Abstract
Background:
Functional electrical stimulation (FES) therapy has been applied to achieve functional benefits post spinal cord injury (SCI), but little is known about its effects on well-being.
Objective:
Using a parallel-group randomized controlled trial (RCT) design (NCT00201968), the effects of a FES-assisted walking intervention on quality of life and participation post SCI were compared to a non-FES exercise program.
Methods:
Individuals with chronic (≥18 months) incomplete SCI (level C2 to T12, AIS C or D) were randomized to a FES-assisted walking (intervention) or aerobic/resistance training (control) sessions 3 times a week for 16 weeks. The Spinal Cord Independence Measure (SCIM), Satisfaction With Life Scale, Lawton Instrumental Activities of Daily Living, Craig Handicap and Assessment Reporting Technique, Reintegration to Normal Living Index, and perceptions of intervention(s) outcomes were completed at baseline, 4, 6, and 12 months. Repeated measures general linear models were used to assess between-group differences. Perceptions of intervention(s) were analyzed using qualitative content analysis.
Results:
Thirty-four individuals were randomized (17 per group); 27 remained at 12 months. The FES group had a significant increase (P < .01) on SCIM mobility subscores (mean [SD] = 17.27 [7.2] to 21.33 [7.6]) compared to the exercise group (mean [SD] = 19.9 [17.1] to 17.36 [5.5]). Although no significant between-group differences were detected for other outcomes, both groups reported positive gains in well-being from trial participation.
Conclusions:
The present study provides insight into the perceived benefits acquired by participating in an RCT comparing exercise to FES therapy and serves as a model for pinpointing domains of well-being that could be targeted for assessment in future SCI trials.
Key words: activities of daily living, community participation, exercise, functional electrical stimulation, qualitative research, quality of life, spinal cord injuries, therapy, walking
Functional electrical stimulation (FES) has been used for over 40 years as a method to generate contraction in paralyzed muscles and restore functions, such as walking, by applying bursts of short electrical pulses to the muscles or the peripheral nerves that innervate the muscles of interest.1 Originally, FES was applied as a permanent orthotic device to restore function, where the patient depended on the FES system for the rest of his or her life to perform the function. Recent application of FES as a short-term therapy in regular training sessions has been used to improve or recover voluntary function, and the reliance on FES to achieve muscle contraction is reduced over time. As such, the benefits of FES therapy could be maintained over time without the continued use of an FES therapeutic device.2 FES therapy has been shown to improve gait in persons with chronic incomplete spinal cord injury (SCI). In a convenience sample of 5 persons with incomplete SCI, Thrasher and colleagues3 reported that their FES therapy gait-training regimen was effective for improving voluntary walking function in a chronic SCI patient population for whom significant functional changes were not expected.
Whether the functional gains achieved by FES therapy translate into clinically meaningful improvements in quality of life (QoL) and community participation for people with chronic SCI is unknown. Our research team conducted a randomized controlled trial (RCT) with the primary aim of testing the hypothesis that the application of our FES-assisted walking protocol 3 times a week for 16 weeks could improve functional mobility in individuals with chronic motor incomplete SCI and that the improvement would be maintained 8 months after cessation of training. Given the expectation of greater functional gains (ie, mobility) from the FES-assisted walking protocol, we hypothesized that the FES therapy group would report significant gains in QoL and community participation compared to the conventional exercise control group.
Methods
Study design and sample
The design was a parallel group RCT (www.clinicaltrials.gov; NCT00201968) conducted at a large SCI rehabilitation hospital. The study was approved by the Research Ethics Board of the Toronto Rehabilitation Institute, and we certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed. Recruitment for the study commenced in March 2005, and the last subject completed follow-up in December 2010. All recruitment, outcome assessment, and intervention group and control group activities were conducted at our center.
Individuals with a chronic (≥18 months) traumatic spinal cord lesion between C2-T12 (American Spinal Injury Association Impairment Scale [AIS] C or D) were eligible. Individuals were excluded if they had any contraindications for FES (see box, Contraindications to Functional Electrical Stimulation). Medical clearance was obtained from potential subjects’ family physicians for trial participation.
Randomization
Upon completion of baseline assessments, subjects were randomly assigned to the intervention or control group in a 1:1 allocation ratio. The randomization sequence was generated using the randperm.m function in Matlab (The MathWorks, Inc., Natick, MA). Envelopes were prepared by a research assistant who was not involved in enrolling subjects. Each subject selected an unmarked, sealed envelope from a box containing envelopes with unique reference numbers. Each reference number corresponded to another sealed envelope in a separate location that indicated group allocation for that subject.
Contraindications to Functional Electrical Stimulation
Cardiac pacemakers
Skin lesions or rashes at potential electrode sites or denervation of targeted muscles
Lower extremity grade IV pressure ulcers, or grade or III pressure ulcers at locations where FES or the harness was applied
Uncontrolled hypertension
Symptoms of orthostatic hypotension upon standing for 15 minutes
Susceptibility to autonomic dysreflexia requiring medication
Intervention group and control group activities
Due to the nature of the treatment, it was not possible to blind the subjects (ie, no placebo group). The patient’s physician was blind to group allocation unless a serious adverse event occurred. Both groups were provided the same level of attention and were engaged in a form of physical activity for 45 minutes per session, 3 days a week, for 4 months (48 sessions in total). A physically active control protocol was selected so that any intervention effects were attributed to the FES walking protocol rather than an improved fitness level. Adherence was determined by counting the number of sessions completed. Subjects who missed sessions were allowed to make them up.
