Abstract
Background:
Individuals with chronic tetraplegia prioritize recovery of hand function as an important factor in improving their quality of life. Interventions that may improve hand function and increase corticomotor excitability are functional electrical stimulation (FES), somatosensory stimulation (SS), and task-oriented training.
Objective:
We compared functional and corticomotor outcomes in a control condition to changes associated with FES (triggered via electromygraphic signals) and with SS (constant trains), each combined with either unimanual or bimanual training.
Methods:
Using a randomized, clinical trial design, comparisons were made to a delayed intervention control group. Participants (n = 24) had chronic tetraplegia, with the ability to activate thenar muscles, and were randomly assigned to either the immediate intervention (intervention) or control/ delayed intervention groups. Primary analyses compared intervention (FES or SS) to control/delayed intervention. Secondary analyses compared subgroups of FES versus SS (regardless of uni- or bilateral training) and uni- versus bimanual training (regardless of stimulation type). Outcomes were assessed before and after the control and the intervention period.
Results:
Compared to control/delayed intervention, the intervention group had greater changes in unimanual function and corticomotor area, regardless of whether practice was combined with FES or with SS. Irrespective of stimulation type, the bimanual subgroups improved to a greater extent than the unimanual subgroups on the bimanual hand function test.
Conclusions:
Hand training combined with either SS or FES was associated with improved hand use and corticomotor activity in persons with chronic tetraplegia. Both interventions appear to be equally effective.
Key words: functional electrical stimulation, hand function, neuroplasticity, somatosensory stimulation, spinal cord injury, tetraplegia
There are approximately 259,000 individuals living with chronic tetraplegia due to cervical spinal cord injury (SCI) in the United States.1 For persons with tetraplegia, recovery of hand function is an important meaningful goal.2,3 Studies of neuroplasticity have shown that corticomotor reorganization likely contributes to impairment in addition to that imposed by the damaged spinal cord. Maladaptive corticomotor plasticity occurs after SCI, wherein areas of the body affected by the injury have less corticomotor representation4,5 and less corticomotor excitability to muscles that are paretic as a result of the injury.6
The repetitive practice of task-specific activities using a massed practice (MP) schedule has been shown to induce corticomotor reorganization and improve the performance of activities in persons with tetraplegia.7–10 When considering interventions for persons with tetraplegia, the literature indicates that more changes in functional and corticomotor measures are found when MP training is augmented by electrical stimulation.7,8 Somatosensory stimulation (SS) is a form of low-level, continuous electrical stimulation with a long pulse width wherein the goal is to indirectly activate the corticomotor system through the sensory system.11 There is evidence that the long pulse width has been shown to preferentially activate the Ia afferent fibers.12 In persons with weakness due to spinal cord injury (SCI)7,8 or stroke,13 prolonged application of SS alone improved pinch force and unimanual hand function. Further, SS is associated with increased corticomotor excitability5,7,8 and is hypothesized to prepare the system for reorganization.14
Despite the apparent value of SS in augmenting effects of practice, it is conceivable that because the SS provides continuous stimulation, it administers inappropriate sensory information. The individual receives stimulation regardless of the phase of the activity in which they are engaged. As an alternative, functional electrical stimulation (FES) is a form of electrical stimulation wherein the stimulation activates the muscles and acts as a neural prosthesis to improve grasping function.15 Evidence suggests that improvements in activities persist even when the FES is not in use.16,17 As the degree of neural reorganization is dependent on the demands of the task in which the individual engages, more opportunities for practice of challenging activities may result in greater improvement in function and corticomotor reorganization. Further, most stimulators used in the clinical setting have a maximal pulse width of 400 microseconds, which is appropriate for FES; it has been suggested that longer pulse durations are required to activate large-diameter sensory fiber, as is the goal of SS.11 If the shorter pulse width used in FES is effective in promoting hand function compared to SS, then there is no need for the more expensive laboratory stimulators with the capability of increasing the pulse width to 1 millisecond.
The purpose of this study was to evaluate the effectiveness of FES or SS, each combined with either unimanual (Uni) or bimanual (Bi) MP training, as compared to a control condition on the primary outcome measure, the Jebsen Taylor Hand Function Test (JTHF). Because persons with tetraplegia typically have bilateral deficits, bimanual training may be more beneficial than unimanual training.9,10 A pilot study comparing these interventions has been published,10 but it lacked a control condition, which limited the ability to draw conclusions about overall effects of the intervention. We hypothesized that the participants assigned to the FES group would have greater changes in functional outcomes and corticomotor map area. We further hypothesized that participants assigned to the SS group would have a greater increase in corticomotor excitability compared to participants in the FES group.
Methods
This study utilized a delayed intervention design (Figure 1). Participants were randomly assigned to the control/delayed intervention group or immediate intervention group. Simultaneously, participants were also randomly assigned to an intervention of FES or SS, with either uni- or bilateral practice (ie, Uni+SS, Uni+FES, Bi+SS, Bi+FES).
Figure 1. Flow chart of participant group assignment. Twenty-four participants were recruited for the study with 9 to 11 individuals in each intervention group, as well as the delayed intervention control group. FES = functional electrical stimulation; SS = somatosensory stimulation.
