Abstract
Background:
Candidates for activity-based therapy after spinal cord injury (SCI) are often selected on the basis of manual muscle test scores and the classification of the injury as complete or incomplete. However, these scores may not adequately predict which individuals have sufficient residual motor resources for the therapy to be beneficial.
Objective:
We performed a preliminary study to see whether dynamometry and quantitative electromyography (EMG) can provide a more detailed assessment of residual motor resources.
Methods:
We measured elbow extension strength using a hand-held dynamometer and recorded fine-wire EMG from the triceps brachii muscles of 4 individuals with C5, C6, or C7 level SCI and 2 able-bodied controls. We used EMG decomposition to measure motor unit action potential (MUAP) amplitudes and motor unit (MU) recruitment and firing-rate profiles during constant and ramp contractions.
Results:
All 4 subjects with cervical SCI (cSCI) had increased MUAP amplitudes indicative of denervation. Two of the subjects with cSCI had very weak elbow extension strength (<4 kg), dramatically reduced recruitment, and excessive firing rates (>40 pps), suggesting profound loss of motoneurons. The other 2 subjects with cSCI had stronger elbow extension (>6 kg), more normal recruitment, and more normal firing rates, suggesting a substantial remaining motoneuron population.
Conclusions:
Dynamometry and quantitative EMG may provide information about the extent of gray matter loss in cSCI to help guide rehabilitation strategies.
Key words: dynamometry, electromyography, motor units, spinal cord injury, triceps brachii
Restoring upper limb function is a high priority for individuals with tetraplegia due to cervical spinal cord injury (cSCI).1 Triceps brachii muscle function is critical to upper limb performance. The inability to produce adequate elbow extension force reduces the available workspace in which it is possible to position and stabilize the hand, hindering the ability to perform activities of daily living. Triceps brachii strength is also helpful for pressure relief activities and transfers into and out of a wheelchair. Weakness of the triceps brachii can also lead to elbow flexion contractures, which limit the ability to use compensatory strategies.2 Unfortunately, it is common for individuals with cSCI to have weak or absent elbow extension strength as a result of their injury.
There is increasing interest in the use of activity-based therapy involving intensive exercise schedules coupled with somatosensory augmentation to improve upper limb function in persons with incomplete tetraplegia.3 This type of exercise has been shown to increase both cortical4–6 and spinal excitability7 and to improve upper limb function.4–7 However, it is not clear that all individuals with incomplete tetraplegia are necessarily good candidates for these types of programs. If the motoneuron pool of a particular muscle remains largely intact, then an activity-based therapy program that targets the re-establishment of neural connections to activate those motoneurons may increase strength and contribute to improved function. On the other hand, if there has been a major loss of lower motoneurons (gray matter), then the remaining ones may not be able to support an intense schedule of exercise. For these individuals, activity-based therapy might result in overuse weakness and fatigue, as it does in post-polio syndrome.8,9
The conventional clinical measures for assessing motor impairment after cSCI may not be adequate for distinguishing the level of gray matter damage. Overall motor impairment is classified according to the American Spinal Injury Association (ASIA) Standard Neurological Classification of SCI (ASIA classification),10 which identifies a single level at and above which motor function is normal and classifies the injury as either complete or incomplete. This classification by itself does not thoroughly characterize the motor resources in the zone of injury. The strength of key muscle groups is assessed by manual muscle testing, which has been shown to be a poor predictor of voluntary strength.11–13 Moreover, although the triceps brachii is associated in manual muscle testing as the key muscle group of the 7th cervical motor nerve (C7), the muscle actually has 3 heads with multiple innervation levels (C6-8), each of which contributes to elbow extension. Thus more precise knowledge about the deficits and capabilities of particular muscles is needed for making choices about rehabilitation strategies.
In this article, we consider 2 more precise modalities for assessing available motor resources: dynamometry and quantitative EMG. Dynamometry presents a quantitative measure of muscle strength during voluntary actions. The intramuscular EMG signal represents the final output of the motoneuron pool and so provides a direct view of neural function at the spinal level.14–16 In particular, EMG signals from muscles affected by SCI can show reduced numbers of active motor units (MUs) and increased motor unit action potential (MUAP) amplitudes, which are signs of motoneuron loss and subsequent collateral reinnervation, and irregular recruitment and firing patterns, which can indicate impairment of the descending or peripheral drive to the motoneuron pool. We studied 4 individuals with C5, C6, or C7 level SCI to explore the use dynamometry and quantitative EMG for characterizing motor resources in their triceps brachii muscles.
Methods
Subjects
Four individuals with chronic cSCI and 2 nonimpaired control subjects participated in this study (Table 1). Neither of the control subjects had any history of neuromuscular disease or muscular trauma. The experimental procedures were approved by the Stanford University Committee on the Use of Human Subjects in Research and conformed to the Declaration of Helsinki. Informed written consent was obtained from all the subjects.