Intervention group
Individuals assigned to the intervention group received FES stimulation while ambulating on a body weight support treadmill (BWST) (Loko 70; Woodway USA Inc.). This apparatus included an overhead harness that attached to cables and pulleys such that a constant upward force could be applied to the subject while walking. The harness also served as a safety device. Minimal amount of body weight support (in increments of 8 kg) was used to facilitate walking. This amount differed between subjects and from session to session. As the therapy progressed, the amount of body weight support decreased. However, the subjects wore the harness until the end of therapy, either for BWST or safety considerations. Walking exercises were performed at a speed selected by the attending physiotherapist with input from the subject. During therapy, manual assistance was applied by up to 3 assistants to the subject’s lower extremities and lower back when needed to facilitate walking and ensure that movements were carried out in a physiological way. The use of BWST was subjectdependent and varied over time; the amount of weight support was just enough to assist the subject to achieve standing without knees buckling. The amount of body weight support and manual assistance was progressively decreased over time with the goal of achieving no support or assistance.
FES was delivered using 2 Compex Motion transcutaneous electric stimulators (Compex SA, Switzerland), which used surface self-adhesive stimulation electrodes. The 2 stimulators worked independently, each stimulating only 1 leg (ie, 4 electrodes from 1 stimulator were applied to 1 leg only). The stimulators were not synchronized. Instead, each stimulator behaved as an independent system controlling gait sequence of a designated leg and was manually triggered using a push button. The therapist activated the push button shortly after heel-off but before the toe-off phase of the gait cycle. The stimulation sequence was developed such that following the push button activation the entire stimulation sequence was delivered to the targeted muscles in an open-loop control manner. In the majority of the subjects, the push buttons were triggered by the physical therapist or therapist assistant. In few isolated cases, the push buttons were triggered by patients (ie, high-functioning individuals). In cases where a subject had good balance and control and did not need to hold the handrails, they were given the option to control the stimulation when they initiated steps.
The electrodes were placed on the subject’s skin at the motor points above the nerves corresponding to the muscles targeted with FES. Muscles stimulated were quadriceps (electrode size, 5 x 10 cm), hamstrings (electrode size, 5 x 10 cm), tibialis anterior (electrode size, 2.5 x 2.5 cm) and gastrocnemius (electrode size, 2.5 x 2.5 cm). The stimulation pulses used were balanced, biphasic, and current regulated. Pulse-width modulation was used to regulate temporal activity of the muscles, and the pulse amplitude was used to regulate muscle contraction strength. Pulse amplitudes were in the range from 8 to 125 mA (they were subject- and muscle-specific), and pulse durations were in the range of 0 to 300 µs. The pulse frequencies were from 20 to 50 Hz. For example, in the case of 1 subject, the following stimulation parameters were used: maximum pulse duration was 300 µs; pulse frequency was 40 Hz; and maximum pulse amplitudes for the stimulated muscles (left leg) were quadriceps at 40 mA, hamstrings at 46 mA, tibialis anterior at 56 mA, and gastrocnemius at 30 mA. During stimulation, the targeted muscles were stimulated bilaterally and in a physiologically correct sequence that mimicked the muscle activation sequence observed during ambulation in ablebodied individuals.
Control group
Individuals assigned to the control group participated in an exercise program consisting of 20 to 25 minutes of resistance (using hand weight, cables, and uppertone) and 20 to 25 minutes aerobic training (arm cycling, leg cycling, and walking with parallel bars or on a treadmill). Sessions were supervised by trained kinesiologists. Two to 3 sets of resistance training were performed at 12 to 15 repetition maximum resistance for all muscle groups that were capable of voluntary activity. The intensity was progressively increased according to tolerance. Aerobic exercise was performed at a moderate pace (3-5 on the Modified Borg Rating of Perceived Exertion Scale). The control group had an opportunity to exercise on a treadmill if they were able to walk unassisted.
Outcome measures
The registered trial listed the primary outcomes as the following health complications: spasticity, muscle atrophy, and bone loss (osteoporosis). The current report outlines the effects of the intervention on subjects’ activities of daily living, community participation, and life satisfaction. Outcomes were assessed at baseline, 4 months of intervention/control activities, at 6-month follow-up (2 months after intervention/control activities had ceased), and at 12-month follow-up (8 months after intervention/control activities had ceased). Other trial outcomes will be reported elsewhere. Data on body composition, side effects of intervention/control activities, and adverse events were a secondary outcome and are reported by Giangregorio et al.4 Subjects were monitored for side effects during and between training sessions, and they were instructed to report any event to the trial coordinator. Each event was reviewed by a physician adjudicator who was not a member of the research team to determine whether it was related to intervention or control activities.
With regard to the outcomes of interest presented in the current report, subjects were assessed on all outcome measures at baseline, exit (4 months), 6 months, and 12 months except for the Spinal Cord Independence Measure (collected at baseline and at 12 months). For each outcome measure, responses by the subjects were collected by a research assistant blinded to group allocation.
Spinal Cord Independence Measure
The Spinal Cord Independence Measure (SCIM) is a comprehensive ability rating scale that has been designed specifically for patients with spinal cord lesions.5 SCIM score ranges from 0 to 100 and assesses 3 areas of function: selfcare, respiratory and sphincter management, and mobility. The present study used the third version of the scale, which has been shown to be reliable and valid for SCI.6,7 For this study, only the 8-item mobility subscale was analyzed (scores ranging from 0 to 40), because it was the domain that we hypothesized would change with the intervention. The SCIM was collected by a research assistant who was blinded to group allocation through selfreport. It was only administered at baseline and at 12 months, because this was a secondary outcome measure of the clinical trial.
