, but not in normal non-target cells.
Figure 4. CXCR4 promoter activity in cell lines.
CXCR4 promoter activity was assessed in SKOV3.ip1, OV-4 ovarian cancer cells, fibroblasts and THLE-3 normal hepatocytes by staining with fluorescent CXCR4 antibody followed by FACS analysis. Percent of cells with CXCR4 promoter activity are provided in each quadrant.
Expression of TIMP2 does not inhibit the oncolytic potency of a replicating adenovirus in vitro
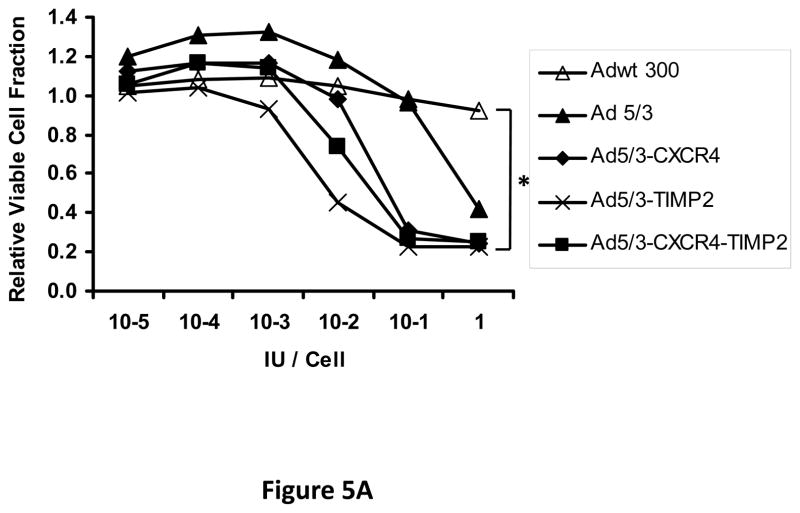
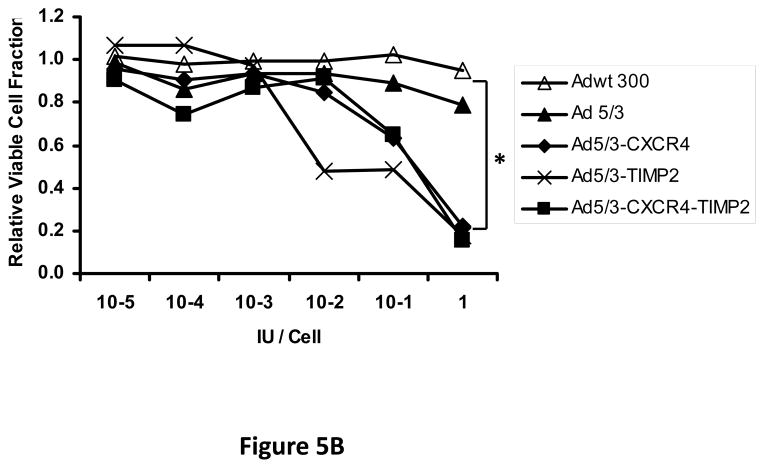
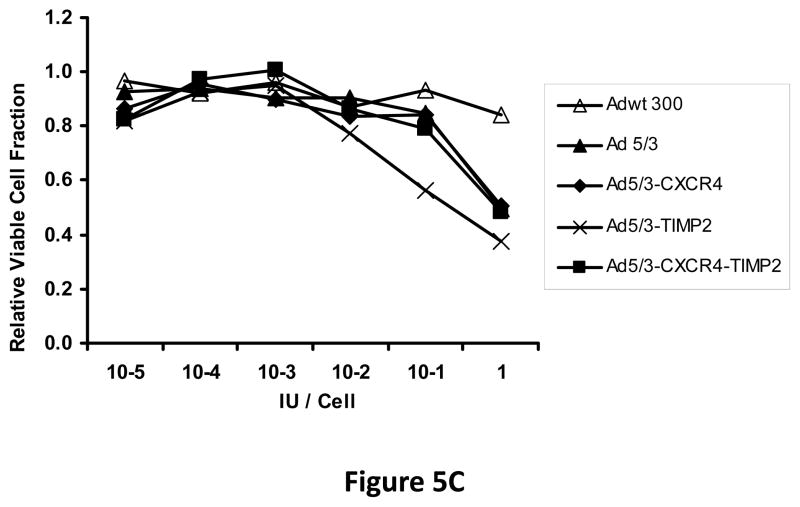
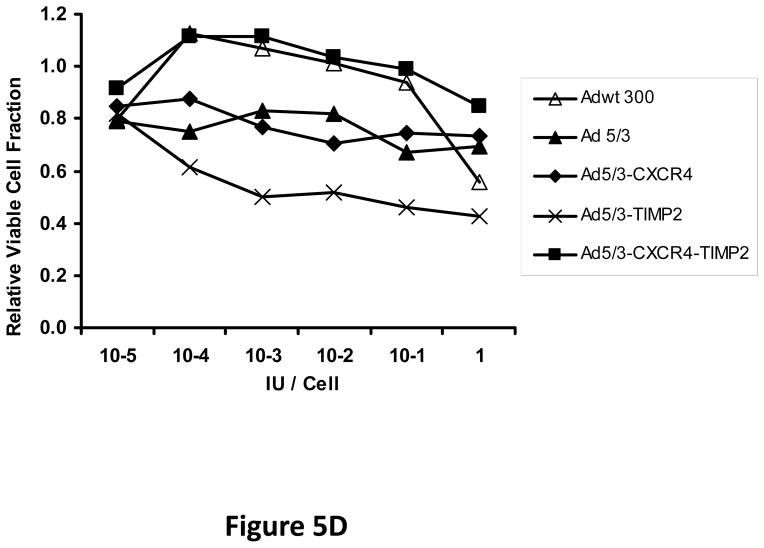
Next, oncolytic potency of the viruses was examined quantitatively using the MTS assay on ovarian cancer cell lines SKOV3.ip1 (Fig. 5A) and OV-4 (Fig. 5B). Results of this assay further demonstrated the oncolytic potency of the TIMP2 armed viruses, which exhibited enhanced killing of ovarian cancer cells when compared to the unarmed viruses in both SKOV3.ip1 and OV-4 cells. Furthermore, selectivity of oncolysis was assessed with MTS assay on control fibroblasts (Fig. 5C) and THLE-3 (Fig. 5D) normal human hepatocytes. Enhanced killing of fibroblasts was noted with Ad5/3-CXCR4-TIMP2, when compared to Adwt 300 (Fig 5C). This is likely due to the Ad3 receptor expression on fibroblasts, rather than the lack of selectivity with CXCR4 promoter, as cells infected with Ad5/3 exhibit similar level of fibroblast killing. In THLE-3 hepatocytes, there was minimal cell killing with the Ad5/3-CXCR4-TIMP2 virus. In contrast, infection with Adwt300 led to a 40% killing of normal hepatocytes in vitro (Fig. 5D). Again, the MTS data for the cell lines correlated with both the viral replication and CXCR4 promoter activity.
Figure 5. Oncolytic potency of the armed CRAds.
SKOV3.ip1 (A), OV-4 (B) ovarian cancer cells, control fibroblasts (C) and THLE-3 normal human hepatocytes (D) were infected at indicated MOIs. Eight days postinfection, cell viability was assessed quantitatively using the MTS assay. Results were normalized to the uninfected cells and plotted as relative viable cell fraction. All samples were performed in triplicate. *p< 0.05.