Table 1. Subject characteristics.
| Subject | Age, years | Gender | Time since injury, years | Neurological level | ASIA classification | Triceps MMT grade |
|---|---|---|---|---|---|---|
| S1 | 45-49 | M | 20-24 | C6 | A | 2+ |
| S2 | 35-39 | F | 10-14 | C7 | D | 3– |
| S3 | 50-54 | F | 15-19 | C5 | D | 3+ |
| S4 | 45-49 | M | 15-19 | C6 | A | 4– |
| C1 | 25-29 | M | — | NI | E | 5 |
| C2 | 25-29 | F | — | NI | E | 5 |
Note: Age and time since injury are expressed as a range rather than a specific number to comply with the journal’s privacy policy. ASIA = American Spinal Injury Association; MMT = manual muscle test; NI = non-impaired.
Dynamometry
Elbow extension strength was measured in the cSCI participants utilizing a hand-held dynamometer.17–19 Each participant was seated in his or her wheelchair. Strength was measured in 3 positions that were chosen to test the 3 heads of the triceps brachii and to evaluate performance in functional postures. They included “overhead” extension with the shoulder fully abducted and externally rotated and the elbow extended, “forward” extension with the shoulder abducted 90° in neutral rotation and the elbow extended (in the horizontal plane), and “downward” extension with the shoulder in neutral and the elbow flexed 90°. For each position, a break test was performed by pushing the elbow into flexion. The dynamometer (Lafayette Model 01165, Lafayette, IN) was placed just proximal to the wrist ulnar styloid process. Each position was tested 3 times with 1 minute of rest between each test.
EMG signal collection
Subjects sat comfortably with their arm supported in 90° shoulder abduction, 90° elbow flexion, and neutral forearm rotation. The subjects with cSCI sat in their wheelchairs. A cuff was placed around the subject’s distal forearm and connected to a force transducer (Gould-Statham UT03 with 45-kg load cell). Subjects were asked to produce 20-seconds-long isometric elbow extension contractions at 0.5, 1, and 1.5 kg force and isometric ramp contractions that increased in force from 0 to 3 kg (or maximal effort) over the course of 20 seconds. These tasks allowed us to examine MU firing behavior over the full range of voluntary effort for the participants with SCI and to make comparisons in terms of either absolute or normalized force. Subjects were provided with visual feedback of contractile force and auditory feedback of the EMG signal. Two of the subjects with cSCI were not able to perform the 1.5 kg contraction, but otherwise all the subjects performed all the tasks with reasonable accuracy.
For the subjects with cSCI, fine-wire electrodes were inserted into each of the 3 heads (lateral, medial, and long) of the triceps brachii muscle. For the control subjects, electrodes were inserted at 3 different locations 10 mm apart in the lateral head. Each insertion consisted of a pair of 50-µm-diameter stainless steel wires, insulated except for a 1-mm exposed recording surface at the tip (Jari Electrode Supply, Gilroy, CA). If the EMG activity from a particular electrode was not sufficiently crisp, a second electrode was inserted nearby. The signals from each wire were recorded in monopolar fashion with respect to a surface electrode close to the insertion site. The ground electrode was placed over the lateral epicondyle. The signals were amplified (Nicolet Viking, Madison, WI) with filter settings of 5 Hz and 5 kHz, sampled at 10 kHz, and stored on a computer for further analysis.
EMG signal analysis
The EMG signals were decomposed into trains of MUAPs by an experienced investigator using the computer-aided EMG lab decomposition program.20 This involved forming templates of each MUAP spike and then classifying each discharge in the signal as an occurrence of one of the templates or a superposition of multiple templates. The completeness of the decomposition was judged by the absence of residual activity. The MUAP waveforms were averaged from the signal using the identified discharge times as triggers. The peakto-peak MUAP amplitude and mean firing rate of each MU during the constant force contractions were calculated. For each ramp contraction, the instantaneous firing rates, recruitment profile, and total activity were calculated. The instantaneous firing rate was calculated for each MU by interpolating the reciprocals of the interdischarge intervals using a zero-order hold and then lowpass filtering at 2 Hz. The recruitment profile was calculated by counting the total number of active MUs from all recording sites at each instant in time. The total activity is a measure of the total neural drive to the muscle.21 It was calculated by summing the instantaneous firing rates of all the active MUs. For the constant force contractions of the control subjects, the recruitment level at each recording site was calculated by counting the number of active MUs with spike amplitudes greater than 100 µV.