Satisfaction with Life Scale
The Satisfaction with Life Scale (SWLS) is a 5-item scale that assesses people’s satisfaction with their life as a whole.8 Scores range from 1 to 35, and higher scores reflect greater life satisfaction. The SWLS has a test–retest reliability of 0.82 over a 2-month interval and an internal consistency reliability of 0.87.8 It has been shown to be valid and reliable measure for SCI.9
Lawton Instrumental Activities of Daily Living Scale
The Lawton Instrumental Activities of Daily Living (IADL) scale is a 9-item instrument to assess independent living skills.10 (Item 8, related to medication use, has 2 subitems.) Scores range from 11 to 29, with higher scores representing higher ability. The Lawton IADL provides selfreported information about functional skills that are necessary to live in the community, and it has been shown to be a responsive measure for SCI.11
Craig Handicap and Assessment Reporting Technique
The Craig Handicap and Assessment Reporting Technique (CHART) assesses the construct of “handicap” (loss or limitation of opportunities to take part in the life of the community on an equal level with others).12 The CHART evaluates physical independence, mobility, occupation, social integration, and economic self-sufficiency. It collects information on the degree to which the respondent fulfills the roles typically expected of people without disabilities. Each domain has a maximum score of 100, with higher scores indicating greater role equivalence to an ablebodied person. The CHART is a reliable and valid measure for SCI.13
For the current study, data from all the subscales except for economic self-sufficiency were utilized, as we hypothesized that these domains would be the most sensitive to the effects of the intervention.
Reintegration to Normal Living (RNL) Index
The Reintegration to Normal Living (RNL) Index is an 11-item scale of community participation that assesses involvement in meaningful activities (employment, recreational, and social activities), perceived ability to move within the community, and the degree of comfort with social roles.14 Scores range from 0 to 21, with higher scores representing higher levels of participation. Each item is rated using a 3-point scale (0 = does not describe my situation; 1 = partially describes my situation; and 2 = fully describes my situation), which has been validated for collection in chronic SCI.15
Treatment regimen perceptions
Subjects in both groups completed a series of open-ended questions at baseline asking them about the motivation for and expectations of their participation in the program (see Appendix). At the follow-ups, they were asked about their perspectives on the outcomes of the treatment regimens and the study in general. Subjects completed these questions with a research assistant either on paper or by audiotape.
Statistical analyses
The trial reporting was done in accordance with the CONSORT criteria, and subject flow through the study was depicted using a CONSORT flow diagram (http://www.consortstatement.org/). Descriptive statistics were used to characterize subject demographics, medical history information, and all outcomes. Mean (SD) was used for continuous variables and number (%) was used for categorical variables. Sample size was determined using the outcome that was expected to demonstrate the smallest effect size for the registered trial, namely tibia cortical bone mineral density (BMD; not reported here). In a report on FES-assisted cycling, Eser et al16 reported SD of 0.03 to 0.06 g/cm3 for the tibial cortical BMD in individuals with SCI, and the estimated clinically meaningful effect was 0.6% per month, corresponding with a sample size of 13 per group, assuming that alpha is 0.05. Our target was 17 subjects per group to account for attrition. Between-group differences, differences over time, and the Time x Group interaction after 4 months and 12 months were analyzed by a per protocol analysis using a repeated measures general linear model. An alpha of 0.05 (2-tailed) was used for all tests. Bonferroni corrections were performed in the case of multiple comparisons. IBM SPSS version 19 (Armonk, NY, USA) was used for the analyses.
The open-ended response data on the perceptions of the treatment regimens were analyzed using a qualitative content analysis approach (QCA). QCA is a dynamic form of analysis of verbal and visual data that is oriented toward summarizing the informational contents of that data.17,18 For this approach, responses from the surveys and transcribed audio interviews were reviewed. Through QCA, it is possible to distil words into fewer content-related categories. It is assumed that when classified into the same categories, words and phrases share the same meaning.19
For this study, the responses on the surveys and interview transcripts were read to obtain a general sense of the information collected and to reflect on emerging content areas. The surveys and transcripts were then reviewed line by line, and words and passages were highlighted and labeled with a code. Codes were compared based on similarities and differences and sorted into categories using Microsoft Excel. Some codes were merged to eliminate duplicates or redundancies. These steps were conducted by 2 of the authors (S.L.H., A.P.).
Results
Of the 34 individuals who entered the study, 27 (16 intervention, 11 control) returned for the final assessment (Figure 1). Demographic information, medical history, and impairment characteristics of the intervention and control groups are presented in Table 1, and data on the outcome measures of interest are presented in Table 2. There were no significant differences between groups at baseline for any of the outcomes of interest (SCIM, SWLS, IADL, CHART, RNL Index). On average, fewer sessions were completed by control group subjects (34.1 sessions; 71%) than intervention group subjects (42.1; 88%).
Figure 1. CONSORT flow diagram. FES = functional electrical stimulation.