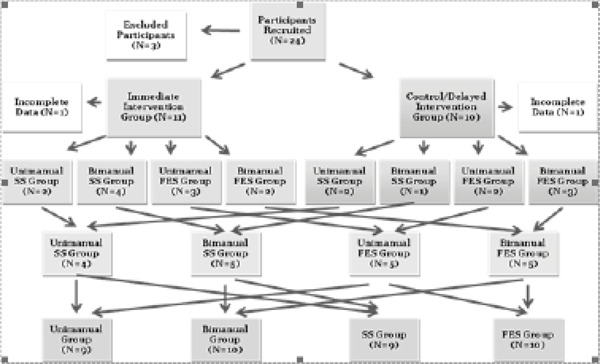
Participants assigned to the immediate intervention group started the intervention phase immediately after their first testing session. Participants assigned to the control/delayed intervention group completed a first testing session and then continued their regular activities for a 3-week period before completing a second session of testing. After the intervention period, all participants received a final session of testing. By comparing participants who received immediate intervention to those assigned to the control/delayed intervention group, we examined the effectiveness of different forms of combined intervention of MP training and electrical stimulation compared to the control condition. Further, the comparison of pre- and postintervention data of all participants enabled us to compare different combinations of interventions.
Twenty-four participants were recruited, as pilot work suggested that 12 individuals per group would have 80% power to detect a between-group difference at α ≤ 0.05 based on an effect size of 0.61. The 24 participants met the inclusion criteria: diagnosis of cervical SCI (C7 or above), chronic injury (at least 1 year post injury), activation of thenar muscles (measured through manual muscle testing), and ability to perform at least 2 items on the JTHF or Chedoke Arm and Hand Activity Inventory (CAHAI). We included participants who had a C7 injury to ensure that we recruited individuals who had difficulty with reaching and grasping activities. To ensure that they were not denevated at the median nerve, we only included participants who could voluntarily activate their thenar muscles. We excluded individuals who had seizure disorder, epilepsy, head injury, or stroke or who demonstrated reasonably good hand function as indicated by a score equal to or greater than 73/77 on the CAHAI. Through the use of 2 activity level outcomes (JTHF and CAHAI) as our inclusion and exclusion criteria, we captured individuals who were less likely to experience a ceiling effect on our outcomes (particularly on the ordinal measure CAHAI) and were able to perform the intervention.
The clinical testing was performed by a physical therapist using a standardized protocol. The testing physical therapist did not participate in the training sessions but was not restricted from the training area, so blinding may have been incomplete. All clinical tests were videotaped to be reviewed and scored at a later time to verify data. Clinical tests included measures of unimanual and bimanual hand function, key pinch strength, sensation, and a participation questionnaire. Activity limitations were measured using the JTHF and CAHAI.18 The JTHF test is reliable and valid for persons with tetraplegia.19 The time to perform each item was recorded and summed. CAHAI was used to evaluate use of both hands together.18 CAHAI has good reliability in persons post stroke,18 and concurrent validity has been established in persons with tetraplegia.10 Each item was given a score from 1 to 7, with 1 being dependent or unable and 7 being independent. The sum of all items examined was used as the final score.
Key pinch strength was measured with a digital handheld dynamometer (Microfet4; Hoggan Health Industries, West Jordan, UT). The average force produced from 3 trials was recorded. This method of measuring pinch strength has been shown to be reliable in persons with SCI.19 Sensation was evaluated using the Semmes-Weinstein Monofilament Test (SWMT).20 The SWMT has good reliability and is responsive to change.21 Five monofilaments ranging in diameter from 2.83 mm to 6.65 mm were used. Each monofilament correlates to a target force on a logarithmic scale. The region of the hand innervated by the median nerve (tip of the thumb, tip of index, and base of index finger) was evaluated. The 3 scores were summed (maximum score is 15). A Health Assessment Questionnaire (HAQ)22 was completed by each participant. The questionnaire was limited to items related to dressing, grooming, eating, reaching, and gripping. The individual items were summated, where a low score indicates greater participation and a high score indicates less participation (higher disability).
Corticomotor maps of the thenar muscles were constructed by recording motor evoked potentials (MEPs) in response to a stimulus evoked by transcranial magnetic stimulation (TMS).9,10 Two examiners were present during the mapping process and consistently performed the same tasks. The first examiner performed the stimulating procedures and monitored the participant, while the second examiner evaluated the signal and recorded the frame number and the intensity of the stimulus or site of the map. Participants sat in their wheelchairs while wearing a cap marked with 1-cm squares. Silver/silver chloride surface electromyographic (EMG) electrodes were placed over their thenar muscles. Monophasic singlepulse TMS was delivered by a Magstim 200 stimulator (The Magstim Co. Ltd, Wales, UK) using a figure-eight-shaped magnetic coil. The weaker limb was tested unless the participant had no voluntary motor function in the thenar muscles in their weaker hand, in which case the stronger limb was tested. The region overlying the cortical hand area was stimulated and the coil moved until the “hot spot” (greatest amplitude, shortest latency MEP) was identified.
MEPs were evoked while participants maintained a muscle contraction of 10% of maximum voluntary contraction23 during groups of 3 stimuli. The participant’s maximum voluntary contraction of abductor policis brevis was measured using a force transducer (Flexiforce Economical Load and Force System, Tekscan Inc., Boston, MA) with force displayed on a computer screen. During mapping, the stimulus intensity was 120% of active motor threshold (AMT).24 Starting at the hot spot, each site on the cap grid was stimulated 3 times. The coil was moved laterally by 1-cm increments until no MEP response was evoked. The MEP values were normalized to the maximum response of the median nerve to direct stimulation (M-wave).