Results
Dynamometry
The triceps brachii strength measurements differed considerably between the subjects with cSCI (Table 2). The measurements were in the same rank order as the manual muscle test grades, although there was a large difference in strength between subjects S2 and S3, who had manual muscle test grades of 3- and 3+, respectively. The strength measurements also differed by as much as 3 kg when the same participant was tested in different postures. This difference was particularly noticeable for the weakest subject (S1), who was able to produce substantially more force in the downward posture than in the others.
Table 2. Dynamometry results.
| Subject | Triceps MMT grade | Overhead, kg | Downward, kg | Forward, kg |
|---|---|---|---|---|
| S1 | 2+ | 0.7 | 3.7 | 1.1 |
| S2 | 3− | 1.2 | 2.2 | 2.3 |
| S3 | 3+ | 6.4 | 8.7 | 9.5 |
| S4 | 4− | 15.1 | 14.8 | 17.7 |
Note: MMT = manual muscle test.
EMG
All 4 of the subjects with cSCI had EMG findings that differed markedly from those of the control subjects. In general, the EMG findings in the 2 weaker subjects (S1 and S2) also differed from those in the 2 stronger subjects (S3 and S4).
All 4 of the subjects with cSCI had MUAPs with amplitudes higher than the highest value seen in the control subjects (2.5 mV) (Figure 1A, dotted line). The largest MUAPs of each subject with cSCI ranged from 5.5 to 23 mV. Subject S3 had large MUAPs in the lateral head but normally sized MUAPs in the long head, whereas subject S4 had the opposite – normal MUAPs in the lateral head and large ones in the long head. Subject S2 had large MUAPs in both heads, and subject S1 had no detectable MUAPs in the long head. The large MUAPs in the subjects with cSCI also tended to exhibit a considerable degree of shape instability from discharge to discharge (Figure 1A, inset).
Figure 1. Motor unit action potential (MUAP) amplitudes (A) and firing rates (B) during 1 kg constant force contractions of all 6 subjects. The dotted lines show the range of values of the control subjects. The filled circles indicate motor units (MUs) from the lateral and medial heads, and the open circles indicate MUs from the long head. The inset in (A) shows 10 occurrences each of a large MUAP from subject S2 and a normal MUAP from subject C1 to illustrate the difference in amplitude and shape variability.
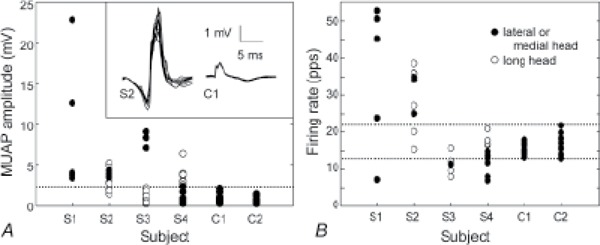
All 4 of the subjects with cSCI also had firing rates that fell outside the range observed in the control subjects (13 to 22 pps during the 1 kg constant force contractions) (Figure 1B, dotted lines). In the 2 weaker subjects (S1 and S2), most of the MUs fired faster than 22 pps, with rates ranging up to 40 pps for S2 and up to 54 pps for S1. In the 2 stronger subjects (S3 and S4), many of the MUs fired slower than 13 pps, with rates ranging down to 6 pps. Subject S1 also had one MU that fired at a very low rate (7 pps).
The recruitment and firing-rate behavior during the ramp contractions are shown in Figures 2, 3, and 4 for one of the control subjects, one of the stronger subjects with cSCI, and one of the weaker subjects with cSCI, respectively.
Figure 2. Ramp contraction of control subject C2. Top panel: Recruitment profile, showing the number of active motor units (MUs; across all recording sites) at each instant during the contraction. Middle panel: Instantaneous firing rates of each MU. Bottom panel: Contractile force (solid) and total activation (dotted). The total activation is the sum of the instantaneous firing rates of all the MUs that are active at each instant in time and thus represents the total neural drive to the muscle. The total activation has been scaled to match the force profile.
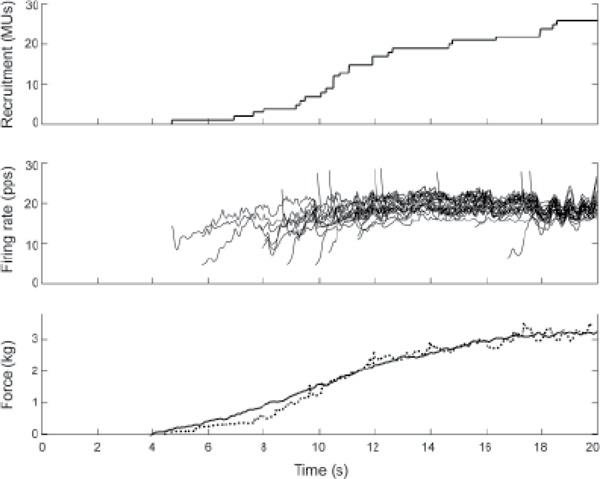
Figure 3. Ramp contraction of subject S3. The different traces are as described in Figure 2. The downward steps in the recruitment profile (top panel) indicate instances in which motor units (MUs) are temporarily de-recruited.