Table 1. Demographic and impairment characteristics (N = 34).
| Variable | FES (n=17) | Control (n=17) |
|---|---|---|
| Mean age, years (SD) | 56.6 (14.0) | 54.1 (16.5) |
| No. of males (%) | 14 (82.4) | 12 (70.6) |
| Mean UEMS (SD) | 38.3 (7.4) | 37.5 (13.8) |
| Mean LEMS (SD) | 30.4 (8.2) | 27.9 (9.8) |
| Mean duration of injury, years (SD) | 8.75 (9.7) | 10.3 (11.1) |
| AIS C, n (%) | 6 (35.2) | 7 (41.2 ) |
| AIS D, n (%) | 11 (64.7) | 10 (58.8) |
| Mean height (SD) | 174.3 (7.9) | 173.6 (9.2) |
| Mean weight (SD) | 81.3 (13.1) | 90.7 (39.0) |
| Mean no. of training sessions completed (SD) | 42.1 (10.7) | 34.1 (17.6) |
Note: In the functional electrical stimulation (FES) group, the person who dropped out had an AIS D score and a mean Spinal Cord Independence Measure (SCIM) mobility score of 13. Of the 6 people who dropped out of the study from the control group, 4 had an AIS D score and 2 had an AIS C score. Only 5 completed the SCIM, which yielded a mean (SD) SCIM mobility subscore of 21 (12). LEMS = lower extremity mobility score; UEMS = upper extremity mobility score.
Table 2. Life satisfaction scores, activities of daily living, and community participation scores across time by group.
| Group | Group x Time interaction | ||
|---|---|---|---|
| FES (n = 16) Mean (SD) | Control (n = 11) Mean (SD) | P values | |
| SWLS baseline | 18.81(7.17) | 20.18 (8.61) | .682 |
| SWLS post | 18.69 (9.60) | 17.82 (6.79) | |
| SWLS 6 months | 18.63 (9.79) | 18.45 (6.53) | |
| SWLS 12 months | 18.63 (9.79) | 18.45 (6.53) | |
| IADL baseline | 20.25 (4.06) | 22.18 (3.87) | .322 |
| IADL post | 18.50 (3.67) | 19.91 (2.94) | |
| IADL 6 months | 20.44 (3.78) | 21.55 (3.62) | |
| IADL 12 months | 20.44 (3.78) | 21.55 (3.62) | |
| RNL baseline | 16.44 (4.59) | 17.18 (3.46) | .373 |
| RNL post | 16.81 (3.87) | 17.00 (4.15) | |
| RNL 6 months | 16.37 (4.27) | 17.91 (2.88) | |
| RNL 12 months | 16.37 (4.27) | 17.91 (2.88) | |
| CHART Mobility baseline | 79.81 (21.00) | 82.09 (19.31) | .840 |
| CHART Mobility post | 85.28 (13.81) | 84.27 (11.89) | |
| CHART Mobility 6 months | 86.56 (14.44) | 88.45 (15.25) | |
| CHART Mobility 12 months | 86.56 (14.44) | 88.45 (15.25) | |
| CHART Social baseline | 89.94 (13.12) | 72.73 (24.00) | .065 |
| CHART Social post | 90.31 (18.02) | 89.64 (12.63) | |
| CHART Social 6 months | 91.13 (12.39) | 73.00 (30.61) | |
| CHART Social 12 months | 88.69 (17.10) | 75.73 (31.15) | |
| CHART Physical baseline | 92.32 (11.75) | 97.94 (2.49) | .214 |
| CHART Physical post | 93.72 (8.02) | 94.99 (7.30) | |
| CHART Physical 6 months | 93.90 (6.16) | 93.85 (5.01) | |
| CHART Physical 12 months | 93.81 (6.16) | 93.85 (5.01) | |
| SCIM Mobility baselinea | 17.27 (7.25) | 19.09 (7.08) | .003 |
| SCIM Mobility 12 monthsa | 21.33 (7.62) | 17.36 (5.46 ) | |
Note: CHART = Craig Handicap and Assessment Reporting Technique; FES = functional electrical stimulation; IADL = Lawton Instrumental Activities of Daily Living Scale; RNL = Reintegration to Normal Living Index; SCIM = Spinal Cord Independence Measure; SWLS = Satisfaction With Life Scale.
One FES subject did not complete the SCIM at baseline and is thus excluded from the analysis.
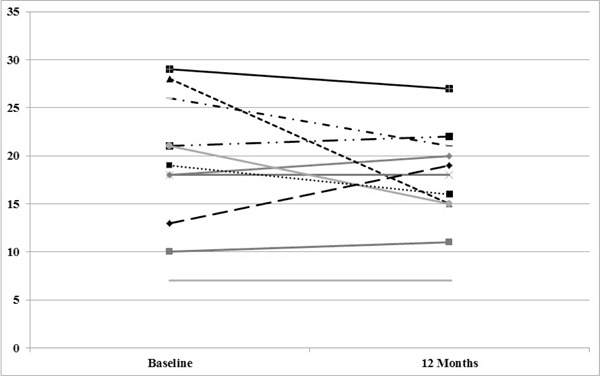
No significant differences were detected between groups on the SWLS, IADL, CHART, or RNL Index; there were no within-subject changes detected on these measures (Table 2). For the SCIM, there was 1 participant in the FES group with missing data at baseline, who was thus excluded from the analysis on this outcome. There was no significant main effect for time (F1,24 = 1.747, P = .199) and no difference between the groups (F1,24 = 0.166, P = .687) on the SCIM mobility subscore. However, the interaction was significant (F1,24 = 10.716, P = .003). The mean (SD) score at baseline for the intervention group was 17.27 (7.25) and 19.09 (7.08) for the control group. The scores at 12 months post intervention were 21.33 (7.62) for the intervention group and 17.36 (5.46) for the control group. When the interaction was examined, it was determined that the SCIM mobility scores improved over time for the intervention group (P < .01) but not for the control group. Figures 2 and 3 provide the trajectories for each subject on the SCIM mobility subscores in each condition (intervention vs control).
Figure 2. Spinal Cord Independence Measure (SCIM) mobility subscores for each subject in the functional electrical stimulation (FES) intervention group.