CRAd armed with TIMP2 replicates efficiently in primary ovarian cancer tissues
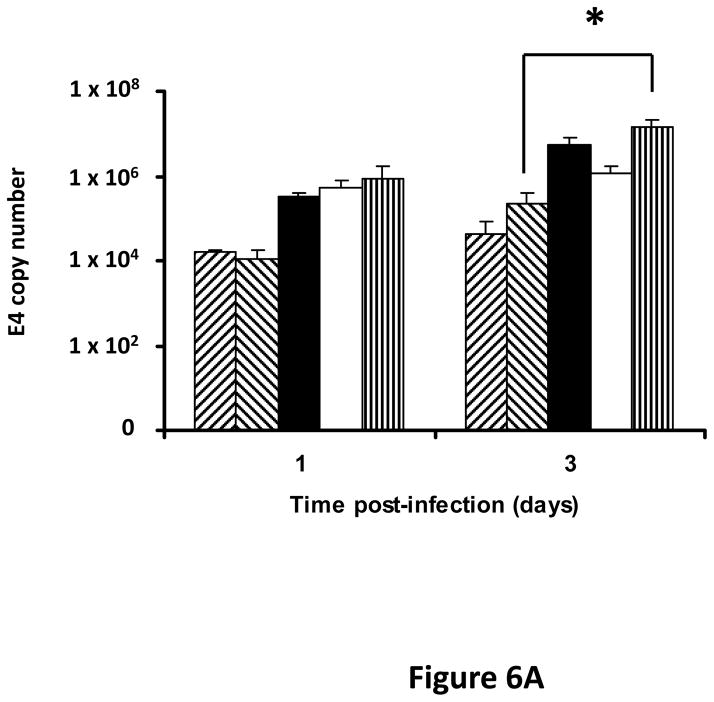
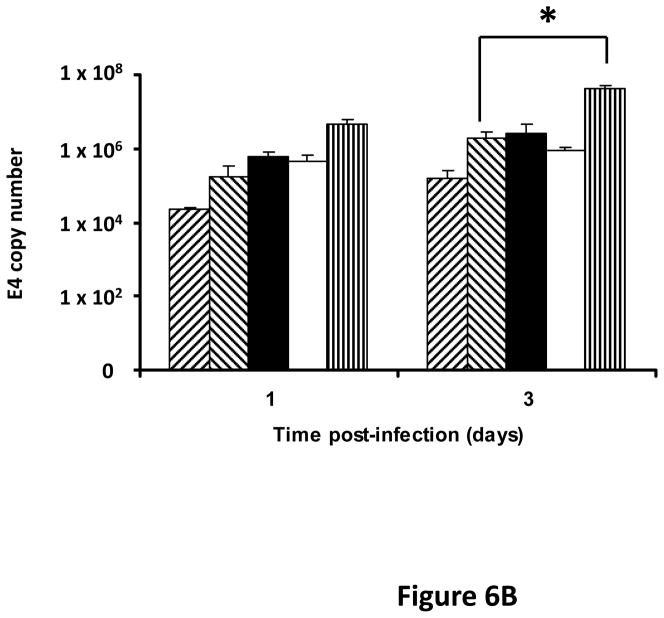
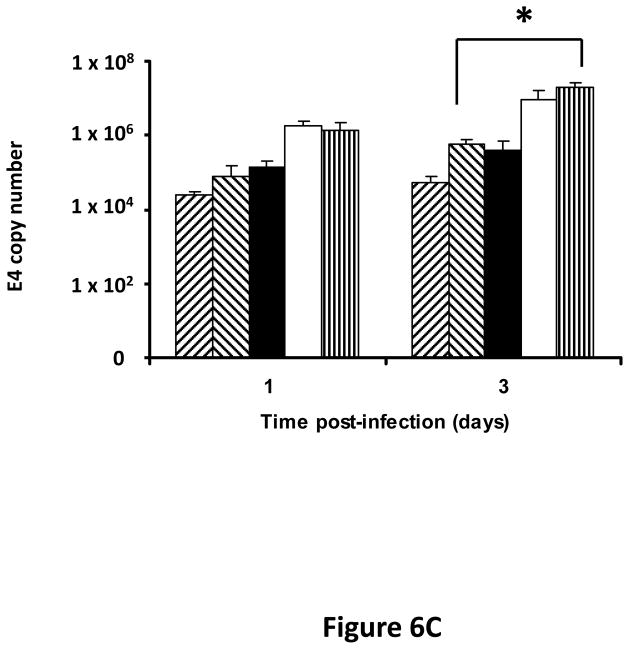
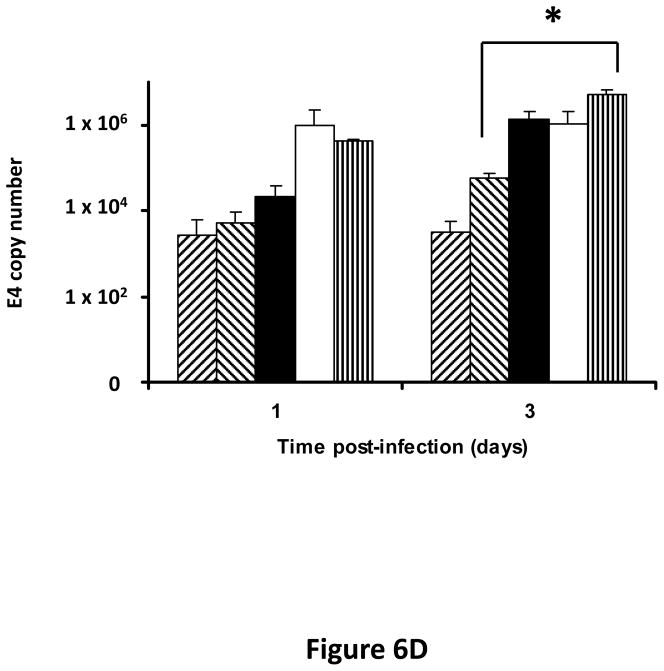
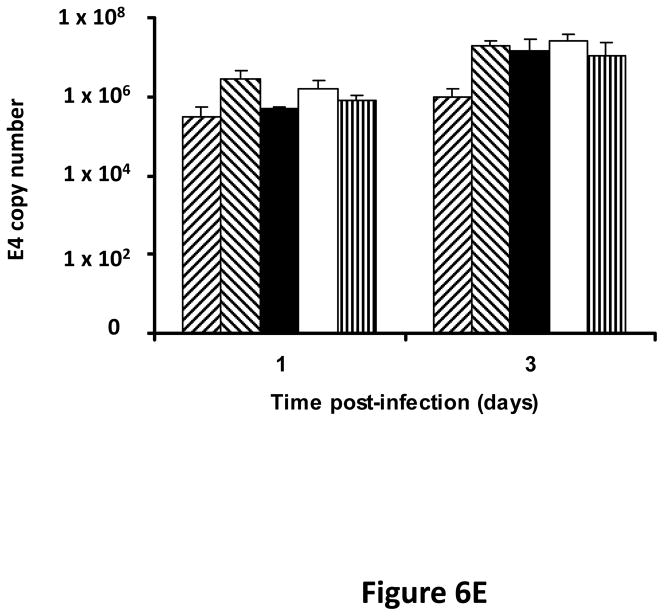
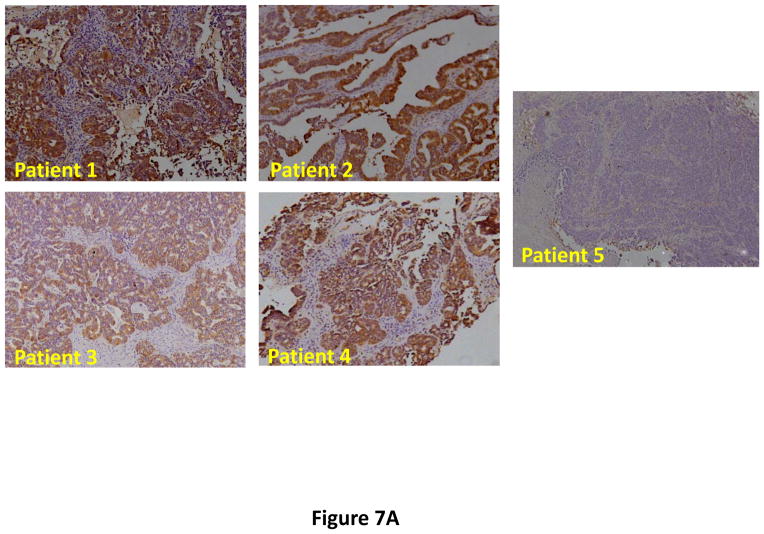
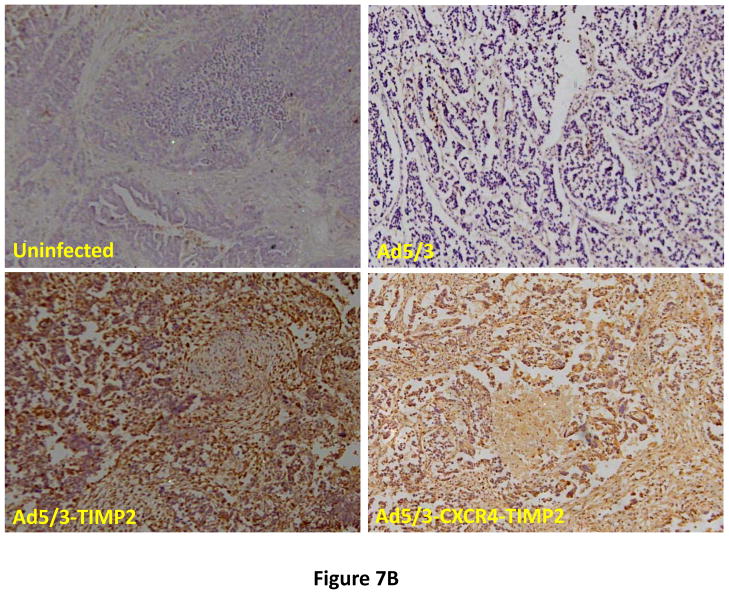
Following successful testing of the armed CRAd (Ad5/3-CXCR4-TIMP2) in established human ovarian cancer cell lines that indicated both its therapeutic transgene expression and oncolytic potential, we sought to determine if these effects would be similar in ovarian cancer tissues as a prelude to clinical translation of this virus. To this end, we employed an ex vivo model system to further examine replication efficacy of Ad5/3-CXCR4-TIMP2 and the control viruses. Five primary epithelial ovarian tumors were collected and examined for viral replication from patients with advanced disease. The median patient age was 60 years old (range 49 – 69) and all patients were Caucasian. Four of the five patients were diagnosed with stage IIIC papillary serous adenocarcinoma