The EMG data were analyzed using Signal 2 software (Cambridge Electronic Design, Cambridge, UK). The difference between the RMS amplitude at 20 to 40 milliseconds following the stimulus and the RMS amplitude of a 20-millisecond time interval prior to the stimulus was calculated. The RMS amplitude (at each site) was averaged across the 3 MEPs. Corticomotor measures included the corticomotor map area, defined as the number of sites that elicited a MEP,25 and the normalized corticomotor map volume, defined as the sum of the normalized MEPs.25 The test–retest reliability for corticomotor map area in nondisabled individuals is moderate with an intercorrelation coefficient (ICC) of 0.63-0.8626; reliability of this technique in persons with SCI has not been evaluated.
The intervention schedule was 5 days per week, 2 hours per day, for 3 weeks. During each session, participants received the assigned electrical stimulation to his/her weaker limb. For participants in the SS group, silver/silver chloride surface electrodes were placed over the median nerve at the wrist. The SS was delivered using a constant current stimulator (Digitimer model DS7A; Digitimer Ltd, Hertfordshire, UK) according to a previously published protocol7–10 wherein participants received trains of stimulation, set below motor threshold with a long pulse width. Each train comprised 5 single pulses of 1 millisecond duration at 10 Hz. The intensity was set below motor threshold, which elicited no hand movement or visible muscle twitches (approximately 1-2 milliamps) and therefore did not interfere with MP. While the participant performed the MP activities, the trains of stimulation were delivered concurrently.
For participants in the FES group, the electrical stimulation was triggered only when muscle activation exceeded the threshold value. To accomplish this, the stimulating electrodes were placed in the same location as for SS, and recording electrodes were placed on the thenar muscles with a ground electrode over the styloid process. The EMG was amplified and digitized using the analogto-digital converter and visualized in computerized software (Spike; Cambridge Electronic Design, Cambridge, UK). The software triggered the stimulator when the participant activated his/ her thenar muscles. When threshold was reached, trains of electrical stimuli were delivered at 40 pulses per second, 200 microseconds duration, and at 120% of the intensity required to elicit a twitch in the thenar muscles. The stimulation was designed to assist with grasping at the time in the movement when the stimulation would be most useful.
Each training session was divided into 20- to 25-minute increments in each of the activity categories.9,10 Activity categories included finger isolation, pinching, pinch with rotation, grasping, and grasping with rotation tasks. Each of the 5 movement categories had 10 associated activities in which the participant selected 2 tasks from each category during a single training session. The intent was to practice a variety of tasks with the goal that improvements in motor control would be transferrable to other activities performed in daily life. The physical therapist who designed the intervention also performed all intervention procedures but did not perform the clinical testing protocols.
The unimanual MP protocol was modeled after the unimanual training described previously.7,8 When necessary, objects were stabilized by external support to allow tasks to be accomplished unimanually. The participants practiced the manipulative portion of the task with their weaker limb. For example, in the container opening task, jars were glued onto a large board and participants were instructed to remove the lid. The bimanual tasks were as similar as possible to the unimanual tasks, with slight modifications such that the tasks were practiced bimanually. Using the previous example, participants in the bimanual group practiced opening containers using their stronger hand to stabilize the container and their weaker hand to remove the lid.
The intent of the intervention was to provide a challenging environment for the participant and encourage typical movement patterns. If the individual was unable to perform the activity using a typical movement pattern, then assistance was provided by the physical therapist. If the individual performed the activity in a similar manner to a nondisabled individual and rated the task as not challenging, then the task was modified to increase the difficulty.
Statistical analysis was performed using SAS software (SAS Institute, Cary, NC). As data were collected, summary statistics and histograms were generated to ensure equality of groups in terms of voluntary control over intrinsic hand muscles, upper extremity motor score, severity of injury, and duration of injury. A repeated measure analysis of variance (ANOVA) (Time x Group) was used to compare the effects of the interventions (using actual scores), and data were further analyzed according to stimulation type (SS vs FES) and according to MP type (Uni vs Bi). The level of significance was accepted at α ≤ 0.05.
Results
Twenty-four participants were recruited based on information available in the volunteer registry at The Miami Project to Cure Paralysis and demographic information can be examined in Table 1. Nineteen participants completed the study. Three participants did not meet the inclusion criteria (participants 12, 20, and 23 had scores equal to or greater than 73/77 on the CAHAI), and 2 did not complete the study (participants 21 and 24). Four of these individuals were in the control/ delayed intervention group, 2 were in the uni group, and 3 were in the SS group. In the control condition, the severity of injuries was classified as follows: 50% American Spinal Injury Association Impairment Scale (AIS) C, 20% AIS B, 20% AIS D, and 10% AIS A. In the intervention group, the severity was classified as 55% AIS D, 40% AIS C, 10% AIS B, and 0% AIS A. The levels of injury for participants in the control/delayed intervention group were 40% C6, 30% C7, 20% C4, and 10% C5 as compared to the intervention group at 30% C4, 30% C6, 20% C7, 10% C3, and 10% C5. The mean (SD) duration of injury for the control condition was 6.40 (7.60) years, and for the intervention group it was 4.70 (6.38) years. Both groups were 70% men.
