Abstract
Membrane-bound voltage-gated Ca2+ channels (VGCCs) are targets for specific signaling complexes, which regulate important processes like gene expression, neurotransmitter release and neuronal excitability. It is becoming increasingly evident that the so called “resistant” (R-type) VGCC Cav2.3 is critical in several physiologic and pathophysiologic processes in the central nervous system, vascular system and in endocrine systems. However its eponymous attribute of pharmacologic inertness initially made in depth investigation of the channel difficult. Although the identification of SNX-482 as a fairly specific inhibitor of Cav2.3 in the nanomolar range has enabled insights into the channels properties, availability of other pharmacologic modulators of Cav2.3 with different chemical, physical and biological properties are of great importance for future investigations. Therefore the literature was screened systematically for molecules that modulate Cav2.3 VGCCs.
Keywords: drug sensitivity, anticonvulsive drugs, experimentally induced epilepsy
1. The Cav2.3 Voltage-Gated Ca2+ Channel
Cav2.3 belongs to the family of voltage-gated Ca2+ channels which comprises ten different genes for ion conducting pore proteins (Figure 1). The ion conducting pore protein of the Cav2.3 VGCCs was initially cloned from a rabbit brain cDNA library [1]. After functional expression of the rat Cav2.3 clone, it was initially speculated that it may represent the low voltage-activated T-type Ca2+ channel, which was not yet structurally identified at that time [2]. However, consecutive cloning and expression of human Cav2.3 splice variants in X. laevis oocytes or HEK-293 cells revealed a VGCC with properties closer resembling a high-voltage-gated Ca2+ channel [3,4].
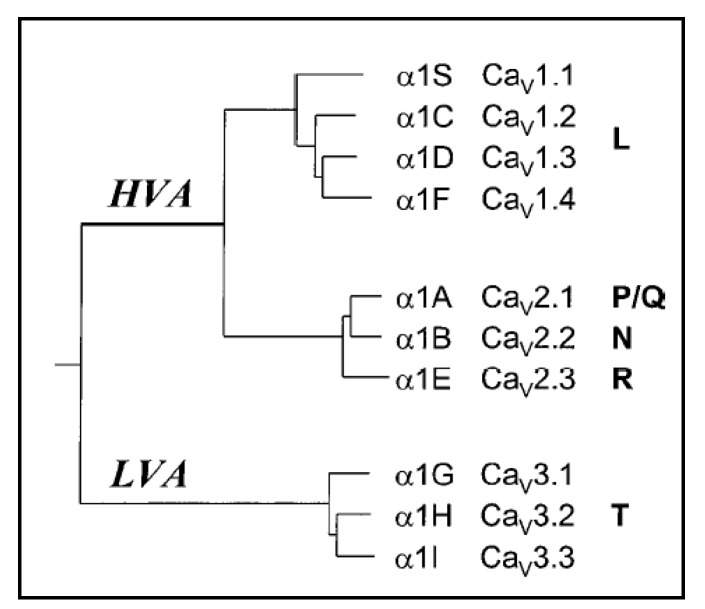
Figure 1.
Evolutionary tree of voltage-gated Ca2+ channels (modified according to [5]). The cDNA of the putative membrane-spanning regions including the pore loops of the human sequences were aligned.
Although the structure of Cav2.3 deduced from sequencing of cDNA has now been known for several years [6,7], its physiological and pathophysiological roles are far from fully understood [8,9,10]. Evolutionarily, Cav2.3 may have developed very early [5,11], which may underline its great significance in vivo. The total quaternary structure of a Cav2.3-containing native VGCC is still unknown, but may contain additional subunits including the well known auxiliary β-subunits, which have been shown to modulate Cav2.3-mediated inward currents in heterologous expression systems [12,13]. Molecular properties of Cav2.3 have been characterized on the amino acid level for functional protein-protein interaction [14,15,16] however to date, Cav2.3 VGCCs have yet to be purified as has been done for L-type Ca2+ channels from rabbit skeletal muscle [17,18,19,20], and bovine heart [21] and for the neuronal N-type Ca2+ channels [22,23].
Sequence comparison of the deduced primary sequence revealed the well known intramolecular homology pattern, which is known for all voltage-gated Ca2+ as well as for voltage-gated Na+ channels. It contains four internal repeats, which have been termed domains I, II, III, and IV. Secondary structure analysis predicts 6 transmembrane segments including a random coiled short part between transmembrane segment 5 and 6, the pore forming segment (P-loop) [24]. Many of these structure predictions resemble the confirmed structural elements in the bacterial and rat voltage-gated K+-channel [25,26] and a bacterial Na+-channel [27,28].
Additional elements may contribute to the kinetic properties of Cav2.3-mediated inward currents as reported for structurally similar ion channels. The segments S6 participate in gating the ion channels [29,30,31,32], and the P-loops form essential parts of the selectivity filters, thereby also influencing the speed of the ion flux through the pore [33,34,35,36,37,38,39,40]. The segment S4 acts mainly as the voltage sensor [41,42], and its detailed orientation to the pore region has been elucidated in crystals from the bacterial K+ channel to a great extent [43].
Only segments of the cytosolic loops from Cav1.2 L-type VGCCs have been co-crystallized with functionally auxiliary subunits [44] or functionally interacting calmodulin [45,46,47,48]. Few protein interactions of Cav2.3 have been reported such as with a β-subunit [15,16] or with novel partners in heterologous expression systems [49,50,51,52], however, they have yet to be investigated by crystallization. The β-subunit interaction site with Cav1.1 and Cav1.2 is located in a conserved region between domain I and II [53,54], which also contains the interaction site of Cav2.3 with β-subunits [14,15,16].
The II-III linker harbors a unique site located within the arginine-rich stretch, which is responsible for Ca2+-mediated modulation of the Cav2.3 voltage-gated Ca2+ channel [55]. It may be involved in the protein kinase C (PKC)-mediated signaling to Cav2.3 [56], linking Cav2.3 signaling to muscarinic receptor activation [57,58,59,60,61] and perhaps also to muscarinic enhancement of the “toxin-resistant” R-type Ca2+ current in hippocampal CA1 pyramidal neurons [62]. Cav2.3 also contains the better known, carboxyterminal Ca2+/calmodulin interaction site, which was not only found for the members of the Cav2/non-L-type but also for members of the classical L-type Ca2+ channel subfamily [63].
Structurally, a broad set of splice variants can be predicted from the different cloning approaches (Table 1), which result from alternate use of exon 19 encoded arginine-rich segment in the II-III loop, as well as from the alternate use of exon 45 in the carboxyterminal region [7]. Cav2.3d was originally cloned as a fetal splice variant from human brain [4]. Interestingly, the major splice variants (Table 2) deduced from RT-PCR studies differ between brain regions [64] in mice. Splice variants of Cav2.3 from different species (see also Table 1, Table 2) as well as auxiliary subunits are tissue-specifically expressed [9]. In addition to expression in neuronal [65,66,67,68,69] and endocrine tissues [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85], Cav2.3 transcripts have also been detected in mamalian heart [86,87,88], kidney [70,86,89], sperm [90,91,92,93], spleen [3], and retina [94,95,96,97]. Furthermore, the subcellular distribution of Cav2.3 has been investigated revealing both somatodendritic and presynaptic expression [98] with additional functional specificities [99].
Table 1.
Splice variants of voltage-gated Cav2.3 R-type Ca2+ channels. Exon 19 is encoding an arginine-rich segment of the cytosolic loop between domain II and III, which is responsible for a transient positive Ca2+ feedback, when cytosolic Ca2+ is increasing by Ca2+ influx through the channel itself. Exon 45 is encoding a carboxyterminal insertion of unknown function. Details of exon 20 sequence are found in [7].
| Nomenclature, splice variant | Structure related to alternate exons expressed (+) | Expression (tissue and species) | Ref. | |||
|---|---|---|---|---|---|---|
| Novel terms | Old terms | Exon 19 (57 nts) | Segment (21 nts) in exon 20 | Exon 45 (129 nts) | ||
| Cav2.3a | alpha1E-1 | - | + | - | Rat cerebellum | [100] |
| Cav2.3b | alpha1E-2 | + | - | - | Less important in CNS | [3] |
| Cav2.3c | alpha1E-3 | + | + | - | Dominant in CNS | [3] |
| Cav2.3d | alpha1Ed | + | + | + | Human fetal brain | [4] |
| Cav2.3e | alpha1Ee | - | + | + | Pancreas, kidney, heart | [70,101] |
| Cav2.3f | alpha1Ef | + | - | + | Rat cerebellum | [100] |
Table 2.