of the ovary, where tumors larger than 2 cm were present in the peritoneal cavity. The fifth patient was diagnosed with stage IV metastatic ovarian carcinoma. The tumors were obtained during debulking surgeries and maintained in culture immediately following surgery. Tumor slices of 250 μm -thick were made, and were infected at MOI of 5×106 IU/slice with Ad5/3-CXCR4-TIMP2 or each of the control viruses. Viral replication was assessed through determination of E4 copy number. Consistently, Ad5/3-CXCR4-TIMP2 exhibited high levels of viral replication (Fig. 6A–E). The CXCR4 promoter was active in all five tumor samples, and we observed a 8- to 16- fold increase in viral replication from day 1 to day 3 when cells were infected with Ad5/3-CXCR4-TIMP2. Moreover on day 3, in four of the five patient samples, Ad5/3-CXCR4-TIMP2 exhibited 21-to 89- fold increase in viral replication when compared to the Ad5/3 virus, suggesting the potential of this vector for ovarian cancer therapy. Furthermore, we examined by immunohistochemistry, the expression level of the CXCR4 receptor as an indicator of the CXCR4 promoter activity, which we have used to achieve selective replication. As shown in Fig. 7A, there is a strong CXCR4 staining in the four of the five primary ovarian tumors examined, indicating that CXCR4 promoter is a good promoter for targeted replication in ovarian cancer cells. Once again, the viral replication data were further supported by the results of the immunohistochemistry for CXCR4. Sample from patient 5 was negative for CXCR4 expression and this correlated with the viral replication data, indicating no advantage in viral replication with Ad5/3-CXCR4-TIMP2 when compared to Ad5/3. Examination of primary tumor sample from patient 4 reveled low level of endogenous TIMP2 expression. However, 48 hours after infection with the TIMP2 armed viruses, the level of TIMP2 present in the tissue increased significantly (Fig. 7B). Since TIMP2 is a secretory protein, results of immunohistochemistry also indicated positive reactivity in the stromal compartment following transduction with vector containing the TIMP2 transgene (Figure 7B).
Figure 6. TIMP2 armed CRAd exhibit enhanced viral replication in primary epithelial ovarian tumor samples.