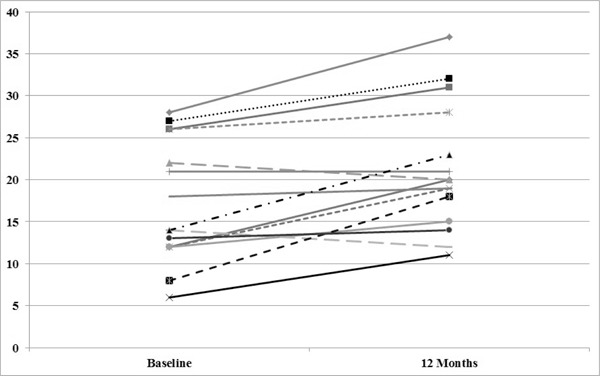
Figure 3. Spinal Cord Independence Measure (SCIM) mobility subscores for each subject in the control group.
Subject perceptions on treatment regimens
Figure 4 provides an overview of the subjects’ perceptions of the program and details the themes and subthemes that emerged for both groups and over time. Except for 1 individual in the intervention group who only did the baseline assessment (survey), all the subjects completed all of the interviews and surveys. There were 16 interviews (7 intervention, 5 control), and the rest of the data were obtained through the responses to the survey questions. It should be noted that some responses from the surveys were recorded by a research assistant; in some instances, this was done using a third-person narrative.
Figure 4. Themes and subthemes of subjects’ perspectives on the program at post intervention, 6 months, and 12 months.
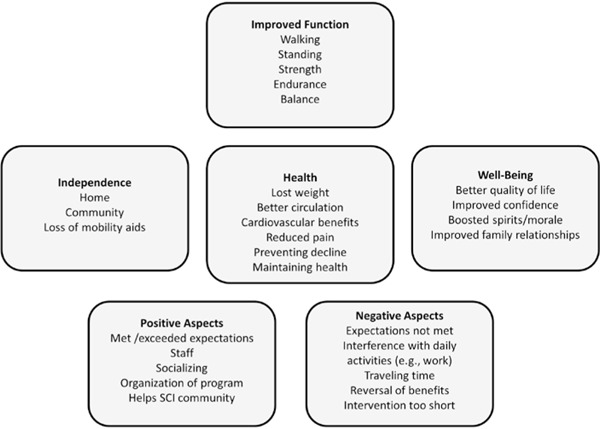
With regard to the baseline interviews, a salient theme that emerged for both groups was the desire for improving physical function and health (ie, walking, standing, balance, strength and endurance, weight loss). In addition, 2 related themes with regard to increasing independence (ie, losing aids) and improving well-being (ie, increased self-confidence, better QoL) were noted:
Give me back my life, improve mobility in the community. Improve everyday activities around the house. Provide me with a higher level of self-confidence. [EL, intervention group, baseline]
Participate with grandchildren and young friends – would like to be able to catch up with them and not feel like a hinderance. [FA, intervention group, baseline]
To get out of the chair; get dressed in and out of bed. Be able to be more independent. [FB, control group, baseline].
Some subjects in both groups were motivated to participate in order to help others:
If we all help and put the information we can together, we might come up with some solutions. [FI, control group, baseline]
At post intervention (exit, 6 months, and 12 months), many improvements in function and health were noted by both the intervention and control groups (ie, walking, standing, transferring, flexibility). As a result of these functional improvements, increased independence was reported by the sample:
Has helped to improve confidence in walking and standing, can get up on his neighbors’ stairs (New Years Eve, required help from 2 people to get up stairs, at Easter, he could climb on his own). [EK, intervention group, exit]
Because the more he walks now, the better he feels and gets greater independence. Can walk or visit friends further away from home. [EB, intervention group, exit]
I am doing things I never did before, groceries, cleaning the house. [EH, control group, 12 months]
Many persons in both groups noted that their dependence on aids had lessened:
Has changed from using a cane to a walker and finds has more stability. [EI, control group, exit]
Hence, the comments provided by many subjects indicated that their ability to be mobile in the home and in the community had improved as a result of participating in either exercise or FES treatment.
With regard to improvements in well-being, many comments indicated that QoL had improved in various ways:
Gave me back my life. [EL, intervention group, 12 months]
Makes life more fulfilling. More independent. [EA, intervention group, 6 months]
Most comments were not so generic in terms of how the program improved well-being. Rather, 2 subthemes emerged under improved well-being: improved confidence and boosted spirits/morale.
Given me more confidence and no longer fears falling. [EK, intervention group, 6 months]
More confident on the street that balance has improved. [EZ, control group, 12 months]
Emotionally – looks forward to coming, increased spirit, increased hopes of walking. [FM, intervention group, exit]
Feels “peppier” overall. [EP, intervention group, exit]
As such, the hope for increased self-confidence was realized post intervention and at subsequent follow-ups, and reports of increased mood and morale emerged over time.
In general, the program was viewed favorably by both the FES (intervention) and exercise (control) groups. Some of the negative perceptions’ were that the interventions affected work schedules and other daily activities and the travelling time required to receive the therapy and to do the follow-up assessments at the center was an inconvenience. Some subjects suggested that the duration of the program should have been longer.
Discussion
The present study attempted to determine the benefits of FES-assisted walking on various domains pertinent to well-being (QoL, community participation, etc) compared to a conventional exercise program for persons with SCI. Overall, neither treatment regimen resulted in a statistically significant improvement in these areas, with the exception of mobility, which improved in the intervention group.