Transcripts of major splice variants of voltage-gated Cav2.3 R-type Ca2+ channels expressed in different brain regions [64].
| Brain region (mouse) | Major splice variant | Miscellaneous |
|---|---|---|
| Neocortex | Cav2.3c | Minor amounts of Cav2.3e |
| Hippocampus | Cav2.3c | Minor amounts of Cav2.3e |
| Thalamus | Cav2.3c | Substantial amounts of Cav2.3e and Cav2.3f |
| Cerebellum, mesencephalon, medulla oblongata | Cav2.3e | minor amounts of Cav2.3a |
In heterologous expression systems, Cav2.3c [3] and Cav2.3d [4,102] inward currents are activated at test potentials of about −30 mV. The single channel conductance is about 14 pS [103], and the channel kinetics measured by patch-clamp recordings reveal a fast activating and inactivating channel type with transient inward current characteristics [7,55], similar but not as fast as observed for T-type voltage-gated Ca2+ channels [13].
2. Selective and Non-Selective Antagonists of Cav2.3
The first “pharmacoresistant” Ca2+ current in vivo was recorded and published in 1993 [104,105], which means it occured between the years 1987 (the first cloning of a VGCC subunit [106]) and 1994 (final cloning of the remaining high-voltage gated Ca2+ channels). Doe-1, cloned from Discopyge ommata, represented a novel Ca2+ channel type, which was insensitive towards dihydropyridines, but was antagonized rather than activated by 5 µM Bay K. This channel type was only slightly and readily reversibly inhibited by 5 µM ω-conotoxin-MVIIC, was insensitive towards ω-agatoxin-IVA, and fully reversibly blocked by ω-conotoxin-GVIA, an irreversible antagonist of N-type Ca2+ channels [104]. Interestingly, the same group identified a similar Ca2+ current component in rat cerebellar granule neurons and called the doe-1-like component “R-type current” [105].
The peptide antagonist SNX-482, which was initially purified from the venom of the tarantula Hysterocratis gigas [107] blocks Cav2.3 with an IC50 value of 15–30 nM and was the first and still is the only Cav2.3-prevalent antagonist,. At concentrations higher than 500 nM SNX-482 also inhibits N-type Ca2+ currents [107], wherease L-type Ca2+ currents are inhibited by about 25% at concentrations of 200 nM SNX-482 [108]. Therefore, it only can be regarded as Cav2.3-prevalent, but not as Cav2.3-specific or -selective.
In cerebellar granule cells, two Cav2.3 isoforms could be distinguished from eachother by their varying SNX-482 IC50 values of 6 nM and 81 nM, and a third R-type Ca2+ current component by its insensitivity to SNX-482 [109].
The first gene inactivation of Cav2.3 led to knock-out mice, which in cerebellar granule cells and in DRG neurons still expressed a drug insensitive Ba2+ current. The peak inward current (IBa) was even larger than in cultured mouse neurons from contol mice (knock-out IBa 113 ± 27 pA (n = 5 ); control 85 ± 21 pA (n = 9)) [110]. Only the wild type cultured neurons were inhibited by SNX-482, but not the neurons from Cav2.3-deficient mice, leading to the conclusion that a non-Cav2.3-dependent R-type current may exist.
In murine hippocampal and neocortical neurons, Cav2.3 contributes not only to the SNX-482-sensitive component of the R-type Ca2+ current, which was recorded in the presence of combination of Ca2+ channel antagonists (ω-conotoxin-GVIA, 2 µM; ω-conotoxin-MVIIC, 3 µM; ω-agatoxin-IVA, 200 nM; nifedipine, 10 µM), but also to the SNX-482-insensitive part [66]. Interestingly, the voltage of half-maximal activation (V1/2, act) was shifted to more positive voltages in all three cell types investigated (dissociated CA1 pyramidal cells, dentate gyrus cells, neocortical neurons), specially in the neocortex, where it was reduced from –68 ± 2 mV to –58 ± 7 mV [66]. Overall, it may be useful to keep in mind that the R-type Ca2+ current may be more than only the Cav2.3-gene encoded Ca2+ channel in neuronal tissues [8,111,112].
Divalent and trivalent heavy metal cations were often used to antagonize either all voltage-gated Ca2+ inward currents (Cd2+, La3+) or to specifially inhibit some T-type and the R-type Ca2+ current (Ni2+). Unfortunately, the half maximal concentrations for Cav2.3 and Cav3.2 are close to each other (10–30 µM), rendering Ni2+ blockade unsuitable for distinction of Cav2.3 currents in tissue in which Cav3.2 is also expressed. Physiologically, homeostasis of other divalent cations like Cu2+ and Zn2+ may play an important role [10,113,114], notably also in neurodegenerative disease [115].
Table 3 summarizes the effect of drugs and toxins on Cav2.3 reported in the literature. Most drugs in the table are non-selective, in the sense that currents through other Ca2+ channel Cavα1 subunits are also antagonized with an IC50 not larger than tenfold. Many substances show inhibitory effects on Cav2.3 or on R-type Ca2+ currents. One set of drugs is related to anticonvulsive effects, others are used as anesthetic drugs. Even high concentrations of classical Ca2+ channel antagonists can inhibit Cav2.3 induced inward currents as shown for the dihydropyrdines isradipine [87] and nicardipine [116]. Routinely, in order to block L-type voltage-gated Ca2+ channels, a dihydropyridine concentration of around 10 µM is chosen by electrophysiologists. Considering that such high concentrations of isradipine or nicardipine substantially block E-/R-type Ca2+ currents, lower concentrations of e.g., isradipine of 0.5 µM are more suitable, in order to observe antagonism by low concentrations of SNX-482 as shown for cardiac E-/R-type Ca2+ currents in murine myocytes [101]. However, one has to keep in mind that SNX-482 may block L-type Ca2+ current at elevated concentrations [108].
Table 3.
Selected antagonists of Cav2.3 (modified according to: Wrubel, 2009 [127]). Recombinant Cav2.3 was expressed in different cell lines and was cotransfected with auxiliary subunits (β-subunits from different species). Note, trace metals must be applied under well defined conditions, which provide buffering of the cation of interest [10]. Abbreviations: n.t. = not tested.
| Substance | Application | IC50 or Kd [µM] | Amount of max. Inhibition | Selectivity | Ref. |
|---|---|---|---|---|---|
| SNX-482 | Peptide toxin | 0.015–0.030 | > 80 % | Cav2.3-prevalent | [107,108,128,129,130] |
| ω-Aga-IVA | Peptide toxin | 0.051 | 80% | non-selective | [116] |
| ω-Aga-IIIA | Peptide toxin | 0.003–0.010 | 100% | non-selective | [107] |
| Ni2+ | Unphysiological | 27.4/303 | 100% | non-selective | [3,131] |
| Cd2+ | Unphysiological | 0.8 | 100% | non-selective | [3] |
| Zn2+ | Trace element | 31.8 | >90% | non-selective | [132] |
| Zn2+ (calibrated) | Trace element | 1.3 | 100% | non-selective | [10] |
| Cu2+ | Trace element | 0.018 | 100% | non-selective | [10] |
| Topiramate | Anticonvulsive | 50.9 | >70% | non-selective | [133] |
| Lamotrigine | Anticonvulsive | >10 | non-selective | [134] | |
| Sipatrigine | Anticonvulsive | 10 | 100% | non-selective | [134] |
| 202W92 | Anticonvulsive | 56 | 100% | [134] | |
| Ethosuximide | Anticonvulsive | 20000 | 100% | non-selective | [135] |
| MPS (α-methylphenylsuccinimide) | Anticonvulsive | 2300 | 100% | [135] | |
| Phenytoin | Anticonvulsive | 360 | 100% | [135] | |
| Phenobarbital | Anticonvulsive | 2700 | >80% | [135] | |
| Pentobarbital | Anticonvulsive | 600 | 100% | [135] | |
| Halothane | Inhalation anaesthetic | [136,137] | |||
| Isoflurane | Inhalation anaesthetic | 206 | 100% | [136,138,139] | |
| Fomocaine | Local anaestetic | 95 | 100% | [140] | |
| Procaine | Local anaestetic | [140] | |||
| Octanol | Organic solvent | 206 | 100% | [135] | |
| (+)-ACN | Steroid anaestetic | 5.3–10.2 | 100% | [141] | |
| (+)-ECN | Steroid anaestetic | 9.9–16.1 | >70% | [141] | |
| Flecainide | Antiarrhythmic | 320 | [140] | ||
| Penfluridol | Antipsychotic | 13 | [140] | ||
| Verapamil | Antihypertensive | 100 | 100% | non-selective | [142] |
| Diltiazem | Antihypertensive | 220 | 100% | non-selective | [4,142] |
| Isradipine | Antihypertensive | 9.1 | 100% | non-selective | [87] |
| Nicardipine | Antihypertensive | 1 | n.t. | non-selective | [116] |
| Mibefradil | Antihypertensive | 0.4/6.5 | 100% | non-selective | [143] |
| Amiloride | Diuretic | 7400 | 100% | non-selective | [135] |
| Ethoxyzolamide | Carboanhydrase inhibitor/anticonvulsive | 1 | 70% | [144] | |
| Eugenol | Analgetic | [145] | |||
| Bisphenol A | Environmental pollutant | 26 | 50% | non-selective | [146] |
3. Physiological Functions, in Which Cav2.3 may be Involved, as Deduced from Cav2.3-Deficient Mice
Many of the experimental results from gene-inactivated mice cannot automatically transferred to human physiology and pathophysiology of human diseases. But some basic conclusions may be drawn from these investigations of Cav2.3-deficient mice, which were generated and analysed in several different laboratories (for detail, see Kamp et al. [8]).