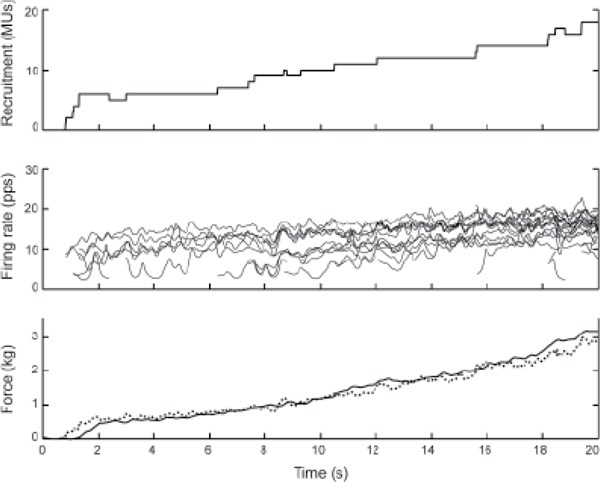
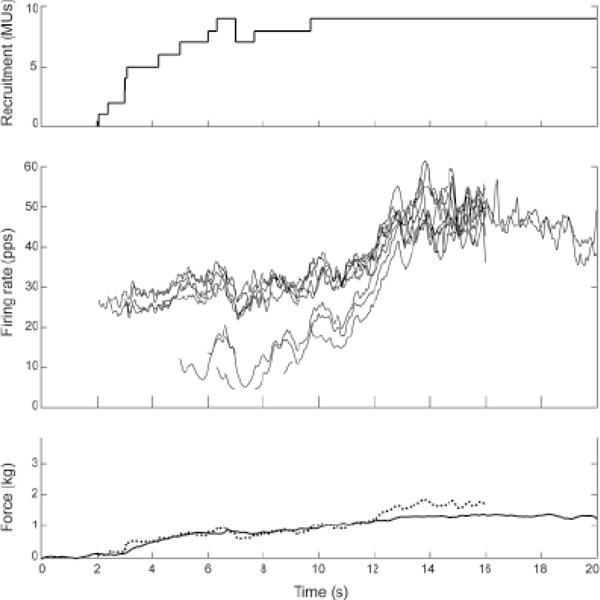
Figure 4. Ramp contraction of subject S2. The different traces are as described in Figure 2. The recruitment and firing rate profiles include all the motor units (MUs) detected in all 3 heads. Because of the high firing rates and motor unit action potential (MUAP) variability, it was only possible to decompose the full firing patterns of 2 MUs beyond 16 seconds, and so it was not possible to calculate the total activity after 16 seconds. The total activity has been scaled to match the force profile over the interval from 0 to 12 seconds. After that, the increase in total activity did not match the force.
Control subject C2 recruited MUs continuously throughout the ramp contraction, with the recruitment profile roughly matching the force profile (Figure 2). Most of the MUs began firing at a low rate but increased their firing rate over the first several discharges until they reached a rate between about 18 and 22 pps. They then stayed at this rate for the rest of the contraction. Several MUs began firing at a higher rate because of an initial doublet. The total activity, which is an estimate of the total neural drive to the muscle (Figure 2, bottom graph, dotted line), increased continuously throughout the contraction with roughly the same profile as the force. The other control subject (C1) exhibited very similar recruitment and firing behavior.
Subject S3, one of the stronger subjects with cSCI, also recruited MUs continuously throughout the contraction (Figure 3). Unlike the control subject, however, the firing rates of this subject increased somewhat throughout the contraction. Several of the MUs fired unstably at rates around 5 pps for several seconds before increasing to a more stable firing rate. As in the case of the control subject, the total activity (Figure 3, bottom graph, dotted line) increased throughout the contraction with approximately the same profile as the force. The other stronger subject with cSCI (S4) exhibited similar behavior.
Subject S2, one of the weaker subjects with cSCI, was only able to achieve a maximum force of 1.3 kg (Figure 4). This subject recruited MUs continuously over the first 6 seconds, with a recruitment profile that roughly matched the force profile, achieving a force of 0.8 kg. Then between 10 and 12 seconds, the force of contraction increased by an additional 0.5 kg due to increasing firing rates without any additional detectable recruitment. The total activity (Figure 4, bottom graph, dotted line) matched the force profile over the interval from 0 to 12 seconds. After 12 seconds, the firing rates and total activity increased further, but there was no appreciable increase in force. The other weaker subject with cSCI (S1) exhibited similar behavior.