Given that the mobility subscale of the SCIM assesses an individual’s capacity with regard to ambulation and transfers, it is not surprising that this scale would be sensitive to the potential benefits of FES. Our findings on improved mobility are consistent with other studies that found improvements in gait using surface FES2,3 and implantable FES approaches post SCI.20 The attribution that the improvement was due to FES, however, must be tempered by a number of study limitations with regard to our research design. One is the possibility that the intervention delivered in the FES condition provided more task-specific training related to walking compared to the exercise session. A comparison group receiving a similar intervention without FES might have better served to attribute causality to the FES.
Another issue is the timing of the SCIM assessments. Unlike the other outcome measures, the SCIM was only collected at baseline and at 12 months. As such, it is not known how both groups fared on the SCIM mobility subscale at end of the intervention (4 months). Further, it is possible that the FES group undertook additional exercise activities that might have led to improved function while those in the control group did not. Future studies should track the physical activities of participants outside of treatment interventions to account for this possibility. The higher rates of dropout in the control arm of the study compared to the intervention arm might have also affected our outcomes. Hence, it appears that participating in the FES arm of the study led to increased and sustained gains in mobility as measured by the SCIM at 12 months post intervention, but further work is required to conclusively demonstrate this.
The lack of effects in both the FES and exercise groups on the IADL has been noted in other similar BWST trials.21 Conversely, the lack of effects in life satisfaction and participation in our sample conflicts with other reports that show improvements in well-being following physical activity interventions post SCI.21–26 For instance, Semerjian and colleagues24 assessed adapted exercise devices on QoL and body satisfaction in persons with SCI and reported that subjects had significant improvements in total QoL and other indices of well-being (health and functioning, social and economic, psychological factors). In our study, the lack of changes in the scores on the SWLS, CHART, and RNL Index for both groups might be due to the fact that some of the scores for the sample were at the upper end of the scales at baseline or were at levels that were comparable to, or even higher than, general reports in the SCI literature.9,15,27–29 Thus, it is possible that the sample was comprised of persons who were generally satisfied with life and actively participating in their communities prior to undergoing the interventions.
Another possibility for the lack of findings is that the measures we used were not sensitive for detecting the appropriate changes in well-being. The RNL Index, SWLS, and CHART are reliable and valid measures for SCI, but their design parameters might have limited our ability to detect changes in well-being for a variety of reasons. For instance, the 3-point rating scale of the RNL Index may not have been sensitive enough to detect gains in functional status in both the FES and exercise groups. Similarly, the subscales on the CHART contain items that may not be sensitive to improvements in gait. In particular, the social subscale only assesses the extent of an individual’s social network and does not assess other important dimensions such as frequency of supportive behaviors offered by the support network or the individual’s personal evaluation on the quality of their social support.30
With regard to life satisfaction, the SWLS is a global measure of subjective well-being that is widely endorsed by the SCI community, but it assesses subjective well-being from a cognitive perspective and not an affective one.31 Further, the SWLS does not differentiate between domains that respondents may be more or less satisfied with.32 These features of the SWLS suggest that it may not have been ideal for our study. For instance, our qualitative reports indicate that the interventions improved affect and self-confidence in our sample. As such, the use of a subjective well-being measure that is oriented to specific life domains and/or has a stronger affective component might have been better suited for detecting changes in our sample. Moreover, scores on the SWLS have been found to be relatively insensitive to the degree of physical impairment in persons with SCI.9
Despite the lack of effects on measures of participation and Qol, many of the open-ended responses indicate that positive gains were experienced by subjects in both groups, leading to increased independence, home and community mobility, and psychological well-being (ie, self-confidence, positive mood). A qualitative study33 exploring the perceptions of subjects with SCI who were implanted with standing FES prostheses reported similar themes and outcomes as our sample. A promising domain for investigation, which was salient in the implantable FES study33 and in this study, are variables related to psychological well-being.
Other studies have shown reduced depression and anxiety and higher self-esteem after FES or exercise.21–24 For instance, Guest and colleagues22 demonstrated improvements related to physical self-concept and depressive symptomatology in a FES-assisted walking study. Similarly, qualitative data by Semerjian and colleagues24 from their exercise SCI study revealed that subjects were most impressed by the psychological implications of exercise (eg, the opportunities to stand and walk). Overall, engaging in interventions that are aimed at improving walking can offer a number of benefits for psychological well-being.34 Qualitative approaches highlight “how subjects’ own perceptions of their improvements – whether big or small – have a far greater impact on subjects’ perceptions on subjective well-being than experimenter-measured improvements.”21(p297)
In general, the qualitative findings indicate that therapy, irrespective of modality in these circumstances, is beneficial for subjects’ well-being. Some caution is warranted with regard to the qualitative data. The majority of data was comprised of open-ended responses on a survey with only a few interview transcripts. This limited our ability to provide an in-depth qualitative analysis. Regardless, we used a systematic approach toward coding the data, which increases the trustworthiness of the quality of the data.
Given these findings, and the themes of improved mood/morale and self-confidence, it may be prudent to assess subjective perceptions related to mood and self-confidence in future exercise intervention studies and trials. These domains may be more sensitive to gains obtained by subjects and may potentially detect between-group changes. For instance, there is evidence that self-efficacy (an individual’s assessment of his/her ability to perform behaviors in specific situations)35 is related to mobility and walking ability in SCI and non-SCI populations.36,37 The measurement of self-efficacy may allow for clearer findings on changes in well-being after individuals participate in an FES-assisted walking protocol to enhance gait versus conventional exercise.