Cav2.3 is expressed in many regions of the CNS and also in peripheral organs and tissues, which makes it difficult to explore its full function in vivo. Cav2.3 triggers or participates in the release of several neurotransmitters such as dopamine in the substantia nigra [117]. In the hippocampus Cav2.3 contributes to fast glutamatergic transmission [118], where it is also involved in long term potentiation at the mossy fiber – CA3 synapses. Therefore, Cav2.3 participates in basic processes related to learning and memory formation [67,119,120,121]. Furthermore, Cav2.3 is an important regulator in spines: activation of Cav2.3 triggers opening of small conductance Ca2+-activated K+-channels in CA1 hippocampal pyramidal neurons [122,123,124], suggesting spine-restricted local microdomains, which are important for synaptic signalling [125]. R-type Ca2+ currents, which were recorded as Ni2+-sensitive tail currents, are available at resting potential and contribute to after-depolarization, and thus to the initiation of burst firing in CA1 hippocampal neurons [126].
The existance of a fetal brain Cav2.3 isoform [4] and the changes in expression of Cav2.3 during neuronal development point to an important role of Cav2.3 during early prenatal stages [147,148,149]. At nerve terminals of the calyx of Held, N- and R-type Ca2+ channels are replaced by P-/Q-type Ca2+ channels during development [150].
Cav2.3-deficient mice reveal altered pain response [151], and transcripts of two different splice variants of Cav2.3 could be identified in rat nociceptive neurons [152]. The major splice variant was Cav2.3e, which was also detected in the cerebellum, heart and endocrine system (Table 1, Table 2).
Cav2.3 is highly expressed in the amygdala, in which the R-type Ca2+ current represents the largest component of high-voltage gated Ca2+ currents. Cav2.3-deficient mice exhibited signs of enhanced fear assuming that Cav2.3-based R-type Ca2+ currents in the amygdala may be associated with fear [153].
Cav2.3-deficient mice represent an important model for convulsive and non-convulsive seizures as was summarized in [9]. Based on the initial detection of Cav2.3 transcripts in the insulinoma cell line INS-1 [70,73], additional investigations were performed with Cav2.3-deficient mice, which showed disturbance not only of glucose-induced insulin release [72,75], but also of glucose-mediated glucagon suppression [74], and more important even disturbances of glucose-mediated somatostatin-release [80].
SNX-482 sensitive R-type Ca2+ current was related to the release of gonadotropin-releasing hormone [81] and of oxytocin [76,77]. Overall, peptide hormone release often appears to be triggered by Cav2.3 VGCCs, possibly by producing the global increase in cytoxolic Ca2+ required for refilling of the readily releasable pool of granules during the second phase of insulin release [75,154].
After cerebral aneurysm rupture and subarachnoidal hemorrhage Cav2.3 has been shown to contribute to cerebral artery constriction i.e., vasospasm [155], a devastating delayed event causing often fatal strokes. Accordingly intracisternal administration of SNX-482 reduced delayed vasospasm in a rat model of subarachnoid hemmorhage [156].
The expression of Cav2.3 in cardiomyocytes is still under discussion: Cav2.3 protein has yet to be detected reliably in murine cardiomyocytes, but transcripts could be amplified by single cell RT-PCR from microscopically identified murine cardiomyocytes [87,88]. Furthermore, Cav2.3 ablation causes cardiac arhythmia and disturbances in autonomic cardiac control, suggesting that Cav2.3 in pacemaker cells as well as in autonomic nerve endings may participate in cardiac signalling [101].
In future, more specific Cav2.3 modualtors will be a key in establishing the exact role of Cav2.3 in the physiological and pathophysiological processes, that it contributes to. Furthermore, recent evidence points to Cav2.3 as a potential pharmacologic target in therapy of epilepsy, chronic pain, endocrine disturbances and vasospasms after subarchnoid hemmorhage. In this light, non-ion selective Cav2.3 inhibitors with favourable pharmakokinetics could represent new therapeutic strategies for these disorders.
Acknowledgments
Our research was supported by the Center of Molecular Medicine Cologne/Zentrum für Molekularbiologische Medizin Köln (BMBF 01 KS 9502 to T.S. and J.H.), and by the German Research Council (DFG, to T.S.). We are grateful to Ms. Renate Clemens for her technical assistance.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Niidome T., Kim M.-S., Friedrich T., Mori Y. Molecular cloning and characterization of a novel calcium channel from rabbit brain. FEBS Lett. 1992;308:7–13. doi: 10.1016/0014-5793(92)81038-N. [DOI] [PubMed] [Google Scholar]
- 2.Soong T.W., Stea A., Hodson C.D., Dubel S.J., Vincent S.R., Snutch T.P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 3.Williams M.E., Marubio L.M., Deal C.R., Hans M., Brust P.F., Philipson L.H., Miller R.J., Johnson E.C., Harpold M.M., Ellis S.B. Structure and functional characterization of neuronal α1E calcium channel subtypes. J. Biol. Chem. 1994;269:22347–22357. [PubMed] [Google Scholar]
- 4.Schneider T., Wei X., Olcese R., Costantin J.L., Neely A., Palade P., Perez-Reyes E., Qin N., Zhou J., Crawford G.D., et al. Molecular analysis and functional expression of the human type E α1 subunit. Receptors Channels. 1994;2:255–270. [PubMed] [Google Scholar]
- 5.Perez-Reyes E. Molecular Physiology of Low-Voltage-Activated T-type Calcium Channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Reyes E., Schneider T. Calcium channels: Structure, function, and classification. Drug Dev. Res. 1994;33:295–318. doi: 10.1002/ddr.430330311. [DOI] [Google Scholar]
- 7.Pereverzev A., Leroy J., Krieger A., Malecot C.O., Hescheler J., Pfitzer G., Klockner U., Schneider T. Alternate Splicing in the Cytosolic II-III Loop and the Carboxy Terminus of Human E-type Voltage-Gated Ca2+ Channels: Electrophysiological Characterization of Isoforms. Mol. Cell Neurosci. 2002;21:352–365. doi: 10.1006/mcne.2002.1179. [DOI] [PubMed] [Google Scholar]
- 8.Kamp M.A., Krieger A., Henry M., Hescheler J., Weiergräber M., Schneider T. Presynaptic “Cav2.3 containing” E-type Ca2+ channels share dual roles during neurotransmitter release. Eur. J. Neurosci. 2005;21:1617–1625. doi: 10.1111/j.1460-9568.2005.03984.x. [DOI] [PubMed] [Google Scholar]
- 9.Weiergräber M., Kamp M.A., Radhakrishnan K., Hescheler J., Schneider T. The Cav2.3 Voltage-gated calcium channel in epileptogenesis. Shedding new light on an enigmatic channel. Neurosci. Biobehav. Rev. 2006;30:1122–1144. doi: 10.1016/j.neubiorev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Shcheglovitov A., Vitko I., Lazarenko R.M., Orestes P., Todorovic S.M., Perez-Reyes E. Molecular and biophysical basis of glutamate and trace metal modulation of voltage-gated Ca(v)2.3 calcium channels. J. Gen. Physiol. 2012;139:219–234. doi: 10.1085/jgp.201110699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spafford J.D., Zamponi G.W. Functional interactions between presynaptic calcium channels and the neurotransmitter release machinery. Curr. Opin. Neurobiol. 2003;13:308–314. doi: 10.1016/S0959-4388(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 12.Parent L., Schneider T., Moore C.P., Talwar D. Subunit Regulation of the Human Brain α1E Calcium Channel. J. Membrane Biol. 1997;160:127–140. doi: 10.1007/s002329900302. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima Y.M., Todorovic S.M., Pereverzev A., Hescheler J., Schneider T., Lingle C.J. Properties of Ba2+ currents arising from human α1E and α1Eβ3 constructs expressed in HEK293 cells: Physiology, pharmacology, and comparison to native T-type Ba2+ currents. Neuropharmacology. 1998;37:957–972. doi: 10.1016/S0028-3908(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 14.Berrou L., Bernatchez G., Parent L. Molecular Determinants of Inactivation within the I-II Linker of alpha1E (Cav2.3) Calcium Channels. Biophys. J. 2001;80:215–228. doi: 10.1016/S0006-3495(01)76008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berrou L., Dodier Y., Raybaud A., Tousignant A., Dafi O., Pelletier J.N., Parent L. The C-terminal residues in the alpha-interacting domain (AID) helix anchor CaVbeta subunit interaction and modulation of CaV2.3 channels. J. Biol. Chem. 2005;280:494–505. doi: 10.1074/jbc.M410859200. [DOI] [PubMed] [Google Scholar]