To assess how well the EMG measurements obtained from a single recording site were representative of the state of the entire muscle, we also compared the recruitment levels and mean firing rates measured at the different recording sites during the constant force contractions of the control subjects (Figure 5). The precise values of these quantities differed between recording sites, but the overall trend was the same at each recording site. For example, the recruitment levels during the 0.5 kg contractions ranged from 1 to 6 across all the recording sites of both subjects, but at each site the number of active MUs increased in the 1 kg contraction and then again in the 1.5 kg contraction.
Figure 5. Recruitment (A) and mean firing rate (B) during the constant force contractions of the control subjects. Each plot shows the results from one of the 3 recording sites in subject C1 (filled circles) or C2 (open circles). MUs = motor units.
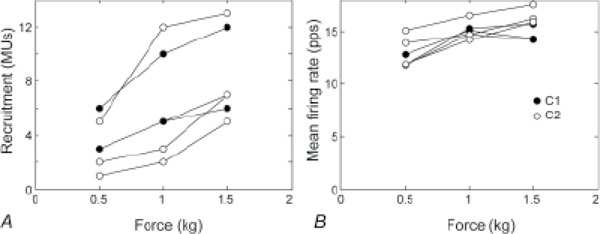
We also performed a second set of recordings in subject S1 in which we recorded from 2 sites in the lateral head separated by 1 cm. The muscle was very atrophied, and this represented the farthest apart that we could insert 2 fine-wire electrodes within the palpable belly. In this experiment, both electrodes recorded exactly the same 2 MUs and nothing more. So at least in this subject, the 2 recording sites 1 cm apart gave comparable results.
Discussion
EMG assessment of motor resources
In this study, we used intramuscular EMG to evaluate motor resources in muscles weakened by SCI. Specifically, we used EMG decomposition to look for signs of denervation and reinnervation and to assess the remaining capacity of the motoneuron pool to modulate force production during voluntary contraction. This was a preliminary study involving only a very limited number of subjects, but it nevertheless illustrates the potential of quantitative EMG analysis.
MUAP amplitude
The amplitudes of the MUAPs in the EMG signal provide an indication of whether denervation has taken place. Damage to the spinal cord in cSCI often results in the death of motoneurons, sometimes across a region that may extend for several spinal segments.22 The axons of surviving motoneurons sprout and reinnervate muscle fibers that have lost their innervation. This results in MUs with an increased number and density of muscle fibers and consequently an increased MUAP amplitude.23–26 We found MUAPs with large amplitudes in all 4 of the cSCI subjects, indicating that they had all suffered partial denervation and reinnervation. In 2 of the subjects, increased MUAP amplitudes were found only in some heads of the triceps, suggesting that the loss of gray matter had been confined to the motoneuron pools innervating those heads (Figure 1A).
The waveforms of many of the large MUAPs from the subjects with cSCI also exhibited a large variability in their shape from discharge to discharge (Figure 1A, inset). This variability is usually considered a sign of unstable transmission in immature nerve twigs or endplates during the acute stages of reinnervation.27 The observation of increased waveform variability in all 4 of the subjects with cSCI suggests that remodeling processes may still be ongoing in chronic cSCI.23,26
Recruitment and firing rates
The recruitment and firing-rate profiles extracted from the EMG signal directly reflect the capacity and limitation of the motoneuron pool. The nervous system controls the force of a muscular contraction by modulating both the number of active MUs (recruitment) and the MU firing rates. During the ramp contractions, the control subjects and the 2 stronger subjects with cSCI modulated force primarily by recruitment (Figures 2 and 3). They recruited more and more MUs continuously throughout the contraction in direct proportion to the desired force without substantially modulating firing rate.
One distinguishing feature of the firing behavior of the 2 stronger subjects with cSCI was that the firing rates of many of their MUs fell below the range observed in the 2 control subjects (Figure 1B). Other investigators have reported lower firing rates during low force contractions in normal triceps than the ones observed in our 2 control subjects (10.2 ± 1.4 pps at 10% MVC28; 9.9 ± 1.4 pps at less than 5% MVC29). Nevertheless, several of the MUs in Figure 3 fired at rates around 5 pps for several seconds after they were first recruited. Such very low firing rates are uncommon in able-bodied individuals. This suggests a deficit in the neural drive to these motoneurons that is insufficient to bring them rapidly up to a more stable firing rate.
The 2 weaker subjects with cSCI (S1 and S2) exhibited considerably different firing behavior (Figure 4). For these subjects, the 3 kg target of the ramp contractions exceeded their maximum strength. They used recruitment to increase force at first, but they ran out of MUs before reaching the target force level. After that they were able to achieve a small further increase in force by increasing firing rates.