Conclusions
This study provides insight into the perceived benefits that individuals with SCI acquired by participating in an RCT comparing exercise to FES therapy, and it serves as a model for pinpointing domains of well-being that could be targeted for assessment in future trials.
Acknowledgments
This project was supported by the Ontario Neurotrauma Foundation and by the Toronto Rehabilitation Institute, which receives funding under the Provincial Rehabilitation Research Program from the Ministry of Health and Long-Term Care in Ontario. The views expressed do not necessarily reflect those of the Ministry. Salary support for Dr. S.L. Hitzig was provided by the Ontario Neurotrauma Foundation and the Rick Hansen Institute (grant 2010-RHI-MTNI-836). Salary support for Dr. L. M. Giangregorio was provided by the Ontario March of Dimes and the Canadian Institutes of Health Research.
The authors declare no conflict of interest.
Additional contributions: We would like to thank the participants for their dedication to the research.
Appendix
Interview Guides for Baseline, 4- and 6-Month Follow-ups, and 12-Month Follow-up
Baseline Interview Questions
-
Please tell me why you agreed to participate in this study?
Prompt: motivation, expectations of treatment
-
Please describe what you hope to accomplish?
Prompt: specific goals and objectives (ie, related to function, ADLs, etc)
-
Can you explain to me why these accomplishments are important to you?
Prompt: significance, practical, day-to-day living
4- and 6-Month Follow-up Interview Questions
-
Please tell me how the program is going? What was it about the program you like or dislike?
Prompt: determine the positive and negative features of the program
-
Is the experience in our program matching your expectations?
Prompt: motivation; goal attainment, etc
If expectations are not realized, can you explain to me why not?
-
Can you describe how the program has or has not benefited you?
Prompt: physical, mental, emotional, social (emphasis on change and impact)
-
Can you explain to me why these accomplishments are important to you?
Prompt: significance, implications for daily living
12-Month Follow-up Interview Questions
-
Now that the program has been completed, can you tell what you think about it? What was it about the program you liked or disliked?
Prompt: determine the positive and negative features of the program
-
Did the program match your expectations?
Prompt: motivation; goal attainment, etc
If expectations are not realized, can you explain to me why not?
Do you have any suggestion for change?
-
Can you describe how the program has or has not benefited you?
Prompt: physical, mental, emotional, social
-
Can you explain to me why these accomplishments are important to you?
Prompt: significance, implications for daily living
-
Considering everything we have talked about so far, please compare the program to other interventions/ treatments.
Prompt: comparison/contrast; positive and negative features including emotional, physical, and social motivation factors, self-image, etc
Would you consider your participation in the program successful or unsuccessful? Please explain.
Is there anything else you would like to tell me about the program?
References
- 1.Popovic MR, Curt A, Keller T, Dietz V. Functional electrical stimulation for grasping and walking: Indications and limitations. Spinal Cord. 2001; 39:403–412 [DOI] [PubMed] [Google Scholar]
- 2.Thrasher TA, Popovic MR. Functional electrical stimulation of walking: Function, exercise and rehabilitation. Ann Readapt Med Phys. 2008;51:452–460 [DOI] [PubMed] [Google Scholar]
- 3.Thrasher TA, Flett HM, Popovic MR. Gait training regimen for incomplete spinal cord injury using functional electrical stimulation. Spinal Cord. 2006;44:357–361 [DOI] [PubMed] [Google Scholar]
- 4.Giangregorio LM, Craven BC, Richards K, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on body composition. J Spinal Cord Med. 2012; 35:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catz A, Itzkovich M, Agranov E, Ring H, Tamir A. SCIM--spinal cord independence measure: A new disability scale for patients with spinal cord lesions. Spinal Cord. 1997;35:850–856 [DOI] [PubMed] [Google Scholar]
- 6.Catz A, Itzkovich M, Tesio L, et al. A multi-center international study on the Spinal Cord Independence Measure, Version III: Rasch psychometric validation. Spinal Cord. 2007:45;1–17 [DOI] [PubMed] [Google Scholar]
- 7.Itzkovich M, Gelernter I, Biering-Soerensen F, et al. The Spinal Cord Independence Measure (SCIM) Version III: Reliability and validity in a multi-center international study. Disabil Rehabil. 2007:29;1926–1933 [DOI] [PubMed] [Google Scholar]
- 8.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Personality Assess. 1985;49:71–75 [DOI] [PubMed] [Google Scholar]
- 9.Dijkers MP. Correlates of life satisfaction among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:867–876 [DOI] [PubMed] [Google Scholar]
- 10.Lawton M, Brody E. Assessment of older people: Selfmaintaining and instrumental activities of daily living. Gerontology. 1969;9:179–186 [PubMed] [Google Scholar]
- 11.Lawton G, Lundgren-Nilsson A, Biering-Sorensen F, et al. Cross-cultural validity of FIM in spinal cord injury. Spinal Cord. 2006;44:746–752 [DOI] [PubMed] [Google Scholar]