(A–E) Five human primary ovarian tumor tissues were obtained from debulking surgeries of patients with either stage III or stage IV disease. Slices from the tissues were made, then infected with the indicated viruses and maintained in culture for up to four days. DNA was extracted from the media at the indicated times and subjected to Q-PCR to detect the E4 gene copy number as a measure of viral DNA replication. Data indicate mean ± SD from triplicate assays for each patient tissue. *p< 0.05.
Figure 7. Immunohistochemistry in primary epithelial ovarian tumor slices.
(A) Five human primary ovarian tumor tissues from patients with advanced cancer were examined for expression of CXCR4. Primary ovarian tumor from patient 4 was examined for TIMP2 (B) expression with the indicated viruses at 48 hrs postinfection.
Discussion
In recognition of the oncolytic potential of replicating adenoviruses, these microbiologicals have been rapidly translated into human clinical trials for patients with advanced cancer. These studies have demonstrated safety, but suggest the need for strategies to improve their efficacy for successful clinical translation (27). One strategy used to improve the efficacy of adenoviruses is to engineer the virus to deliver a therapeutic transgene (28). Most of these “armed” oncolytic adenoviruses have been designed to carry suicide genes, such as cytosine deaminase or herpes simplex virus thymidine kinase, that augment virus mediated killing of the infected tumor cells (29, 30). However, the timing of the prodrug administration is extremely crucial, as bad timing could potentially inhibit viral spread due to premature killing of the virus prior to the completion of the viral replication cycle. Instead of focusing solely on enhanced killing of the infected cancer cells, we hypothesized that it would be rational to arm the replicating adenovirus with a secreted protein that can also modulate the tumor microenvironment and thereby limit or prevent tumor progression.
Toward this goal, we sought to enhance the efficacy of a replicating adenovirus by arming it with TIMP2. TIMP2 possesses a number of attractive features that favor its use in this therapeutic strategy. TIMP2 is a small, 21 kDa unglycosylated protein that is naturally secreted. TIMP2 binds in a 1:1 stoichiometric ratio to the active forms of MMPs, including MMP-2 and MMP-9, thereby inhibiting the MMP activity associated with tumor growth, angiogenesis and invasion (9). Thus, overexpressing TIMP2 in the context of a tumor microenvironment should directly block tumor growth. A distinct advantage of TIMP2 over other members of the TIMP family is that it has also been shown to inhibit tumor growth and angiogenesis by a variety of mechanisms independent of direct MMP-inhibition (14–16). TIMP2 can inhibit the proliferation of endothelial cells in response to angiogenic stimuli such as fibroblast growth factor 2 or vascular endothelial growth factor A by binding to α3β1 integrin and cause the induction of protein tyrosine phosphatase activity (16). TIMP2 also inhibits migration of endothelial cells through an indirect MMP inhibitor effect that is mediated by the upregulation of the RECK protein, a membrane anchored MMP inhibitor (15). Finally, tumors overexpressing TIMP2 have reported to have an increased activity of mitogen-activated protein kinase phosphatase 1, which leads to an increase dephosphorylation of p38 mitogen-activated protein kinase that, in turn, results in inhibition of tumor growth and angiogenesis (14). Studies with non-replicative adenoviral vectors expressing TIMP2 have proved their utility for cancer therapy, resulting in the reduction of tumor growth, angiogenesis and metastasis in various cancer models (31–33). Collectively, these studies provide a clear rationale for a therapeutic strategy exploiting the localized overexpression of TIMP2 in the tumor microenvironment.