A notable feature of the firing behavior in both subject S1 and S2 is that many of the firing rates were extremely high. These 2 subjects typically exhibited sustained firing rates between 30 and 50 pps, which is considerably above the maximum normal firing rate in triceps reported by Del Valle and Thomas30 (24.7 ± 7.1 pps). Moreover, the increases in firing rate above about 40 pps were not accompanied by increases in force (Figure 4). This suggests that 40 pps was the tetanic twitch fusion rate of the muscle fibers, above which further increases in firing rate were not effective in increasing force.
EMG assessment of motor resources
The EMG results thus suggest important differences in the motor resources between the subjects. Although all subjects have sustained some loss of motoneurons, a substantial population of motoneurons still survives in subjects S3 and S4. These motoneurons are under voluntary control, although there has been some damage to descending tracks that affects the ability to activate them effectively. These subjects can be presumed to have adequate motor resources to produce elbow extension contractions of sufficient strength to be able to accomplish activities of daily living and to benefit from rehabilitation exercise intervention.
On the other hand, subjects S1 and S2 have an adequate descending drive but lack a sufficient number of surviving motoneurons to be able to produce adequate force. When these subjects attempt to make strong contractions, they end up driving their MUs far above their normal firing rates and, in fact, beyond the rate that is even effective for increasing force. These high rates can be expected to produce metabolic fatigue at the neuromuscular junctions and in the muscle fibers. For these subjects, elbow extension activities of daily living may require their maximal voluntary effort and be highly fatiguing. Moreover, aggressive exercise intervention may overtax their limited resources
Inadequacy of conventional clinical assessments
Whether an injury is complete or incomplete is an important consideration in determining whether to choose a restorative versus a compensatory rehabilitation approach. The patient’s prognosis and plan of care is guided by this classification. Current practice uses evidence of sacral sparing in combination with sensory testing and manual muscle test scores to assign complete or incomplete status. Our findings raise several concerns with this method of classification. The examples in this article suggest that neither the grade determined by manual muscle testing nor the ASIA classification as complete or incomplete provide a sufficient assessment of motor resources to reliably guide rehabilitation planning.
Although the rank ordering of the subjects by manual muscle test grade agreed with the dynamometry results, measuring strength in 3 different postures provided additional information about the strength differences in the 3 separate heads of the triceps. The quantification of strength using the dynamometer was also useful for comparing the subjects with cSCI with nonimpaired subjects. In combination with the EMG results, strength differences between subjects could be better understood. For example, both subjects S2 and S3 had muscle grades of 3 (active movement against gravity), even though S2 exhibited EMG signs of profound gray matter loss and was only able to produce about a fourth as much force.
The ASIA classification of SCI has long been the basis for determining prognosis for recovery of motor function and developing a realistic plan for conventional rehabilitation. However, the recent interest in activity-based therapy represents a paradigm shift in treating individuals with cSCI.31 The aim is to restore neural connections that may expand functional cortical representations and improve neural activation by intense practice,32 exploiting intrinsic neural networks (locomotor training),31 or augmenting practice with peripheral nerve stimulation.4,5 These approaches can only be expected to improve functional performance if the motor resources and the force-producing capacity of muscles are intact. Thus the selection of candidates for these approaches will require a more extensive evaluation of available motor resources than is possible using the ASIA classification protocol.
In this article, the use of quantitative EMG provided information about available motor resources in the zone of partial preservation that was not provided by the ASIA classification. In particular, the EMG analysis was able to distinguish between the more severely limited motor resources of subject S2 and the better preserved resources of subject S3, both of whom were classified as ASIA D (incomplete). It was also able to distinguish between the profound loss of motor resources in subject S1 and the well-preserved resources of subject S4, both of whom were classified as ASIA A (complete).
Thus our results suggest that EMG analysis of MU recruitment and firing-rate modulation may provide an indication of whether an individual has sufficient motor resources to benefit from activity-based therapy and may provide a useful adjunct to existing electrophysiological tests that are used to characterize connectivity in SCI.33 It should be mentioned that sensory status is also an important aspect of functional assessment after cSCI that was not specifically addressed in this study.
Does intramuscular EMG adequately sample the entire muscle?
Needle and fine-wire electrodes sample only a small cross-section of the muscle, and it is difficult to know whether the EMG signal adequately represents the motor resources throughout the entire muscle. We addressed this issue by recording simultaneously from 3 different sites in the muscles of the control subjects (Figure 5) and from 2 sites in one subject with cSCI. Our results showed that each of the recording sites provided sufficient information to estimate the approximate shapes of the recruitment and firing-rate profiles during the ramp contractions and thus to characterize the available motor resources.