- 16.Shakeri B., Bourdin B., Demers-Giroux P.O., Sauve R., Parent L. A quartet of leucine residues in the guanylate kinase domain of CaVbeta determines the plasma membrane density of the CaV2.3 channel. J. Biol. Chem. 2012;287:32835–32847. doi: 10.1074/jbc.M112.387233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flockerzi V., Oeken H.J., Hofmann F. Purification of a functional receptor for calcium-channel blockers from rabbit skeletal-muscle microsomes. Eur. J. Biochem. 1986;161:217–224. doi: 10.1111/j.1432-1033.1986.tb10145.x. [DOI] [PubMed] [Google Scholar]
- 18.Sieber M., Nastainczyk W., Zubor V., Wernet W., Hofmann F. The 165-kDa peptide of the purified skeletal muscle dihydropyridine receptor contains the known regulatory sites of the calcium channel. Eur. J. Biochem. 1987;167:117–122. doi: 10.1111/j.1432-1033.1987.tb13311.x. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi M., Seagar M.J., Jones J.F., Reber B.F., Catterall W.A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc. Natl. Acad. Sci. USA. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Striessnig J., Knaus H.G., Grabner M., Moosburger K., Seitz W., Lietz H., Glossmann H. Photoaffinity labelling of the phenylalkylamine receptor of the skeletal muscle transverse-tubule calcium channel. FEBS Lett. 1987;212:247–253. doi: 10.1016/0014-5793(87)81354-6. [DOI] [PubMed] [Google Scholar]
- 21.Schneider T., Hofmann F. The bovine cardiac receptor for calcium channel blockers is a 195-kDa protein. Eur. J. Biochem. 1988;174:369–375. doi: 10.1111/j.1432-1033.1988.tb14107.x. [DOI] [PubMed] [Google Scholar]
- 22.Witcher D.R., De Waard M., Campbell K.P. Characterization of the purified N-type Ca2+ channel and the cation sensitivity of omega-conotoxin GVIA binding. Neuropharmacology. 1993;32:1127–1139. doi: 10.1016/0028-3908(93)90007-P. [DOI] [PubMed] [Google Scholar]
- 23.Witcher D.R., de Waard M., Sakamoto J., Franzini Armstrong C., Pragnell M., Kahl S.D., Campbell K.P. Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science. 1993;261:486–489. doi: 10.1126/science.8392754. [DOI] [PubMed] [Google Scholar]
- 24.Guy H.R., Conti F. Pursuing the structure and function of voltage-gated channels. TINS. 1990;13:201–206. doi: 10.1016/0166-2236(90)90160-c. [DOI] [PubMed] [Google Scholar]
- 25.Doyle D.A., Cabral J.M., Pfuetzner R.A., Kuo A.L., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 26.Long S.B., Campbell E.B., MacKinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 27.Payandeh J., Scheuer T., Zheng N., Catterall W.A. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payandeh J., Gamal El-Din T.M., Scheuer T., Zheng N., Catterall W.A. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486:135–139. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmann F., Lacinová L., Klugbauer N. Voltage-dependent calcium channels: From structure to function. Rev. Physiol. Biochem. Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 30.Zhen X.G., Xie C., Fitzmaurice A., Schoonover C.E., Orenstein E.T., Yang J. Functional architecture of the inner pore of a voltage-gated Ca2+ channel. J. Gen. Physiol. 2005;126:193–204. doi: 10.1085/jgp.200509292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie C., Zhen X.G., Yang J. Localization of the activation gate of a voltage-gated Ca2+ channel. J. Gen. Physiol. 2005;126:205–212. doi: 10.1085/jgp.200509293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raybaud A., Baspinar E.E., Dionne F., Dodier Y., Sauve R., Parent L. The role of distal S6 hydrophobic residues in the voltage-dependent gating of CaV2.3 channels. J. Biol. Chem. 2007;282:27944–27952. doi: 10.1074/jbc.M703895200. [DOI] [PubMed] [Google Scholar]
- 33.Tang S., Mikala G., Bahinski A., Yatani A., Varadi G., Schwartz A. Molecular localization of ion selectivity sites within the pore of a human L-type cardiac calcium channel. J. Biol. Chem. 1993;268:13026–13029. [PubMed] [Google Scholar]
- 34.Yang J., Ellinor P.T., Sather W.A., Zhang J.-F., Tsien R.W. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- 35.Kim M.-S., Morii T., Sun L.-X., Imoto K., Mori Y. Structural determinants of ion selectivity in brain calcium channel. FEBS Lett. 1993;318:145–148. doi: 10.1016/0014-5793(93)80009-J. [DOI] [PubMed] [Google Scholar]
- 36.Ellinor P.T., Yang J., Sather W.A., Zhang J.F., Tsien R.W. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron. 1995;15:1121–1132. doi: 10.1016/0896-6273(95)90100-0. [DOI] [PubMed] [Google Scholar]
- 37.Parent L., Gopalakrishnan M. Glutamate substitution in repeat IV alters divalent and monovalent cation permeation in the heart Ca2+ channel. Biophys. J. 1995;69:1801–1813. doi: 10.1016/S0006-3495(95)80050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dirksen R.T., Nakai J., Gonzalez A., Imoto K., Beam K.G. The S5-S6 linker of repeat I is a critical determinant of L-type Ca2+ channel conductance. Biophys. J. 1997;73:1402–1409. doi: 10.1016/S0006-3495(97)78172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cibulsky S.M., Sather W.A. Control of ion conduction in L-type Ca2+ channels by the concerted action of S5–6 regions. Biophys. J. 2003;84:1709–1719. doi: 10.1016/S0006-3495(03)74979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cibulsky S.M., Sather W.A. The EEEE Locus Is the Sole High-affinity Ca2+ Binding Structure in the Pore of a Voltage-gated Ca2+ Channel Block by Ca2+ Entering from the Intracellular Pore Entrance. J. Gen. Physiol. 2000;116:349–362. doi: 10.1085/jgp.116.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Y., Ruta V., Chen J., Lee A., MacKinnon R. The principle of gating charge movement in a voltage-dependent K+ channel. Nature. 2003;423:42–48. doi: 10.1038/nature01581. [DOI] [PubMed] [Google Scholar]
- 42.Lacinova L. Voltage-dependent calcium channels. Gen. Physiol Biophys. 2005;24(Suppl. 1):1–78. doi: 10.1152/physiolgenomics.00278.2005. [DOI] [PubMed] [Google Scholar]