Table 1. Demographic information of participants.
| Individual group assignmenta | Age, years | Gender | Severity of injury (AIS) | Neurological level of injury | Duration of injury, years | UE sensory score (light touch) (C5-T1) | UE sensory score (pin prick) (C5-T1) | UE motor score (C5-T1) | Participant identification |
|---|---|---|---|---|---|---|---|---|---|
| Control, Uni, SS | 40-49 | M | C | C4 | 1-4 | 18 | 9 | 23 | 14 |
| Control, Uni, SS | <30 | M | C | C5 | ≥10 | 20 | 12 | 24 | 15 |
| Control, Uni, SS | <30 | M | C | C6 | 1-4 | 18 | 15 | 31 | 16 |
| Control, Uni, SS | <30 | M | A | C7 | 5-9 | 18 | 12 | 44 | 23 |
| Control, Uni, FES | <30 | F | D | C6 | 1-4 | 17 | 16 | 35 | 13 |
| Control, Bi, SS | ≥50 | M | C | C6 | ≥10 | 20 | 12 | 36 | 21 |
| Control, Bi, SS | ≥50 | M | B | C6 | ≥10 | 20 | 20 | 25 | 17 |
| Control, Bi, SS | 30-39 | F | C | C7 | 1-4 | 20 | 19 | 34 | 6 |
| Control, Bi, FES | ≥50 | F | B | C7 | 1-4 | 16 | 11 | 32 | 19 |
| Control, Bi, FES | 40-49 | M | D | C5 | 5-9 | 20 | 17 | 38 | 20 |
| Control, Bi, FES | 40-49 | M | B | C6 | 1-4 | 20 | 11 | 25 | 24 |
| Control, Bi, FES | <30 | M | A | C7 | 1-4 | 15 | 14 | 39 | 5 |
| Control, Bi, FES | ≥50 | M | D | C4 | 1-4 | 8 | 10 | 23 | 9 |
| Intervention, Uni, SS | ≥50 | F | C | C3 | 1-4 | 17 | 7 | 23 | 2 |
| Intervention, Uni, SS | 40-49 | F | D | C3 | 1-4 | 17 | 11 | 37 | 12 |
| Intervention, Uni, SS | <30 | M | D | C5 | 1-4 | 20 | 20 | 36 | 22 |
| Intervention, Uni, FES | <30 | M | D | C7 | 1-4 | 11 | 10 | 33 | 7 |
| Intervention, Uni, FES | <30 | M | B | C7 | 1-4 | 18 | 17 | 36 | 11 |
| Intervention, Uni, FES | 40-49 | M | D | C4 | 5-9 | 11 | 9 | 44 | 18 |
| Intervention, Bi, SS | 40-49 | M | C | C6 | 1-4 | 11 | 6 | 19 | 3 |
| Intervention, Bi, SS | 40-49 | F | C | C4 | ≥10 | 17 | 13 | 26 | 4 |
| Intervention, Bi, SS | ≥50 | M | C | C6 | 1-4 | 15 | 10 | 26 | 8 |
| Intervention, Bi, FES | ≥50 | F | D | C6 | 5-9 | 18 | 19 | 33 | 10 |
| Intervention, Bi, FES | ≥50 | M | D | C4 | 1-4 | 14 | 14 | 25 | 1 |
Note: Nineteen participants completed the study: 3 participants did not meet the inclusion criteria (participants 12, 20, and 23) and 2 did not complete the study (participants 21 and 24). All causes of injury were traumatic. American Spinal Injury Association Impairment Scale = AIS; F = female; M = male; UE = upper extremity.
aControl or intervention group (Control/Intervention), unimanual or bimanual training (Uni/Bi), and somatosensory stimulation or functional electrical stimulation (SS/FES).
We first considered outcomes of all participants in the immediate intervention group together and compared their outcomes to those of the control period of participants in the control/delayed intervention group (Table 2). These groups were similar in their demographic information and their baseline testing scores, except for one measure. At baseline, participants in the control/delayed intervention group performed significantly better on the JTHF than participants in the intervention group (t = 2.37, P = .03), however we will provide evidence that suggests that this did not influence the outcome.
Table 2. Comparison of pre and post-intervention values for participants in the control and intervention, unimanual and bimanual massed practice, and SS and FES groups.
