Abstract
Autologous hematopoietic cell transplantation (AHCT) as initial therapy of patients with multiple myeloma (MM) improves survival. However, data to support this approach for relapsed/progressive disease after initial AHCT (AHCT1) are limited. Using Center for International Blood and Marrow Transplant Research data, we report the outcomes of 187 patients who underwent a second AHCT (AHCT2) for the treatment of relapsed/progressive MM. Planned tandem AHCT was excluded. Median age at AHCT2 was 59 years (range, 28 to 72), and median patient follow-up was 47 months (range, 3 to 97). Nonrelapse mortality after AHCT2 was 2% at 1 year and 4% at 3 years. Median interval from AHCT1 to relapse/progression was 18 months, and median interval between transplantations was 32 months. After AHCT2, the incidence of relapse/progression at 1 and 3 years was 51% and 82%, respectively. At 3 years after AHCT2, progression-free survival was 13%, and overall survival was 46%. In multivariate analyses, those relapsing ≥36 months after AHCT1 had superior progression-free (P = .045) and overall survival (P = .019). Patients who underwent AHCT2 after 2004 had superior survival (P = .026). AHCT2 is safe and feasible for disease progression after AHCT1. In this retrospective study, individuals relapsing ≥36 months from AHCT1 derived greater benefit from AHCT2 compared with those with a shorter disease-free interval. Storage of an adequate graft before AHCT1 will ensure that the option of a second autologous transplantation is retained for patients with relapsed/progressive MM.
Keywords: Second autologous, transplantation, Multiple myeloma, Relapsed multiple myeloma
INTRODUCTION
Data support the use of autologous hematopoietic cell transplantation (AHCT) in the up front treatment of eligible patients with plasma cell multiple myeloma (MM). Pivotal studies from the 1990s and 2000s demonstrated prolonged remission and survival when AHCT was compared with chemotherapy alone [1-3]. A benefit in progression-free survival (PFS) has been confirmed in meta-analyses [4], although the success of salvage therapy in the chemotherapy arms likely mitigated demonstration of an overall survival (OS) benefit. Even in the era of novel agents, AHCT remains a cornerstone of therapy [3,5-7].
When patients relapse/progress after an up front single or tandem transplantation, salvage treatment options include additional chemotherapy, clinical trials with investigational agents, and, in select cases, allogeneic hematopoietic cell transplantation, or a second autologous transplantation. Although lenalidomide and bortezomib improve survival in relapsed/progressive myeloma, the development of chemotherapy resistance is a common feature of this disease, and the survival rate of patients refractory to both bortezomib and lenalidomide is dismal [8].
Data regarding outcomes of a second AHCT (AHCT2) performed as salvage therapy for relapse/progression after AHCT1 are primarily limited to retrospective analyses from single institutions [9-15]. Registry data have the advantage of larger numbers of patients in a multi-institutional context. With this in mind, we analyzed data from 187 patients reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) to clarify the benefits of AHCT2, performed at relapse/progression after AHCT1.
METHODS
Data Source
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), the Autologous Blood and Marrow Transplant Registry (ABMTR), and the National Marrow Donor Program that comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic HCT and AHCT to a statistical center at the Health Policy Institute of the Medical College of Wisconsin in Milwaukee or the National Marrow Donor Program Coordinating Center in Minneapolis, Minnesota. Participating centers are required to register all transplantations consecutively; compliance is monitored by on-site audits. Patients are followed up longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed with a waiver of informed consent and in compliance with Health Insurance Portability and Accountability Act regulations as determined by the institutional review board and the privacy officer of the Medical College of Wisconsin. All CIBMTR centers contribute to the registration data. Research data are collected on a subset of registered patients and include detailed disease and pre-transplantation and post transplantation clinical information.
Patients
We identified 983 patients who underwent a second AHCT for MM between 1995 and 2008. Patients who had AHCT2 for reasons other than relapsed/progressive disease were removed from the study cohort, including planned tandem transplantation (n = 704), graft failure (n = 10), another malignancy (n = 1), or unknown (n = 23). Patients who had undergone a previous allogeneic stem cell transplantation (n = 10), a third subsequent allogeneic stem cell transplantation (n = 2), and those without a minimum of 100 days of follow-up data (n = 46) were also excluded. A total of 187 patients from 55 centers in North America who received an AHCT2 for relapsed/progressive MM after an initial AHCT1 comprised the final study population. Median follow-up of survivors from the second transplantation in this study was 47 months (range, 3 to 97 months).