- 43.Lee S.Y., Banerjee A., MacKinnon R. Two separate interfaces between the voltage sensor and pore are required for the function of voltage-dependent K+ channels. PLoS. Biol. 2009;7:e47. doi: 10.1371/journal.pbio.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Petegem F., Clark K.A., Chatelain F.C., Minor D.L., Jr. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petegem F.V., Chatelain F.C., Minor D.L. Insights into voltage-gated calcium channel regulation from the structure of the Ca(V)1.2 IQ domain-Ca2+/calmodulin complex. Nat. Struct. Mol. Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim E.Y., Rumpf C.H., Fujiwara Y., Cooley E.S., Van Petegem F., Minor D.L., Jr. Structures of CaV2 Ca2+/CaM-IQ domain complexes reveal binding modes that underlie calcium-dependent inactivation and facilitation. Structure. 2008;16:1455–1467. doi: 10.1016/j.str.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dick I.E., Tadross M.R., Liang H., Tay L.H., Yang W., Yue D.T. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature. 2008;451:830–834. doi: 10.1038/nature06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tadross M.R., Dick I.E., Yue D.T. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell. 2008;133:1228–1240. doi: 10.1016/j.cell.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krieger A., Radhakrishnan K., Pereverzev A., Siapich S.A., Banat M., Kamp M.A., Leroy J., Klöckner U., Hescheler J., Weiergräber M., Schneider T. The molecular chaperone hsp70 interacts with the cytosolic II-III loop of the Cav2.3 E-type voltage-gated Ca2+ channel. Cell Physiol Biochem. 2006;17:97–110. doi: 10.1159/000092071. [DOI] [PubMed] [Google Scholar]
- 50.Kamp M.A., Shakeri B., Tevoufouet E.E., Krieger A., Henry M., Behnke K., Herzig S., Hescheler J., Radhakrishnan K., Parent L., Schneider T. The C-terminus of human Ca(v)2.3 voltage-gated calcium channel interacts with alternatively spliced calmodulin-2 expressed in two human cell lines. Biochim. Biophys. Acta. 2012;1824:1045–1057. doi: 10.1016/j.bbapap.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Radhakrishnan K., Kamp M.A., Siapich S.A., Hescheler J., Lüke M., Schneider T. Cav2.3 Ca2+ channel interacts with the G1-subunit of V-ATPase. Cellul. Physiol. Biochem. 2011;27:421–432. doi: 10.1159/000329963. [DOI] [PubMed] [Google Scholar]
- 52.Radhakrishnan K., Krieger A., Dibué M., Hescheler J., Schneider T. APLP1 and Rab5A Interact with the II-III loop of the Voltage-gated Ca2+-channel Cav2.3 and Modulate its Internalization Differently. Cellul. Physiol. Biochem. 2011;28:603–612. doi: 10.1159/000335756. [DOI] [PubMed] [Google Scholar]
- 53.Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T.P., Campbell K.P. Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 54.De Waard M., Pragnell M., Campbell K.P. Ca2+ channel regulation by a conserved β subunit domain. Neuron. 1994;13:495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 55.Leroy J., Pereverzev A., Vajna R., Qin N., Pfitzer G., Hescheler J., Malecot C.O., Schneider T., Klockner U. Ca2+-sensitive regulation of E-type Ca2+ channel activity depends on an arginine-rich region in the cytosolic II-III loop. Eur. J. Neurosci. 2003;18:841–855. doi: 10.1046/j.1460-9568.2003.02819.x. [DOI] [PubMed] [Google Scholar]
- 56.Klöckner U., Pereverzev A., Leroy J., Krieger A., Vajna R., Hescheler J., Pfitzer G., Malecot C.O., Schneider T. The cytosolic II-III loop of Cav2.3 provides an essential determinant for the phorbol ester-mediated stimulation of E-type Ca2+ channel activity. Eur. J. Neurosci. 2004;19:2659–2668. doi: 10.1111/j.0953-816X.2004.03375.x. [DOI] [PubMed] [Google Scholar]
- 57.Mehrke G., Pereverzev A., Grabsch H., Hescheler J., Schneider T. Receptor Mediated Modulation of Recombinant Neuronal Class E Calcium Channels. FEBS Lett. 1997;408:261–270. doi: 10.1016/S0014-5793(97)00437-7. [DOI] [PubMed] [Google Scholar]
- 58.Meza U., Bannister R., Melliti K., Adams B. Biphasic, Opposing Modulation of Cloned Neuronal α1E Ca Channels by Distinct Signaling Pathways Coupled to M2 Muscarinic Acetylcholine Receptors. J. Neurosci. 1999;19:6806–6817. doi: 10.1523/JNEUROSCI.19-16-06806.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melliti K., Meza U., Adams B. Muscarinic stimulation of α1E Ca Channels is selectively blocked by the effector antagonist function of RGS2 and phsopholipase C-β1. J. Neurosci. 2000;20:7167–7173. doi: 10.1523/JNEUROSCI.20-19-07167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bannister R.A., Melliti K., Adams B.A. Differential Modulation of CaV2.3 Ca2+ Channels by G{alpha}q/11-Coupled Muscarinic Receptors. Mol. Pharmacol. 2004;65:381–388. doi: 10.1124/mol.65.2.381. [DOI] [PubMed] [Google Scholar]
- 61.Kamatchi G.L., Franke R., Lynch C., III, Sando J.J. Identification of sites responsible for potentiation of type 2.3 calcium currents by acetyl-beta-methylcholine. J. Biol. Chem. 2004;279:4102–4109. doi: 10.1074/jbc.M308606200. [DOI] [PubMed] [Google Scholar]
- 62.Tai C., Kuzmiski J.B., MacVicar B.A. Muscarinic enhancement of R-type calcium currents in hippocampal CA1 pyramidal neurons. J. Neurosci. 2006;26:6249–6258. doi: 10.1523/JNEUROSCI.1009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang H., DeMaria C.D., Erickson M.G., Mori M.X., Alseikhan B.A., Yue D.T. Unified Mechanisms of Ca2+ Regulation across the Ca2+ Channel Family. Neuron. 2003;39:951–960. doi: 10.1016/S0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- 64.Weiergräber M., Henry M., Krieger A., Kamp M.A., Radhakrishnan K., Hescheler J., Schneider T. Altered seizure susceptibility in mice lacking the Cav2.3 E-type Ca2+ channel. Epilepsia. 2006;47:839–850. doi: 10.1111/j.1528-1167.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- 65.Sochivko D., Chen J., Becker A., Beck H. Blocker-resistant Ca2+ currents in rat CA1 hippocampal pyramidal neurons. Neuroscience. 2003;116:629–638. doi: 10.1016/S0306-4522(02)00777-7. [DOI] [PubMed] [Google Scholar]
- 66.Sochivko D., Pereverzev A., Smyth N., Gissel C., Schneider T., Beck H. The α1E calcium channel subunit underlies R-type calcium current in hippocampal and cortical pyramidal neurons. J. Physiol. 2002;542:699–710. doi: 10.1113/jphysiol.2002.020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dietrich D., Kirschstein T., Kukley M., Pereverzev A., von der Brelie C., Schneider T., Beck H. Functional specialization of presynaptic Cav2.3 Ca2+ channels. Neuron. 2003;39:483–496. doi: 10.1016/S0896-6273(03)00430-6. [DOI] [PubMed] [Google Scholar]
- 68.Osanai M., Saegusa H., Kazuno A.A., Nagayama S., Hu Q., Zong S., Murakoshi T., Tanabe T. Altered cerebellar function in mice lacking CaV2.3 Ca2+ channel. Biochem. Biophys. Res. Commun. 2006;344:920–925. doi: 10.1016/j.bbrc.2006.03.206. [DOI] [PubMed] [Google Scholar]
- 69.Han W., Saegusa H., Zong S., Tanabe T. Altered cocaine effects in mice lacking Ca(v)2.3 (alpha(1E)) calcium channel. Biochem. Biophys. Res. Commun. 2002;299:299–304. doi: 10.1016/S0006-291X(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 70.Vajna R., Schramm M., Pereverzev A., Arnhold S., Grabsch H., Klöckner U., Perez-Reyes E., Hescheler J., Schneider T. New Isoform of the Neuronal Ca2+ Channel α1E Subunit in Islets of Langerhans, and Kidney. Distribution of Voltage-Gated Ca2+ Channel α1 Subunits in Cell Lines and Tissues. Eur. J. Biochem. 1998;257:274–285. doi: 10.1046/j.1432-1327.1998.2570274.x. [DOI] [PubMed] [Google Scholar]
- 71.Vajna R., Klöckner U., Pereverzev A., Weiergräber M., Chen X.H., Miljanich G., Klugbauer N., Hescheler J., Perez-Reyes E., Schneider T. Functional coupling between 'R-type' calcium channels and insulin secretion in the insulinoma cell line INS-1. Eur. J. Biochem. 2001;268:1066–1075. doi: 10.1046/j.1432-1327.2001.01969.x. [DOI] [PubMed] [Google Scholar]
- 72.Pereverzev A., Mikhna M., Vajna R., Gissel C., Henry M., Weiergräber M., Hescheler J., Smyth N., Schneider T. Disturbances in glucose-tolerance, insulin-release and stress-induced hyperglycemia upon disruption of the Cav2.3 (α1E) subunit of voltage-gated Ca2+ channels. Mol. Endocrinol. 2002;16:884–895. doi: 10.1210/me.16.4.884. [DOI] [PubMed] [Google Scholar]