| Control Mean (SD) | Intervention Mean (SD) | Unimanual MP Mean (SD) | Bimanual MP Mean (SD) | SS Mean (SD) | FES Mean (SD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical measure | Pre | Post | Pre | Post | P | Pre | Post | Pre | Post | P | Pre | Post | Pre | Post | P |
| JTHF, seconds |
225.7 (177.9) |
235.2 (174.2) |
482.8 (296.5) |
411.1 (297.4) |
.03 | 291.5 (259.2) |
225.5 (188.7) |
457.3 (276.6) |
412.4 (289.2) |
.57 | 432.4 (254.3) |
365.9 (242.8) |
329.5 (285.8) |
293.4 (271.5) |
.57 |
| CAHAI seconds |
40.7 (13.2) |
39.8 (14.4) |
36.9 (13.8) |
39.0 (14.1) |
.15 | 44.6 (13.9) |
44.6 (17.2) |
33.3 (13.5) |
38.1 (11.9) |
.03 | 34.6 (10.1) |
36.8 (9.5) |
42.3 (17.3) |
45.1 (17.6) |
.68 |
| Pinch, lbs |
4.6 (6.3) |
4.7 (5.8) |
4.4 (4.1) |
4.9 (4.7) |
.44 | 3.8 (4.3) |
4.8 (4.2) |
5.30 (7.0) |
6.1 (8.2) |
.23 | 2.4 (1.9) |
3.1 (1.9) |
6.6 (7.4) |
7.6 (8.4) |
.23 |
| SWMT | 12.5 (3.9) |
13.0 (4.6) |
9.5 (4.1) |
10.0 (4.4) |
.60 | 10.0 (5.6) |
12.0 (5.3) |
8.5 (4.5) |
9.0 (4.2) |
.18 | 7.0 (4.1) |
8.0 (3.8) |
12.5 (3.9) |
14.0 (3.7) |
.18 |
| HAQ score |
27.5 (8.9) |
27.0 (9.2) |
31.2 (9.0) |
30.0 (7.8) |
.46 | 23.6 (10.0) |
21.8 (8.8) |
32.0 (11.8) |
30.3 (11.4) |
.74 | 30.8 (9.9) |
28.6 (9.3) |
28.2 (9.2) |
27.0 (8.7) |
.18 |
Note: CAHAI = Chedoke Arm and Hand Activity Inventory; FES = functional electrical stimulation; HAQ = Health Assessment Questionnaire; JTHF = Jebsen Taylor Hand Function Test; MP = massed practice; SWMT = Semmes-Weinstein Monofilament Test.
For the JTHF, there was a significant Time x Group interaction (F1,18 = 5.31, P = .03) between the control period of the control/delayed intervention group and intervention group. The control/delayed intervention group did not improve after the control period as demonstrated by an increase in time (mean [SD] change = 9.52 [45.55] seconds), whereas the immediate intervention group improved as demonstrated by a decrease in time or negative value (mean [SD] change = -71.63 [100.16] seconds). The effect size was -81.14 seconds. There is 95% confidence that the true mean difference lies between -5.12 and -157.18 seconds. As this confidence interval does not include zero, there is 95% confidence that the intervention group improved more than the control condition.
We next compared outcomes of all participants in the immediate intervention group to outcomes of the intervention period of participants assigned to the control/delayed intervention group. Following the intervention period, participants in the control/delayed intervention group improved by an amount similar to those in the immediate intervention group (mean [SD] = -51.63 [17.08] seconds vs mean [SD] = -71.63 [100.16], respectively). The JTHF change from pre- to postintervention testing in participants in the control/delayed intervention group was significant (t = 3.04, P = .01), indicating improvement following the intervention. Further, the correlation between initial test score and the amount of change in this measure was not significant (r = -0.31, P = .19), indicating that this relationship between the initial score and the amount of change could have happened by chance.
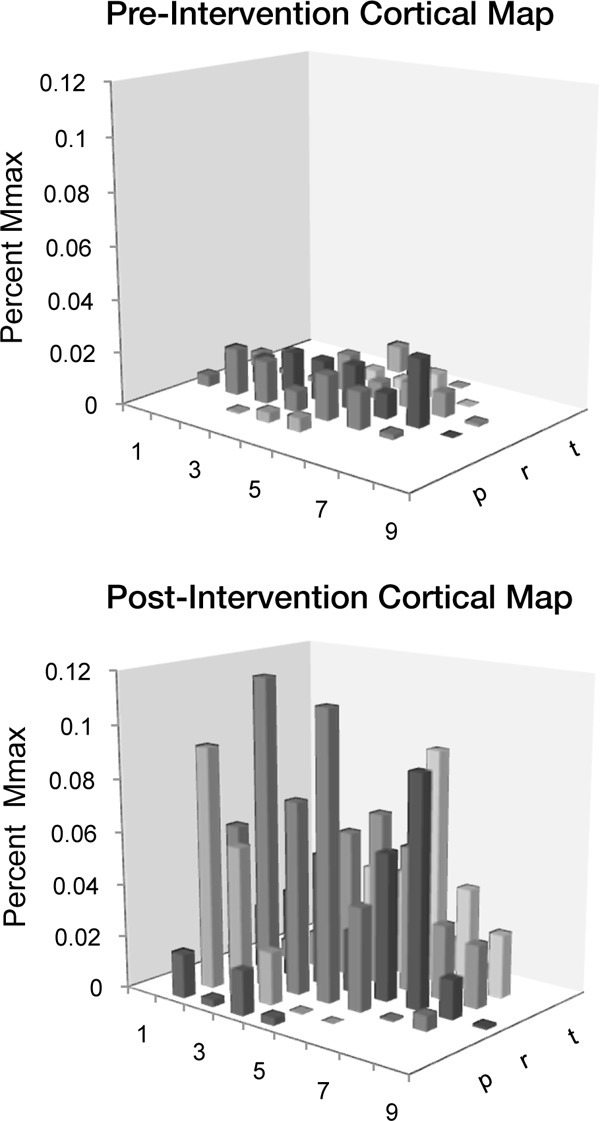
Corticomotor activity data are summarized in Table 3. Of the 19 participants who completed the study, 5 were unable to complete the TMS testing session: 1 did not tolerate the TMS, 3 had excessive stimulation artifact associated with the recording, and 1 participant was excluded from TMS due to possible history of head injury that was revealed after enrollment. An example of a corticomotor map pre and post intervention is illustrated in Figure 2.