Long-term follow-up
The individuals in this study had chronic injuries and were not expected to have additional spontaneous recovery.34,35 However, our preliminary findings show evidence of ongoing reinervation indicative of remodeling and continuing adaptive changes to the C7 motorneuron pool. This demonstrates instability in the nervous system long after the initial injury. A number of studies have reported some success with restoring strength to the triceps brachii in incomplete chronic cSCI by augmenting the sensory input with vibration36 and EMG biofeedback.37 Variable results and heterogeneous subject populations make it difficult to predict who will benefit from these interventions. For individuals with tetraplegia, a critical assessment of the segmental damage and motor impairment due to the injury may help to identify those individuals who will benefit from restorative therapies. Residual motor resources that may be present in persons with incomplete or complete ASIA classifications should be evaluated.
Clinical implications
Better assessment of available motor resources can help us tailor therapeutic interventions for individual patients. Our findings, although based on a limited number of subjects, raise the possibility that this assessment can be obtained from an EMG examination. Intramuscular, rather than surface, EMG recordings will be required to obtain specific information about MU firing behavior. Individuals with few remaining MUs may have only a limited capacity for increasing strength. For these individuals, interventions such as mass practice and functional electrical stimulation that drive already overworked MUs even harder may not only be ineffective, but, to the extent that they exacerbate fatigue, may even be detrimental. For these individuals, submaximal exercises with longer holding periods that attempt to build fatigue resistance may be more appropriate.
Acknowledgments
Supported by the Department of Veterans Affairs, Rehabilitation Research and Development Projects B73656P and B6857W.
The authors declare no conflict of interest.
References
- 1.Anderson KD. Targeting recovery: Priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383 [DOI] [PubMed] [Google Scholar]
- 2.Bryden AM, Kilgore KL, Lind BB, Yu DT. Triceps denervation as a predictor of elbow flexion contractures in C5 and C6 tetraplegia. Arch Phys Med Rehabil. 2004;85(11):1880–1885 [DOI] [PubMed] [Google Scholar]
- 3.Backus D. Exploring the potential for neural recovery after incomplete tetraplegia through nonsurgical interventions. Phys Med Rehabil. 2010;2(12):S279–S285 [DOI] [PubMed] [Google Scholar]
- 4.Beekhuizen KS, Field-Fote EC. Massed practice versus massed practice with stimulation: Effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabil Neural Repair. 2005;19(1):33–45 [DOI] [PubMed] [Google Scholar]
- 5.Beekhuizen KS, Field-Fote EC. Sensory stimulation augments the effects of massed-practice training in persons with tetraplegia. Arch Phys Med Rehabil. 2008;89(4):602–608 [DOI] [PubMed] [Google Scholar]
- 6.Hoffman LR, Field-Fote EC. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: A case report. Phys Ther. 2007;87(2):208–223 [DOI] [PubMed] [Google Scholar]
- 7.Beekhuizen KS, Field-Fote EC. Massed practice and somatosensory stimulation improves arm/hand function in individuals with SCI. Soc Neurosci Abstr. 2004;231:8 [Google Scholar]
- 8.Agre JC. The role of exercise in the patient with post-polio syndrome. Ann N Y Acad Sci. 1995;753:321–334 [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez H, Olsson T, Borg K. Management of postpolio syndrome. Lancet Neurol. 2010;9:634–642 [DOI] [PubMed] [Google Scholar]
- 10.Maynard FM, Bracken MB, Creasey G, et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 1997;35(5):266–274 [DOI] [PubMed] [Google Scholar]
- 11.Jaweed MM, Herbison GJ, Ditunno JF. Wrist extensor muscle strength measurement by force transducer in normal and quadriplegic subjects. ASIA Abstr Digest. 1987;13:135–136 [Google Scholar]
- 12.Needham-Shropshire BM, Klose KJ, Tucker ME, Thomas CK. Manual muscle test score and force comparisons after cervical spinal cord injury. J Spinal Cord Med. 1997;20(3):324–330 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz S, Cohen ME, Herbison GJ, Shah A. Relationship between two measures of upper extremity strength: Manual muscle test compared to hand-held myometry. Arch Phys Med Rehabil. 1992;73(11):1063–1068 [PubMed] [Google Scholar]