- 73.Pereverzev A., Vajna R., Pfitzer G., Hescheler J., Klockner U., Schneider T. Reduction of insulin secretion in the insulinoma cell line INS-1 by overexpression of a Ca(v)2.3 (alpha1E) calcium channel antisense cassette. Eur. J. Endocrinol. 2002;146:881–889. doi: 10.1530/eje.0.1460881. [DOI] [PubMed] [Google Scholar]
- 74.Pereverzev A., Salehi A., Mikhna M., Renstrom E., Hescheler J., Weiergraber M., Smyth N., Schneider T. The ablation of the Ca(v)2.3/E-type voltage-gated Ca2+ channel causes a mild phenotype despite an altered glucose induced glucagon response in isolated islets of Langerhans. Eur. J. Pharmacol. 2005;511:65–72. doi: 10.1016/j.ejphar.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 75.Jing X., Li D.Q., Olofsson C.S., Salehi A., Surve V.V., Caballero J., Ivarsson R., Lundquist I., Pereverzev A., Schneider T., Rorsman P., Renstrom E. Ca(V)2.3 calcium channels control second-phase insulin release. J. Clin. Invest. 2005;115:146–154. doi: 10.1172/JCI22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang G., Dayanithi G., Newcomb R., Lemos J.R. An R-Type Ca2+ Current in Neurohypophysial Terminals Preferentially Regulates Oxytocin Secretion. J. Neurosci. 1999;19:9235–9241. doi: 10.1523/JNEUROSCI.19-21-09235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ortiz-Miranda S., Dayanithi G., Custer E., Treistman S.N., Lemos J.R. Micro-opioid receptor preferentially inhibits oxytocin release from neurohypophysial terminals by blocking R-type Ca2+ channels. J. Neuroendocrinol. 2005;17:583–590. doi: 10.1111/j.1365-2826.2005.01346.x. [DOI] [PubMed] [Google Scholar]
- 78.Albillos A., Neher E., Moser T. R-type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J. Neurosci. 2000;20:8323–8330. doi: 10.1523/JNEUROSCI.20-22-08323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mergler S., Wiedenmann B., Prada J. R-type Ca2+-channel activity is associated with chromogranin A secretion in human neuroendocrine tumor BON cells. J. Membr. Biol. 2003;194:177–186. doi: 10.1007/s00232-003-2039-3. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Q., Bengtsson M., Partridge C., Salehi A., Braun M., Cox R., Eliasson L., Johnson P.R., Renström E., Schneider T., Berggren P.-O., Gopel S., Ashcroft F.M., Rorsman P. R-type calcium-channel-evoked CICR regulates glucose-induced somatostatin secretion. Nat. Cell Biol. 2007;9:453–460. doi: 10.1038/ncb1563. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe M., Sakuma Y., Kato M. High expression of the R-type voltage-gated Ca2+ channel and its involvement in Ca2+-dependent gonadotropin-releasing hormone release in GT1-7 cells. Endocrinology. 2004;145:2375–2383. doi: 10.1210/en.2003-1257. [DOI] [PubMed] [Google Scholar]
- 82.Grabsch H., Pereverzev A., Weiergräber M., Schramm M., Henry M., Vajna R., Beattie R.E., Volsen S.G., Klöckner U., Hescheler J., Schneider T. Immunohistochemical detection of α1E voltage-gated Ca2+ channel isoforms in cerebellum, INS-1 cells, and neuroendocrine cells of the digestive system. J. Histochem. Cytochem. 1999;47:981–993. doi: 10.1177/002215549904700802. [DOI] [PubMed] [Google Scholar]
- 83.Matsuda Y., Saegusa H., Zong S., Noda T., Tanabe T. Mice Lacking Cav2.3 (α1E) Calcium Channel Exhibit Hyperglycemia. Biochem. Biophys. Res. Commun. 2001;289:791–795. doi: 10.1006/bbrc.2001.6051. [DOI] [PubMed] [Google Scholar]
- 84.Holmkvist J., Tojjar D., Almgren P., Lyssenko V., Lindgren C.M., Isomaa B., Tuomi T., Berglund G., Renstrom E., Groop L. Polymorphisms in the gene encoding the voltage-dependent Ca2+ channel Ca (V)2.3 (CACNA1E) are associated with type 2 diabetes and impaired insulin secretion. Diabetologia. 2007;50:2467–2475. doi: 10.1007/s00125-007-0846-2. [DOI] [PubMed] [Google Scholar]
- 85.Muller Y.L., Hanson R.L., Zimmerman C., Harper I., Sutherland J., Kobes S., Knowler W.C., Bogardus C., Baier L.J. Variants in the Cav2.3 (alpha1E) Subunit of Voltage-Activated Ca2+ Channels are Associated with Insulin Resistance and Type 2 Diabetes in Pima Indians. Diabetes. 2007;56:3089–3094. doi: 10.2337/db07-0587. [DOI] [PubMed] [Google Scholar]
- 86.Weiergräber M., Pereverzev A., Vajna R., Henry M., Schramm M., Nastainczyk W., Grabsch H., Schneider T. Immunodetection of α1E voltage-gated Ca2+ channel in chromogranin-positive muscle cells of rat heart, and in distal tubules of human kidney. J. Histochem. Cytochem. 2000;48:807–819. doi: 10.1177/002215540004800609. [DOI] [PubMed] [Google Scholar]
- 87.Lu Z.-L., Pereverzev A., Liu H.-L., Weiergraber M., Henry M., Krieger A., Smyth N., Hescheler J., Schneider T. Arrhythmia in isolated prenatal hearts after ablation of the Cav2.3 (α1E) subunit of voltage-gated Ca2+ channels. Cell. Physiol. Biochem. 2004;14:11–22. doi: 10.1159/000076922. [DOI] [PubMed] [Google Scholar]
- 88.Weiergräber M., Henry M., Südkamp M., De Vivie E.R., Hescheler J., Schneider T. Ablation of Cav2.3/E-type voltage-gated calcium channel results in cardiac arrhythmia and altered autonomic control within the murine cardiovascular system. Basic Res. Cardiol. 2005;100:1–13. doi: 10.1007/s00395-004-0488-1. [DOI] [PubMed] [Google Scholar]
- 89.Natrajan R., Little S.E., Reis-Filho J.S., Hing L., Messahel B., Grundy P.E., Dome J.S., Schneider T., Vujanic G.M., Pritchard-Jones K., Jones C. Amplification and overexpression of CACNA1E correlates with relapse in favorable histology Wilms’ tumors. Clin. Cancer Res. 2006;12:7284–7293. doi: 10.1158/1078-0432.CCR-06-1567. [DOI] [PubMed] [Google Scholar]
- 90.Wennemuth G., Westenbroek R.E., Xu T., Hille B., Babcock D.F. Cav2.2 and Cav2,3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J. Biol. Chem. 2000;275:21210–21217. doi: 10.1074/jbc.M002068200. [DOI] [PubMed] [Google Scholar]
- 91.Carlson A.E., Westenbroek R.E., Quill T., Ren D., Clapham D.E., Hille B., Garbers D.L., Babcock D.F. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. USA. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lievano A., Santi C.M., Serrano C.J., Trevino C.L., Bellve A.R., Hernandez-Cruz A., Darszon A. T-type Ca2+ channels and alpha1E expression in spermatogenic cells, and their possible relevance to the sperm acrosome reaction. FEBS Lett. 1996;388:150–154. doi: 10.1016/0014-5793(96)00515-7. [DOI] [PubMed] [Google Scholar]
- 93.Sakata Y., Saegusa H., Zong S.Q., Osanai M., Murakoshi T., Shimizu Y., Noda T., Aso T., Tanabe T. Cav2.3 (α1E) Ca2+ channel participates in the control of sperm function. FEBS Lett. 2002;516:229–233. doi: 10.1016/S0014-5793(02)02529-2. [DOI] [PubMed] [Google Scholar]
- 94.Kamphuis W., Hendriksen H. Expression patterns of voltage-dependent calcium channel α1 subunits (α1A-α1E) mRNA in rat retina. Mol. Brain Res. 1998;55:209–220. doi: 10.1016/S0169-328X(97)00363-X. [DOI] [PubMed] [Google Scholar]
- 95.Lüke M., Henry M., Lingohr T., Maghsoodian M., Hescheler J., Sickel w., Schneider T. A Ni2+-sensitive component of the ERG-b-wave from the isolated bovine retina is related to E-type voltage-gated Ca2+ channels. Graefes Arch. Clin. Exp. Ophthalmol. 2005;243:933–941. doi: 10.1007/s00417-005-1145-6. [DOI] [PubMed] [Google Scholar]
- 96.Siapich S.A., Banat M., Albanna W., Hescheler J., Lüke M., Schneider T. Antagonists of ionotropic gamma-aminobutyric acid receptors impair the NiCl2-mediated stimulation of the electroretinogramm b-wave amplitude from the isolated superfused vertebrate retina. Acta Ophthalmol. 2009;87:854–865. doi: 10.1111/j.1755-3768.2008.01387.x. [DOI] [PubMed] [Google Scholar]
- 97.Siapich S.A., Wrubel H., Albanna W., Hescheler J., Weiergräber M., Lüke M., Schneider T. Effect of ZnCl2 and chelation of zinc ions by N,N-diethyldithiocarbamate (DEDTC) on the ERG b-wave amplitude from the isolated and superfused vertebrate retina. Curr. Eye Res. 2010;35:322–334. doi: 10.3109/02713680903509410. [DOI] [PubMed] [Google Scholar]