Table 3. Mean (SD) values of TMS outcome measures for participants in the control and intervention group.
| Control | Intervention | ||||
|---|---|---|---|---|---|
| TMS measure | Pre | Post | Pre | Post | P |
| Area, cm2 | 14 (15.45) | 13 (11.33) | 12 (6.53) | 21 (11.02) | .03 |
| Volume, cm3 | 1.24 (1.71) | 0.96 (1.30) | 0.68 (0.53) | 2.05 (2.41) | .08 |
Note: TMS = transcranial magnetic stimulation.
Figure 2. Example of cortical map at pre- (top) and postintervention testing (bottom). The numbers and letters are a coordinate system correlating to the grid on the cap. The bars indicate mean amplitude of 3 motor evoked potentials (MEPs).
For corticomotor map area, there was significant Time x Group interaction (F1,13 = 5.69, P = .03) between the control/delayed intervention and immediate intervention groups. The control/ delayed intervention group did not change (mean [SD] = -1.33 [3.93] cm2), whereas the intervention group increased in corticomotor map area (mean [SD] = 6.33 [7.12] cm2). The effect size was 7.67 cm2. There is 95% confidence that the difference between the control condition and intervention condition is between 0.72 and 14.61 cm2.
The participants who were assigned to the different intervention groups were similar in demographic and baseline measures for all comparisons. For the pre- and postintervention scores on the JTHF, there was a significant effect of time for all the groups combined (F3,15 = 9.87, P = .0006), indicating that participants improved compared to baseline, regardless of group assignment.
When grouped by MP type and stimulation type, the mean change in time decreased for both unimanual (mean [SD] = -69.67 [103.77] seconds) and bimanual (mean [SD] = -44.91 [46.93] seconds) groups, and it also decreased for the SS (mean [SD] = -66.15 [98.20] seconds) and FES (mean [SD] = -48.07 [57.90] seconds) groups. There was not a significant difference in the pre- and postintervention change between SS and FES groups (F1,17 = 0.55, P = .46), nor between unimanual and bimanual groups (F1,17 = 0.33, P = .57). All groups improved to a similar extent.
For the pre- and postintervention score on the CAHAI, there was a significant interaction between the type of MP intervention (F1,17 = 5.57, P = .03) (Table 2), indicating that the bimanual group had significantly greater change than the unimanual group. The mean change in score increased for the bimanual group (mean [SD] = 4.70 [3.27]), but did not change for the unimanual group (mean [SD] = -0.05 [5.39]). The effect size for the difference in the change scores between the groups is 4.70. There is 95% confidence that the difference between the change scores is between 0.44 and 8.96. There was no Time x Group interaction between the electrical stimulation protocols on the CAHAI (Table 2). There was no significant difference between groups for pinch strength or sensation.
For corticomotor map area, there was an effect of time for the pre- and postintervention testing sessions indicating that map area increased for all intervention groups (F3,9 = 5.63, P = .04), but there was no significant interaction between groups (F3,9 = 0.79, P = .40), indicating that all groups changed equally. For the comparison between intervention groups, there were not sufficient data to compare groups statistically. The corticomotor map area increased for both the unimanual (mean [SD] = 10.60 [9.13] cm2) and bimanual groups (mean [SD] = 7.00 [3.89] cm2). The corticomotor map area also increased for both the SS group (mean = 2.80 [3.83]) and FES group (mean [SD] = 11.88 [4.88]).
Discussion
People with chronic SCI can improve in hand function following a combined intervention of task-oriented training with electrical stimulation compared to no intervention, as has been demonstrated previously.7,8,10 Participants assigned to the immediate intervention group performed significantly better on the JTHF compared to the control period of the control/delayed intervention group. When the participants in control/delayed intervention group completed the intervention, they performed better on the postintervention test compared to pre intervention, and the amount of change on the JTHF was similar to that of participants in the immediate intervention group.
The control/delayed intervention and immediate intervention groups were similar in their demographics and baseline testing scores, except for one measure. At baseline, control/ delayed intervention participants performed significantly better on the JTHF than participants in the immediate intervention group. This is likely due to an unanticipated influence of spasticity on the performance of some participants. Spasticity was not formally assessed in these participants, yet for some individuals it appeared to significantly impact their movements. This observation was based on videotaped observation of the participants’ performance on the JTHF and the CAHAI. Of the 6 participants with spasticity in their upper limbs, 4 were randomly assigned to the immediate intervention group. The JTHF is a timebased measure and is dependent on performance speed. People with spasticity have difficulty quickly activating and deactivating muscle, and therefore the time to complete items on the JTHF was greater for these individuals. Despite the difference in their baseline scores, these participants demonstrated improved performance following the intervention.