We hypothesized that Ad5/3-CXCR4-TIMP2, a CRAd armed with TIMP2, should directly kill the cancer cells through viral oncolysis, while secretion of TIMP2 by the infected cells into the tumor microenvironment would augment the therapeutic effect by preventing tumor growth and angiogenesis via both MMP-dependent and MMP-independent mechanisms. We have validated in vitro that our TIMP2 armed viruses are secreting functional TIMP2, as indicated by the inhibition of the enzymatic degradation of gelatin by active MMPs. For the TIMP2 armed CRAd to be efficacious, it is important that the expression of TIMP2 does not impair viral replication nor its oncolytic potency. We have demonstrated that both viral replication and oncolytic potency were not compromised in both SKOV3.ip1 and OV-4 human ovarian cancer cells by arming with TIMP2. Moreover, we showed that with the CXCR4 promoter, there is selectivity in replication, as indicated by higher level of replication in the ovarian cancer cells, when compared to the control fibroblasts. In the hopes of translating this virus into the clinic, we have employed the usage of an ex vivo model system to further examine viral replication. Tumors samples examined from five patients with stage III and IV ovarian cancer revealed a consistent high level of replication with Ad5/3-CXCR4-TIMP2 when compared to the other control viruses. This data is very encouraging, suggesting that Ad5/3-CXCR4-TIMP2 might be effective for the treatment of advanced ovarian cancer. Nonetheless, it should be emphasized, based on minimal expression of CXCR4 in patient 5, that a preliminary evaluation of both Ad receptor level and CXCR4 promoter activity should be determined from individual samples during surgical debulking or by needle aspiration prior to using this vector for therapy. Studies are underway to examine the biodistribution and toxicity of Ad5/3-CXCR4-TIMP2 in a murine model of orthotopic disseminated ovarian cancer.
Translational Relevance.
Ovarian cancer remains the leading cause of gynecological cancer death in the U.S. due to advanced stage of cancer at diagnosis along with the lack of effective therapy for disseminated disease. Clinical trials using adenoviral based gene therapy have exhibited safety but suggest the need to increase vector efficacy for successful clinical translation. Towards this goal, we designed a CRAd which combines the property of tumor selective replication with the delivery of a therapeutic transgene, TIMP2, which targets the tumor microenvironment to inhibit growth and dissemination. Our article is the first study of a CRAd armed with a non-cytotoxic transgene for ovarian cancer therapy. Moreover, we have employed an ex vivo model system using primary ovarian tumors slices to examine efficacy of replication of these viruses. These promising preclinical results provide the basis for clinical translation.
Acknowledgments
Grant support: Financial support from the NIH grant R01CA108585 and the UAB Department of Pathology Research Fund is appreciated.
References
- 1.Ozols RF, Bookman MA, Connolly DC, et al. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573–604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- 3.Krasnykh VN, Douglas JT, van Beusechem VW. Genetic targeting of adenoviral vectors. Mol Ther. 2000;1:391–405. doi: 10.1006/mthe.2000.0062. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Kanerva A, Wang M, Bauerschmitz GJ, et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther. 2002;5:695–704. doi: 10.1006/mthe.2002.0599. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Seki T, Dmitriev I, et al. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus-adenovirus receptor-independent gene transfer efficiency. Hum Gene Ther. 2002;13:1647–53. doi: 10.1089/10430340260201734. [DOI] [PubMed] [Google Scholar]
- 7.Zhu ZB, Makhija SK, Lu B, et al. Transcriptional targeting of adenoviral vector through the CXCR4 tumor-specific promoter. Gene Ther. 2004;11:645–8. doi: 10.1038/sj.gt.3302089. [DOI] [PubMed] [Google Scholar]
- 8.Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642–50. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]
- 9.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 10.Schmalfeldt B, Prechtel D, Harting K, et al. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res. 2001;7:2396–404. [PubMed] [Google Scholar]