- 98.Yokoyama C.T., Westenbroek R.E., Hell J.W., Soong T.W., Snutch T.P., Catterall W.A. Biochemical properties and subcellular distribution of the neuronal class E calcium channel α1 subunit. J. Neurosci. 1995;15:6419–6432. doi: 10.1523/JNEUROSCI.15-10-06419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brenowitz S.D., Regehr W.G. “Resistant” channels reluctantly reveal their roles. Neuron. 2003;39:391–394. doi: 10.1016/S0896-6273(03)00470-7. [DOI] [PubMed] [Google Scholar]
- 100.Schramm M., Vajna R., Pereverzev A., Tottene A., Klöckner U., Pietrobon D., Hescheler J., Schneider T. Isoforms of α1E voltage-gated calcium channels in rat cerebellar granule cells—Detection of major calcium channel α1-transcripts by reverse transcription-polymerase chain reaction. Neuroscience. 1999;92:565–575. doi: 10.1016/S0306-4522(99)00013-5. [DOI] [PubMed] [Google Scholar]
- 101.Galetin T., Tevoufouet E.E., Sandmeyer J., Matthes J., Nguemo F., Hescheler J., Weiergräber M., Schneider T. Pharmacoresistant CaV2.3 (E-/R-type) voltage-gated calcium channels influence heart rate dynamics and contribute to cardiac impulse conduction. Cell Biochem. Funct. 2012 doi: 10.1002/cbf.2918. [DOI] [PubMed] [Google Scholar]
- 102.Olcese R., Qin N., Schneider T., Neely A., Wei X., Stefani E., Birnbaumer L. The amino terminus of a calcium channel β subunit sets rates of channel inactivation independently of the subunit's effect on activation. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 103.Perez-Reyes E., Schneider T. Molecular biology of calcium channels. Kidney Int. 1995;48:1111–1124. doi: 10.1038/ki.1995.395. [DOI] [PubMed] [Google Scholar]
- 104.Ellinor P.T., Zhang J.F., Randall A.D., Zhou M., Schwarz T.L., Tsien R.W., Horne W.A. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J.-F., Randall A.D., Ellinor P.T., Horne W.A., Sather W.A., Tanabe T., Schwarz T.L., Tsien R.W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-L. [DOI] [PubMed] [Google Scholar]
- 106.Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium *channel* blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- 107.Newcomb R., Szoke B., Palma A., Wang G., Chen X.H., Hopkins W., Cong R., Miller J., Urge L., Tarczy-Hornoch K., Loo J.A., Dooley D.J., Nadasdi L., Tsien R.W., Lemos J., Miljanich G. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- 108.Bourinet E., Stotz S.C., Spaetgens R.L., Dayanithi G., Lemos J., Nargeot J., Zamponi G.W. Interaction of SNX482 with Domains III and IV Inhibits Activation Gating of alpha1E (CaV2.3) Calcium Channels. Biophys. J. 2001;81:79–88. doi: 10.1016/S0006-3495(01)75681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tottene A., Volsen S., Pietrobon D. alpha1E Subunits Form the Pore of Three Cerebellar R-Type Calcium Channels with Different Pharmacological and Permeation Properties. J. Neurosci. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilson S.M., Toth P.T., Oh S.B., Gillard S.E., Volsen S., Ren D., Philipson L.H., Lee E.C., Fletcher C.F., Tessarollo L., Copeland N.G., Jenkins N.A., Miller R.J. The Status of Voltage-Dependent Calcium Channels in alpha1E Knock-Out Mice. J. Neurosci. 2000;20:8566–8571. doi: 10.1523/JNEUROSCI.20-23-08566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Neelands T.R., King A.P., Macdonald R.L. Functional expression of L-, N-, P/Q-, and R-type calcium channels in the human NT2-N cell line. J. Neurophysiol. 2000;84:2933–2944. doi: 10.1152/jn.2000.84.6.2933. [DOI] [PubMed] [Google Scholar]
- 112.Striessnig J., Koschak A. Exploring the function and pharmacotherapeutic potential of voltage-gated Ca2+ channels with gene knockout models. Channels (Austin.) 2008;2:233–251. doi: 10.4161/chan.2.4.5847. [DOI] [PubMed] [Google Scholar]
- 113.Frederickson C.J., Koh J.Y., Bush A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 114.Frederickson C.J., Suh S.W., Silva D., Frederickson C.J., Thompson R.B. Importance of zinc in the central nervous system: The zinc-containing neuron. J. Nutr. 2000;130:1471S–1483S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- 115.Mathie A., Sutton G.L., Clarke C.E., Veale E.L. Zinc and copper: Pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol. Ther. 2006;111:567–583. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 116.Stephens G.J., Page K.M., Burley J.R., Berrow N.S., Dolphin A.C. Functional expression at rat brain cloned α1E calcium channels in COS-7 cells. Pflugers Arch. 1997;433:523–532. doi: 10.1007/s004240050308. [DOI] [PubMed] [Google Scholar]
- 117.Bergquist F., Nissbrandt H. Influence of R-type (Cav2.3) and T-type (Cav3.1–3.3) antagonists on nigral somatodendritic dopamine release measured by microdialysis. Neuroscience. 2003;120:757–764. doi: 10.1016/S0306-4522(03)00385-3. [DOI] [PubMed] [Google Scholar]
- 118.Gasparini S., Kasyanov A.M., Pietrobon D., Voronin L.L., Cherubini E. Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J. Neurosci. 2001;21:8715–8721. doi: 10.1523/JNEUROSCI.21-22-08715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Breustedt J., Vogt K.E., Miller R.J., Nicoll R.A., Schmitz D. Alpha1E-containing Ca2+ channels are involved in synaptic plasticity. Proc. Natl. Acad. Sci. USA. 2003;100:12450–12455. doi: 10.1073/pnas.2035117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kubota M., Murakoshi T., Saegusa H., Kazuno A., Zong S., Hu Q., Noda T., Tanabe T. Intact LTP and Fear Memory but Impaired Spatial Memory in Mice Lacking Cav2.3 (α1E) Channel. Biochem. Biophys. Res. Commun. 2001;282:242–248. doi: 10.1006/bbrc.2001.4572. [DOI] [PubMed] [Google Scholar]
- 121.Isomura Y., Fujiwara-Tsukamoto Y., Imanishi M., Nambu A., Takada M. Distance-dependent Ni2+-sensitivity of synaptic plasticity in apical dendrites of hippocampal CA1 pyramidal cells. J. Neurophysiol. 2002;87:1169–1174. doi: 10.1152/jn.00536.2001. [DOI] [PubMed] [Google Scholar]
- 122.Bloodgood B.L., Sabatini B.L. Nonlinear regulation of unitary synaptic signals by CaV2.3 voltage-sensitive calcium channels located in dendritic spines. Neuron. 2007;53:249–260. doi: 10.1016/j.neuron.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 123.Stackman R.W., Hammond R.S., Linardatos E., Gerlach A., Maylie J., Adelman J.P., Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J. Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ngo-Anh T.J., Bloodgood B.L., Lin M., Sabatini B.L., Maylie J., Adelman J.P. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat. Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- 125.Bloodgood B.L., Sabatini B.L. Regulation of synaptic signalling by postsynaptic, non-glutamate receptor ion channels. J. Physiol. 2008;586:1475–1480. doi: 10.1113/jphysiol.2007.148353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Metz A.E., Jarsky T., Martina M., Spruston N. R-type calcium channels contribute to afterdepolarization and bursting in hippocampal CA1 pyramidal neurons. J. Neurosci. 2005;25:5763–5773. doi: 10.1523/JNEUROSCI.0624-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wrubel H. Ph.D. Thesis. Universität zu Köln; Cologne, Germany: 2009. Untersuchungen zur Hemmung des spannungsabhängigen Cav2.3-Calciumkanals mittels Patch-Clamp-Technik an stabil transfizierten HEK-293-Zellen. [Google Scholar]
- 128.Arroyo G., Aldea M., Fuentealba J., Albillos A., García A.G. SNX482 selectively blocks P/Q Ca2+ channels and delays the inactivation of Na+ channels of chromaffin cells. Eur. J. Pharmacol. 2003;475:11–18. doi: 10.1016/S0014-2999(03)02084-3. [DOI] [PubMed] [Google Scholar]