Participants in the SS group received continuous electrical stimulation, whereas those in the FES group received stimulation only when appropriate for the task. It could be argued that the continuous nature of the SS provides afferent input that is not appropriate during all phases of MP. The finding that both forms of stimulation were associated with similar improvements indicates that the continuous nature of the SS does not impede learning compared to the more movement appropriate stimulation associated with EMG-triggered FES. It appears that the timing of the stimulation relative to the movement may not be important for improvements in function. Simply providing electrical stimulation and thereby, presumably, increasing cortical excitability11 is sufficient to induce changes in sensation32 and motor performance.7,8 Evidence suggests that when MP is augmented by SS, both changes in functional and corticomotor-related outcome measures are significantly greater than with either intervention in isolation.7,8
The results from the JTHF are similar to those of other studies from our laboratory,7,8,10 wherein a combination intervention of unimanual MP and SS significantly improved unimanual hand function over a control condition. However, it was not known if the combination intervention would induce changes on a measure of bimanual hand function. On the CAHAI, no difference was found between the immediate intervention group and the control period. It is possible that the changes in the immediate intervention group were diluted by the inclusion of both participants who received unimanual training and those who received bimanual training, as those who received bimanual training improved more on the CAHAI than those receiving unimanual training.
The size of the corticomotor map was significantly greater in the immediate intervention group compared to the control condition (Table 2). Other investigators have found increases in size of the corticomotor map following unimanual training.27,28 Liepert et al27 found an increase in the size of the corticomotor map following 1 session of constraint-induced therapy. The same researchers also investigated the effect of a 2-week intervention and found an increase in corticomotor representation.28 There is less evidence in the literature describing the effects of bimanual training on TMS-evoked corticomotor maps. Lewis and Byblow29 found an increase in corticomotor map area following bimanual training in one participant with hemiparesis due to stroke. However, another participant, following the same intervention, had a decrease in corticomotor map area. Other studies investigating the effects of bimanual training have found trends of increased corticomotor map volume.30
We observed an increase in corticomotor map area; for this reason, it may be surprising that there was no associated increase in corticomotor map volume. However, this is consistent with findings of other investigations.31 Meesen et al31 investigated the influence of sensory stimulation in the form of transcutaneous electrical nerve stimulation in nondisabled participants and found changes in corticomotor map area without changes in corticomotor map volume.31 Alternatively, it is possible that we did not have sufficient power to determine the effect on this outcome measure.
On the bimanual hand function outcome measure (CAHAI), participants who received bimanual training improved to a greater extent than those who received unimanual training (Table 2). This is likely influenced by the task specificity of training. Participants in the unimanual group did not practice with their stronger hand nor did they practice using both the limbs together. All of the items on the CAHAI involve use of the both limbs to accomplish one activity. Alternatively, it is possible that individuals in the unimanual group had less room for improvement, as the preintervention score for individuals in the unimanual group was 44.56 ± 13.94, whereas the preintervention score for individuals in the bimanual group was 34.64 ± 13.60. However, we do not believe that this impacted the interpretation of the findings. The preintervention values for the CAHAI are not statistically different (t = 1.61, P = .13). Further, neither group approached the ceiling of this measure (score = 77), as we excluded individuals who scored 73/77 or higher on the CAHAI. However, differences in baseline scores must be considered.
This study is limited by the small number of subjects and the large number of comparisons, which increases the risk of type II error. There is also a large amount of variability in our data. This stems from both the variability in participants’ performance on clinical tests, as well as the variability in neurophysiology (related to integrity of upper or lower motor neurons) that influences the corticomotor outcomes. Future studies should decrease the variability through narrowing inclusion criteria to include participants who can perform all items on the JTHF. Further, investigators should consider analyzing individuals who have spasticity in their upper extremities separately, as individuals with spasticity perform much differently from individuals without spasticity and this contributes to the variability in outcome.
Conclusion
People with chronic SCI can improve in unimanual hand function with a combined intervention of MP training combined with electrical stimulation, regardless of whether the stimulation consists of continuous somatosensory stimulation or EMG-triggered FES. Persons who practice bimanual MP training seem to have greater improvement in bimanual hand function, whereas those who practice unimanual MP training do not appear to receive the same benefit as an outcome of bimanual performance. Physical therapists working with individuals with chronic SCI should consider interventions that improve hand function and promote neuroplastic changes.
Acnowledgments
The authors declare no conflict of interest.