- 14.Stein RB, Brucker BS, Ayyar DR. Motor units in incomplete spinal cord injury: Electrical activity, contractile properties and the effects of biofeedback. J Neurol Neurosurg Psychiatry. 1990;53(10):880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas CK, Broton JG, Calancie B. Motor unit forces and recruitment patterns after cervical spinal cord injury. Muscle Nerve. 1997;20(2):212–220 [DOI] [PubMed] [Google Scholar]
- 16.Zijdewind I, Thomas CK. Motor unit firing during and after voluntary contractions of human thenar muscles weakened by spinal cord injury. J Neurophysiol. 2003;89(4):2065–2071 [DOI] [PubMed] [Google Scholar]
- 17.Burns SP, Breuninger A, Kaplan C, Marin H. Handheld dynamometry in persons with tetraplegia. Am J Phys Med Rehabil. 2005;84:22–29 [DOI] [PubMed] [Google Scholar]
- 18.Drolet M, Noreau L, Vachon J, Moffet H. Muscle strength changes as measured by dynamometry following functional rehabilitation in individuals with spinal cord injury. Arch Phys Med Rehabil. 1999;80(7):791–800 [DOI] [PubMed] [Google Scholar]
- 19.Noreau L, Vachon J. Comparison of three methods to assess muscular strength in individuals with spinal cord injury. Spinal Cord. 1998;36:716–723 [DOI] [PubMed] [Google Scholar]
- 20.McGill KC, Lateva ZC, Marateb HR. EMGLAB: An interactive EMG decomposition program. J Neurosci Methods. 2005;149(2):121–133 [DOI] [PubMed] [Google Scholar]
- 21.Farina D, Negro F, Gazzoni M, Enoka RM. Detecting the unique representation of motor-unit action potentials in the surface electromyogram. J Neurophysiol. 2008;100(3):1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaefer DM, Flanders A, Northrup BE, Doan HT, Osterholm JL. Magnetic resonance imaging of acute cervical spine trauma. Correlation with severity of neurologic injury. Spine (Phila Pa 1976). 1989;14(10):1090–1095 [DOI] [PubMed] [Google Scholar]
- 23.Marino RJ, Herbison GJ, Ditunno JF., Jr.Peripheral sprouting as a mechanism for recovery in the zone of injury in acute quadriplegia: A single-fiber EMG study. Muscle Nerve. 1994;17(12):1466–1468 [DOI] [PubMed] [Google Scholar]
- 24.McComas AJ, Sica RE, Campbell MJ, Upton AR. Functional compensation in partially denervated muscles. J Neurol Neurosurg Psychiatry. 1971;34(4):453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stålberg EV, Trontelj JV, Sanders DB. Single Fiber EMG. 3rd ed. Fiskebäckskil, Sweden: Edshagen; 2010 [Google Scholar]
- 26.Yang JF, Stein RB, Jhamandas J, Gordon T. Motor unit numbers and contractile properties after spinal cord injury. Ann Neurol. 1990;28(4):496–502 [DOI] [PubMed] [Google Scholar]
- 27.Chang CW. Evident transsynaptic degeneration of motor neurons after spinal cord injury: A study of neuromuscular jitter by axonal microstimulation. Am J Phys Med Rehabil. 1998;77(2):118–121 [PubMed] [Google Scholar]
- 28.Dorfman LJ, Howard JE, McGill KC. Influence of contractile force on properties of motor unit action potentials: ADEMG analysis. J Neurol Sci. 1988;86(2-3):125–136 [DOI] [PubMed] [Google Scholar]
- 29.Wiegner AW, Wierzbicka MM, Davies L, Young RR. Discharge properties of single motor units in patients with spinal cord injuries. Muscle Nerve. 1993;16(6):661–671 [DOI] [PubMed] [Google Scholar]
- 30.Del Valle A, Thomas CK. Firing rates of motor units during strong dynamic contractions. Muscle Nerve. 2005;32(3):316–325 [DOI] [PubMed] [Google Scholar]
- 31.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: An emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86(10):1406–1425 [DOI] [PubMed] [Google Scholar]
- 32.Field-Fote EC. Spinal Cord Injury Rehabilitation. Philadelphia: F.A. Davis; 2009 [Google Scholar]
- 33.Tansey KE, McKay WB, Kakulas BA. Restorative neurology: Consideration of the new anatomy and physiology of the injured nervous system. Clin Neurol Neurosurg. 2012;114(5):436–440 [DOI] [PubMed] [Google Scholar]
- 34.Ditunno JF, Stover SL, Freed MM, Ahn JH. Motor recovery of the upper extremities in traumatic quadriplegia: A multicenter study. Arch Phys Med Rehabil. 1992;73(5):431–436 [PubMed] [Google Scholar]
- 35.Drolet M, Noreau L, Vachon J, Moffet H. Muscle strength changes as measured by dynamometry following functional rehabilitation in individuals with spinal cord injury. Arch Phys Med Rehabil. 1999;80(7):791–800 [DOI] [PubMed] [Google Scholar]
- 36.Ribot-Ciscar E, Butler JE, Thomas CK. Facilitation of triceps brachii muscle contraction by tendon vibration after chronic cervical spinal cord injury. J Appl Physiol. 2003;94(6):2358–2357 [DOI] [PubMed] [Google Scholar]
- 37.Brucker BS, Bulaeva NV. Biofeedback effect on electromyography responses in patients with spinal cord injury. Arch Phys Med Rehabil. 1996;77(2):133–137 [DOI] [PubMed] [Google Scholar]