- 12.Whiteneck GG, Charlifue SW, Gerhart KA, Overholser JD, Richardson GN. Quantifying handicap: A new measure of long-term rehabilitation outcomes. Arch Phys Med Rehabil. 1992;73:519–526 [PubMed] [Google Scholar]
- 13.Hall KM, Dijkers M, Whiteneck G, Brooks CA, Krause JS. The Craig Handicap Assessment and Reporting Technique (CHART): Metric properties and scoring. Top Spinal Cord Inj Rehabil. 1998:4:16–30 [Google Scholar]
- 14.Wood-Dauphinée SL, Opzoomer MA, Williams JI, Marchand B, Spitzer WO. Assessment of global function: The Reintegration to Normal Living Index. Arch Phys Med Rehabil. 1988;69:583–590 [PubMed] [Google Scholar]
- 15.Hitzig SL, Romero Escobar EM, Noreau L, Craven BC. Validation of the Reintegration to Normal Living Index for community-dwelling persons with chronic spinal cord injury. Arch Phys Med Rehabil. 2012;93:108–114 [DOI] [PubMed] [Google Scholar]
- 16.Eser P, de Bruin ED, Telley I, Lechner HE, Knecht H, Stussi E. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest. 2003;33:412–419 [DOI] [PubMed] [Google Scholar]
- 17.Altheide DL. Ethnographic content analysis. Qual Sociol. 1987;10:65–77 [Google Scholar]
- 18.Morgan DL. Qualitative content analysis: A guide to paths not taken. Qual Health Res. 1993;1:112–121 [DOI] [PubMed] [Google Scholar]
- 19.Cavanagh S. Content analysis: Concepts, methods and applications. Nurse Res. 1997;4:5–16 [DOI] [PubMed] [Google Scholar]
- 20.Bailey SN, Hardin EC, Kobetic R, Boggs LM, Pinault G, Triolo RJ. Neurotherapeutic and neuroprosthetic effects of implanted functional electrical stimulation for ambulation after incomplete spinal cord injury. J Rehabil Res Dev. 2010;47:7–16 [DOI] [PubMed] [Google Scholar]
- 21.Hicks AL, Adams MM, Martin Ginis K, et al. Longterm body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: Effects on functional walking ability and measures of subjective well-being. Spinal Cord. 2005;43:291–298 [DOI] [PubMed] [Google Scholar]
- 22.Guest RS, Klose KJ, Needham-Shropshire BM, Jacobs PL. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: Part 4. Effect on physical self-concept and depression. Arch Phys Med Rehabil. 1997;78:804-807 [DOI] [PubMed] [Google Scholar]
- 23.Muraki S, Tsunawake N, Hiramatsu S, Yamasaki M. The effect of frequency and mode of sports activity on the psychological status in tetraplegics and paraplegics. Spinal Cord. 2000;38:309–314 [DOI] [PubMed] [Google Scholar]
- 24.Semerjian TZ, Montague SM, Dominguez JF, Mejy Davidian A, de Leon RD. Enhancement of quality of life and body satisfaction through the use of adapted exercise devices for individuals with spinal cord injuries. Top Spinal Cord Inj Rehabil. 2005;11:95–108 [Google Scholar]
- 25.Sipski ML, Delisa JA, Schweer S. Functional electrical stimulation bicycle ergometry: Patient perceptions. Am J Phys Med Rehabil. 1989; 68:147–149 [DOI] [PubMed] [Google Scholar]
- 26.Twist DJ, Culpepper-Morgan JA, Ragnarsson KT, Petrillo CR, Kreek MJ. Neuroendocrine changes during functional electrical stimulation. Am J Phys Med Rehabil. 1992;71:156–163 [DOI] [PubMed] [Google Scholar]
- 27.Putzke JD, Richards JS, Hicken BL, DeVivo MJ. Predictors of life satisfaction: A spinal cord injury cohort study. Arch Phys Med Rehabil. 2002;83:555–561 [DOI] [PubMed] [Google Scholar]
- 28.Geyh S, Ballert C, Sinnott A, Charlifue S, Catz A, D’Andrea Greve JM, Post MW. Quality of life after spinal cord injury: A comparison across six countries. Spinal Cord. 2013;51:322–326 [DOI] [PubMed] [Google Scholar]
- 29.Hastings J, Robins H, Griffiths Y, Hamilton C. The differences in self-esteem, function, and participation between adults with low cervical motor tetraplegia who use power or manual wheelchairs. Arch Phys Med Rehabil. 2011;92:1785–1788 [DOI] [PubMed] [Google Scholar]
- 30.Hicken BL, Putzke JD, Richards JS. Bladder management and quality of life after spinal cord injury. Am J Phys Med Rehabil. 2001;80:916–922 [DOI] [PubMed] [Google Scholar]
- 31.Dijkers MP. Quality of life of individuals with spinal cord injury: A review of conceptualization, measurement, and research findings. J Rehabil Res Dev. 2005;42:87–110 [DOI] [PubMed] [Google Scholar]
- 32.Post M, Noreau L. Quality of life after spinal cord injury. J Neuro Phys Ther. 2005;29:139–146 [DOI] [PubMed] [Google Scholar]
- 33.Bonder BR, Rohde LM, Triolo RJ. Exploratory study of perceived quality of life with implanted standing neuroprostheses. J Rehabil Res Dev. 2012;49:265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hicks AL, Martin Ginis KA. Treadmill training after spinal cord injury: It’s not just about the walking. J Rehabil Res Dev. 2008;45:241–248 [DOI] [PubMed] [Google Scholar]
- 35.Bandura A. Social Foundations of Thought and Actions: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986 [Google Scholar]
- 36.Collins TC, Lunos S, Ahluwalia JS. Self-efficacy is associated with walking ability in persons with diabetes mellitus and peripheral arterial disease. Vascular Med. 2010;15;19–95 [DOI] [PubMed] [Google Scholar]
- 37.Phang SH, Martin Ginis KA, Routhier F, Lemay V. The role of self-efficacy in the wheelchair skills-physical activity relationship among manual wheelchair users with spinal cord injury. Disabil Rehabil. 2012;34:625–632 [DOI] [PubMed] [Google Scholar]