- 11.Huang S, Van Arsdall M, Tedjarati S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–42. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 12.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–27. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 13.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 14.Feldman AL, Stetler-Stevenson WG, Costouros NG, et al. Modulation of tumor-host interactions, angiogenesis, and tumor growth by tissue inhibitor of metalloproteinase 2 via a novel mechanism. Cancer Res. 2004;64:4481–6. doi: 10.1158/0008-5472.CAN-03-2929. [DOI] [PubMed] [Google Scholar]
- 15.Oh J, Seo DW, Diaz T, et al. Tissue inhibitors of metalloproteinase 2 inhibits endothelial cell migration through increased expression of RECK. Cancer Res. 2004;64:9062–9. doi: 10.1158/0008-5472.CAN-04-1981. [DOI] [PubMed] [Google Scholar]
- 16.Seo DW, Li H, Guedez L, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–80. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 17.Kirby TO, Rivera A, Rein D, et al. A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity. Clin Cancer Res. 2004;10:8697–703. doi: 10.1158/1078-0432.CCR-04-1166. [DOI] [PubMed] [Google Scholar]
- 18.Olinga P, Groen K, Hof IH, et al. Comparison of five incubation systems for rat liver slices using functional and viability parameters. J Pharmacol Toxicol Methods. 1997;38:59–69. doi: 10.1016/s1056-8719(97)00060-9. [DOI] [PubMed] [Google Scholar]
- 19.Rivera AA, Davydova J, Schierer S, et al. Combining high selectivity of replication with fiber chimerism for effective adenoviral oncolysis of CAR-negative melanoma cells. Gene Ther. 2004;11:1694–702. doi: 10.1038/sj.gt.3302346. [DOI] [PubMed] [Google Scholar]
- 20.Rocconi RP, Zhu ZB, Stoff-Khalili M, et al. Treatment of ovarian cancer with a novel dual targeted conditionally replicative adenovirus (CRAd) Gynecol Oncol. 2007;105:113–21. doi: 10.1016/j.ygyno.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 21.Baker AH, Wilkinson GW, Hembry RM, Murphy G, Newby AC. Development of recombinant adenoviruses that drive high level expression of the human metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 and -2 genes: characterization of their infection into rabbit smooth muscle cells and human MCF-7 adenocarcinoma cells. Matrix Biol. 1996;15:383–95. doi: 10.1016/s0945-053x(96)90158-4. [DOI] [PubMed] [Google Scholar]
- 22.Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–13. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borovjagin AV, Krendelchtchikov A, Ramesh N, Yu DC, Douglas JT, Curiel DT. Complex mosaicism is a novel approach to infectivity enhancement of adenovirus type 5-based vectors. Cancer Gene Ther. 2005;12:475–86. doi: 10.1038/sj.cgt.7700806. [DOI] [PubMed] [Google Scholar]
- 24.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kossakowska AE, Edwards DR, Lee SS, et al. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol. 1998;153:1895–902. doi: 10.1016/S0002-9440(10)65703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera AA, Wang M, Suzuki K, et al. Mode of transgene expression after fusion to early or late viral genes of a conditionally replicating adenovirus via an optimized internal ribosome entry site in vitro and in vivo. Virology. 2004;320:121–34. doi: 10.1016/j.virol.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Bell JC, Lichty B, Stojdl D. Getting oncolytic virus therapies off the ground. Cancer Cell. 2003;4:7–11. doi: 10.1016/s1535-6108(03)00170-3. [DOI] [PubMed] [Google Scholar]
- 28.Hermiston T. Gene delivery from replication-selective viruses: arming guided missiles in the war against cancer. J Clin Invest. 2000;105:1169–72. doi: 10.1172/JCI9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freytag SO, Rogulski KR, Paielli DL, Gilbert JD, Kim JH. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9:1323–33. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- 30.Wildner O, Morris JC, Vahanian NN, Ford H, Jr, Ramsey WJ, Blaese RM. Adenoviral vectors capable of replication improve the efficacy of HSVtk/GCV suicide gene therapy of cancer. Gene Ther. 1999;6:57–62. doi: 10.1038/sj.gt.3300810. [DOI] [PubMed] [Google Scholar]
- 31.Brand K. Cancer gene therapy with tissue inhibitors of metalloproteinases (TIMPs) Curr Gene Ther. 2002;2:255–71. doi: 10.2174/1566523024605564. [DOI] [PubMed] [Google Scholar]
- 32.Brand K, Baker AH, Perez-Canto A, et al. Treatment of colorectal liver metastases by adenoviral transfer of tissue inhibitor of metalloproteinases-2 into the liver tissue. Cancer Res. 2000;60:5723–30. [PubMed] [Google Scholar]
- 33.Rigg AS, Lemoine NR. Adenoviral delivery of TIMP1 or TIMP2 can modify the invasive behavior of pancreatic cancer and can have a significant antitumor effect in vivo. Cancer Gene Ther. 2001;8:869–78. doi: 10.1038/sj.cgt.7700387. [DOI] [PubMed] [Google Scholar]