Statement of ethics: The authors certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
References
- 1.National Spinal Cord Injury Statistical Center Annual report for the model spinal cord injury care systems. 2011. https://www.nscisc.uab.edu/PublicDocuments/nscisc_home/pdf/Facts%202011%20Feb%20Final.pdf Accessed August13, 2013
- 2.Anderson KD. Targeting recovery: Priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383 [DOI] [PubMed] [Google Scholar]
- 3.Snoek GJ, IJzerman MJ, Hermens HJ, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: Impact and priority for improvement in hand function in tetraplegics. Spinal Cord. 2004;42(9):526–532 [DOI] [PubMed] [Google Scholar]
- 4.Brouwer B, Hopkins-Rosseel DH. Motor cortical mapping of proximal upper extremity muscles following spinal cord injury. Spinal Cord. 1997;35(4):205–212 [DOI] [PubMed] [Google Scholar]
- 5.Freund P, Weiskopf N, Ward NS, et al. Disability, atrophy and cortical reorganization following spinal cord injury. Brain. 2011;134(Pt 6):1610–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davey NJ, Smith HC, Savic G, Maskill DW, Ellaway PH, Frankel HL. Comparison of input-output patterns in the corticospinal system of normal subjects and incomplete spinal cord injured patients. Exp Brain Res.1999;127(4):382–390 [DOI] [PubMed] [Google Scholar]
- 7.Beekhuizen KS, Field-Fote EC. Massed practice versus massed practice with stimulation: Effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabil Neural Repair. 2005;19(1):33–45 [DOI] [PubMed] [Google Scholar]
- 8.Beekhuizen KS, Field-Fote EC. Sensory stimulation augments the effects of massed practice training in persons with tetraplegia. Arch Phys Med Rehabil. 2008;89(4):602–608 [DOI] [PubMed] [Google Scholar]
- 9.Hoffman LR, Field-Fote EC. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: A case report. Phys Ther. 2007;87(2):208–223 [DOI] [PubMed] [Google Scholar]
- 10.Hoffman LR, Field-Fote EC. Functional and corticomotor changes in individuals with tetraplegia following unimanual or bimanual massed practice training with somatosensory stimulation: A pilot study. J Neurol Phys Ther. 2010;34(4):193–201 [DOI] [PubMed] [Google Scholar]
- 11.Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res. 2000;131(1):135–143 [DOI] [PubMed] [Google Scholar]
- 12.Panizza M, Nilsson J, Roth BJ, Basser PJ, Hallett M. Relevance of stimulus duration for activation of motor and sensory fibers: Implications for the study of H-reflexes and magnetic stimulation. Electroencephalogr Clin Neurophysiol . 1992;85(1):22–29 [DOI] [PubMed] [Google Scholar]
- 13.Conforto AB, Cohen LG, dos Santos RL, Scaff M, Marie SKN. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. J Neurol. 2007;254(3):333–339 [DOI] [PubMed] [Google Scholar]
- 14.Cohen LG, Ziemann U, Mechanisms Chen R, functional relevance and modulation of plasticity in the human central nervous system. Electroencephalogr Clin Neurophysiol. 1999;51(Suppl):174–182 [PubMed] [Google Scholar]
- 15.Popovic MR, Popovic DB, Keller T. Neuroprostheses for grasping. Neurol Res. 2002;24(5):443–452 [DOI] [PubMed] [Google Scholar]
- 16.Mangold S, Keller T, Curt A, Dietz V. Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord. 2005;43(1):1–13 [DOI] [PubMed] [Google Scholar]
- 17.Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: Retraining grasping in spinal cord injury. Spinal Cord. 2006;44(3):143–151 [DOI] [PubMed] [Google Scholar]
- 18.Barreca SR, Stratford PW, Lambert CL, Masters LM, Streiner DL. Test-retest reliability, validity, and sensitivity of the Chedoke Arm and Hand Activity Inventory: A new measure of upper-limb function for survivors of stroke. Arch Phys Med Rehabil. 2005;86(8):1616–1622 [DOI] [PubMed] [Google Scholar]
- 19.van Tuijl JH, Janssen-Potten YJM, Seelen HAM. Evaluation of upper extremity motor function tests in tetraplegics. Spinal Cord. 2002;40(2):51–64 [DOI] [PubMed] [Google Scholar]
- 20.Halar EM, Hammond MC, LaCava EC, Camann C, Ward J. Sensory perception threshold measurement: An evaluation of semiobjective testing devices. Arch Phys Med Rehabil. 1987;68(8):499–507 [PubMed] [Google Scholar]
- 21.Jerosch-Herold C. Assessment of sensibility after nerve injury and repair: A systematic review of evidence for validity, reliability and responsiveness of tests. J Hand Surg Br. 2005;30(3):252–264 [DOI] [PubMed] [Google Scholar]
- 22.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: The health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9(5):789–793 [PubMed] [Google Scholar]
- 23.Darling WG, Wolf SL, Butler AJ. Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp Brain Res. 2006;174(2):376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossini PM, Altamura C, Ferreri F, et al. Neuroimaging experimental studies on brain plasticity in recovery from stroke. Eura Medicophys. 2007;43(2):241–254 [PubMed] [Google Scholar]
- 25.Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 1992;85(1):1–8 [DOI] [PubMed] [Google Scholar]
- 26.Malcolm MP, Triggs WJ, Light KE, Shechtman O, Khandekar G, Gonzalez Rothi LJ. Reliability of motor cortex transcranial magnetic stimulation in four muscle representations. Clin Neurophysiol. 2006;117(5):1037–1046 [DOI] [PubMed] [Google Scholar]
- 27.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31(6):1210–1216 [DOI] [PubMed] [Google Scholar]
- 28.Liepert J, Miltner WH, Bauder H, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250(1):5–8 [DOI] [PubMed] [Google Scholar]
- 29.Lewis GN, Byblow WD. Neurophysiological and behavioural adaptations to a bilateral training intervention in individuals following stroke. Clin Rehabil. 2004;18(1):48–59 [DOI] [PubMed] [Google Scholar]
- 30.Stinear JW, Byblow WD. Rhythmic bilateral movement training modulates corticomotor excitability and enhances upper limb motricity poststroke: A pilot study. J Clin Neurophysiol. 2004;21(2):124–131 [DOI] [PubMed] [Google Scholar]
- 31.Meesen RLJ, Cuypers K, Rothwell JC, Swinnen SP, Levin O. The effect of long-term TENS on persistent neuroplastic changes in the human cerebral cortex. Hum Brain Mapp. 2011;32(6):872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuypers K, Levin O, Thijs H, Swinnen SP, Meesen RLJ. Long-term TENS treatment improves tactile sensitivity in MS patients. Neurorehabil Neural Repair . 2010;24(5):420–427 [DOI] [PubMed] [Google Scholar]