- 129.Khosravani H., Zamponi G.W. Voltage-gated calcium channels and idiopathic generalized epilepsies. Physiol Rev. 2006;86:941–966. doi: 10.1152/physrev.00002.2006. [DOI] [PubMed] [Google Scholar]
- 130.Dai G., Haedo R.J., Warren V.A., Ratliff K.S., Bugianesi R.M., Rush A., Williams M.E., Herrington J., Smith M.M., McManus O.B., Swensen A.M. A high-throughput assay for evaluating state dependence and subtype selectivity of Cav2 calcium channel inhibitors. Assay. Drug Dev. Technol. 2008;6:195–212. doi: 10.1089/adt.2008.136. [DOI] [PubMed] [Google Scholar]
- 131.Zamponi G.W., Bourinet E., Snutch T.P. Nickel Block of a Family of Neuronal Calcium Channels: Subtype- and Subunit-Dependent Action at Multiple Sites. J. Membrane Biol. 1996;151:77–90. doi: 10.1007/s002329900059. [DOI] [PubMed] [Google Scholar]
- 132.Sun H.S., Hui K., Lee D.W., Feng Z.P. Zn2+ sensitivity of high- and low-voltage activated calcium channels. Biophys. J. 2007;93:1175–1183. doi: 10.1529/biophysj.106.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kuzmiski J.B., Barr W., Zamponi G.W., MacVicar B.A. Topiramate inhibits the initiation of plateau potentials in CA1 neurons by depressing R-type calcium channels. Epilepsia. 2005;46:481–489. doi: 10.1111/j.0013-9580.2005.35304.x. [DOI] [PubMed] [Google Scholar]
- 134.Hainsworth A.H., McNaughton N.C., Pereverzev A., Schneider T., Randall A.D. Actions of sipatrigine, 202W92 and lamotrigine on R-type and T-type Ca2+ channel currents. Eur. J. Pharmacol. 2003;467:77–80. doi: 10.1016/S0014-2999(03)01625-X. [DOI] [PubMed] [Google Scholar]
- 135.Nakashima Y.M., Todorovic S.M., Covey D.F., Lingle C.J. The anesthetic steroid (+)-3α-hydroxy-5α-androstane-17β-carbonitrile blocks N-, Q-, and R-type, but not L- and P- type, high voltage-activated Ca2+ current in hippocampal and dorsal root ganglion neurons of the rat. Mol. Pharmacol. 1998;54:559–568. doi: 10.1124/mol.54.3.559. [DOI] [PubMed] [Google Scholar]
- 136.Kamatchi G.L., Chan C.K., Snutch T., Durieux M.E., Lynch C., III Volatile anesthetic inhibition of neuronal Ca channel currents expressed in Xenopus oocytes. Brain Res. 1999;831:85–96. doi: 10.1016/S0006-8993(99)01401-8. [DOI] [PubMed] [Google Scholar]
- 137.Kamatchi G.L., Tiwari S.N., Durieux M.E., Lynch C., III Effects of volatile anesthetics on the direct and indirect protein kinase C-mediated enhancement of alpha1E-type Ca2+ current in Xenopus oocytes. J. Pharmacol. Exp. Ther. 2000;293:360–369. [PubMed] [Google Scholar]
- 138.Kamatchi G.L., Tiwari S.N., Durieux M.E., Lynch C., III Effects of volatile anesthetics on the direct and indirect protein kinase C-mediated enhancement of alpha1E-type Ca2+ current in Xenopus oocytes. J. Pharmacol. Exp. Ther. 2000;293:360–369. [PubMed] [Google Scholar]
- 139.Joksovic P.M., Weiergraber M., Lee W., Struck H., Schneider T., Todorovic S.M. Isoflurane-sensitive presynaptic R-type calcium channels contribute to inhibitory synaptic transmission in the rat thalamus. J. Neurosci. 2009;29:1434–1445. doi: 10.1523/JNEUROSCI.5574-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zamponi G.W., Soong T.W., Bourinet E., Snutch T.P. β Subunit Coexpression and the α1 Subunit Domain I-II Linker Affect Piperidine Block of Neuronal Calcium Channels. J. Neurosci. 1996;16:2430–2443. doi: 10.1523/JNEUROSCI.16-08-02430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakashima Y.M., Pereverzev A., Schneider T., Covey D.F., Lingle C.J. Blockade of Ba2+ current through human α1E channels by two steroid analogs, (+)-ACN and (+)-ECN. Neuropharmacology. 1999;38:843–855. doi: 10.1016/S0028-3908(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 142.Cai D., Mulle J.G., Yue D.T. Inhibition of recombinant Ca2+ channels by benzothiazepines and phenylalkylamines: Class-specific pharmacology and underlying molecular determinants. Mol. Pharmacol. 1997;51:872–881. [PubMed] [Google Scholar]
- 143.Jimenez C., Bourinet E., Leuranguer V., Richard S., Snutch T.P., Nargeot J. Determinants of voltage-dependent inactivation affect Mibefradil block of calcium channels. Neuropharmacology. 2000;39:1–10. doi: 10.1016/S0028-3908(99)00153-7. [DOI] [PubMed] [Google Scholar]
- 144.McNaughton N.C., Davies C.H., Randall A. Inhibition of alpha1E Ca2+ channels by carbonic anhydrase inhibitors. J. Pharmacol. Sci. 2004;95:240–247. doi: 10.1254/jphs.FP0040032. [DOI] [PubMed] [Google Scholar]
- 145.Chung G., Rhee J.N., Jung S.J., Kim J.S., Oh S.B. Modulation of CaV2.3 calcium channel currents by eugenol. J. Dent. Res. 2008;87:137–141. doi: 10.1177/154405910808700201. [DOI] [PubMed] [Google Scholar]
- 146.Deutschmann A., Hans M., Meyer R., Haberlein H., Swandulla D. Bisphenol A inhibits voltage-activated Ca2+ channels in vitro: Mechanisms and structural requirements. Mol. Pharmacol. 2013;83:501–511. doi: 10.1124/mol.112.081372. [DOI] [PubMed] [Google Scholar]
- 147.Benquet P., Le Guen J., Pichon Y., Tiaho F. Differential involvement of Ca2+ channels in survival and neurite outgrowth of cultured embryonic cockroach brain neurons. J. Neurophysiol. 2002;88:1475–1490. doi: 10.1152/jn.2002.88.3.1475. [DOI] [PubMed] [Google Scholar]
- 148.Benquet P., Pichon Y., Tiaho F. In vitro development of P- and R-like calcium currents in insect (Periplaneta americana) embryonic brain neurons. Neurosci. Lett. 2004;365:228–232. doi: 10.1016/j.neulet.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 149.Falk T., Muller Y.L., Yool A.J. Differential expression of three classes of voltage-gated Ca2+ channels during maturation of the rat cerebellum in vitro. Dev. Brain Res. 1999;115:161–170. doi: 10.1016/S0165-3806(99)00060-7. [DOI] [PubMed] [Google Scholar]
- 150.Iwasaki S., Momiyama A., Uchitel O.D., Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. J. Neurosci. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saegusa H., Kurihara T., Zong S.Q., Minowa O., Kazuno A.A., Han W.H., Matsuda Y., Yamanaka H., Osanai M., Noda T., Tanabe T. Altered pain responses in mice lacking α1E subunit of the voltage-dependent Ca2+ channel. Proc. Natl. Acad. Sci. USA. 2000;97:6132–6137. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fang Z., Park C.K., Li H.Y., Kim H.Y., Park S.H., Jung S.J., Kim J.S., Monteil A., Oh S.B., Miller R.J. Molecular basis of Ca(v)2.3 calcium channels in rat nociceptive neurons. J. Biol. Chem. 2007;282:4757–4764. doi: 10.1074/jbc.M605248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee S.C., Choi S., Lee T., Kim H.L., Chin H., Shin H.S. Molecular basis of R-type calcium channels in central amygdala neurons of the mouse. Proc. Natl. Acad. Sci. USA. 2002;99:3276–3281. doi: 10.1073/pnas.052697799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rorsman P., Eliasson L., Kanno T., Zhang Q., Gopel S. Electrophysiology of pancreatic beta-cells in intact mouse islets of Langerhans. Prog. Biophys. Mol. Biol. 2011;107:224–235. doi: 10.1016/j.pbiomolbio.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 155.Ishiguro M., Wellman T.L., Honda A., Russell S.R., Tranmer B.I., Wellman G.C. Emergence of a R-type Ca2+ channel (CaV2.3) contributes to cerebral artery constriction after subarachnoid hemorrhage. Circ. Res. 2005;96:419–426. doi: 10.1161/01.RES.0000157670.49936.da. [DOI] [PubMed] [Google Scholar]
- 156.Wang F., Yin Y.H., Jia F., Jiang J.Y. Antagonism of R-Type Calcium Channels Significantly Improves Cerebral Blood Flow after Subarachnoid Hemorrhage in Rats. J. Neurotrauma. 2010;27:1723–1732. doi: 10.1089/neu.2010.1276. [DOI] [PubMed] [Google Scholar]