Statistical Methods
Outcomes analyzed included relapse/progression, nonrelapse mortality (NRM), PFS, and OS. Relapse/progression was defined according to the standard European Group for Blood and Marrow Transplantation/IBMTR/ABMTR criteria [16]. NRM was defined as death from any cause within the first 28 days after transplantation or death thereafter in the absence of relapse/ progression. OS interval was defined as the time from AHCT2 to death from any cause. Patients alive without evidence of disease relapse/progression were censored at last follow-up and the PFS event summarized by a survival curve. Cumulative incidence of NRM and relapse/progression were calculated using cumulative incidence curves to accommodate competing risks. Associations between patient-, disease-, and transplantation-related factors and survival were assessed using multivariate Cox proportional hazards regression. The variables considered in the multivariate analysis were age (continuous), sex, Karnofsky performance score, Durie-Salmon stage, and immunochemical subtype of MM, disease status before AHCT2, conditioning regimen for AHCT2 (melphalan alone versus others), interval from AHCT1 to relapse/progression, interval from AHCT1 to AHCT2, and the year of AHCT2.
Forward stepwise variable selection at a .05 significance level was used to identify covariates. In the model, the assumption of proportional hazards was tested for each variable using a time-dependent covariate and graphical methods. All variables considered in the multivariate analysis satisfied the proportionality assumption. All computations were made using the statistical package SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Patient characteristics are summarized in Table 1. The median age of the cohort was 59 years at AHCT2 (range, 28 to 74), and 53% were of Karnofsky performance score ≥90. Most patients (78%, n = 146) were white. Median interval from AHCT1 to AHCT2 was 32 months (range, 6 to 122 months) and from AHCT1 to first relapse/progression was 18 months (range, 3 to 121 months). A total of 22 patients (12%) underwent the second transplantation within 12 months of AHCT1. Compared with AHCT1, patients were less likely to be in complete or partial remission before AHCT2.
Table 1.
Characteristics of Patients Receiving AHCT2 for Relapsed/Progressive MM between 1995 and 2008
| Patient Characteristics | First Transplantation |
Second Transplantation |
|---|---|---|
| Demographics | ||
| Number of patients | 187 | 187 |
| Age at transplantation, median (range), yr |
57(28-72) | 59(28-74) |
| Male sex | 118(63%) | |
| Race | ||
| White | 146(78%) | |
| African American | 23(12%) | |
| Other | 18(10%) | |
| Disease related | ||
| Immunochemical subtype of MM | ||
| IgG | 89(48%) | |
| IgA | 37(20%) | |
| Light chain/other/unknown | 61(33%) | |
| Durie-Salmon stage at diagnosis | ||
| Stage I | 12(6%) | – |
| Stage II | 40(21%) | – |
| Stage III | 111(59%) | – |
| Missing | 24(13%) | – |
| International stage at diagnosis | ||
| Stage I | 34(18%) | – |
| Stage II | 26(14%) | – |
| Stage III | 17(9%) | – |
| Missing | 110(59%) | – |
| Karnofsky score before AHCT2 | ||
| ≥90% | 99(53%) | |
| Transplantation related | ||
| Serum albumin before AHCT | ||
| <3.5 g/L | 49(26%) | 59(32%) |
| Serum creatinine before transplantation | ||
| ≥1.5 mg/dL | 13(7%) | 25(13%) |
| Conditioning regimen for transplantation | ||
| Melphalan alone | 149(80%) | 158(84%) |
| Melphalan + TBI ± others | 10(5%) | 4(2%) |
| Melphalan ± others | 9(5%) | 17(9%) |
| TBI (no melphalan) ± others | 5(3%) | 2(1%) |
| Busulfan + cyclophosphamide ± others |
12(6%) | 5(3%) |
| Others | 2(1%) | 1(<1%) |
| Disease status before transplantation | ||
| CR/PR | 153(82%) | 74(40%) |
| MR/NR/SD | 21(11%) | 86(46%) |
| Relapse/progression | 1(<1%) | 27(14%) |
| Missing | 12(6%) | – |
| Cytogenetics at any time before transplantation | ||
| Abnormal | – | 27(14%) |
| Normal | – | 84(45%) |
| Not assessable/unknown | – | 76(41%) |
| Time from AHCT1 to AHCT2, median (range), mo |
– | 32(6-122) |
| 6-12 | – | 22(12%) |
| 12-23 | – | 36(19%) |
| 24-35 | – | 51(27%) |
| ≥36 | – | 78(42%) |
| Time from AHCT1 to relapse/ progression, median (range), mo |
18(3-121) | – |
| <6 | 33(18%) | – |
| 6-11 | 27(14%) | – |
| 12-23 | 55(29%) | – |
| 24-35 | 36(19%) | – |
| ≥36 | 36(19%) | – |
| Year of transplantation | ||
| 1990-1994 | 7(4%) | – |
| 1995-2000 | 50(27%) | 18(10%) |
| 2001-2002 | 50(27%) | 18(10%) |
| 2003-2004 | 38(20%) | 35(19%) |
| 2005-2006 | 36(19%) | 53(28%) |
| 2007-2008 | 6(3%) | 63(34%) |
| Post transplantation | ||
| Best response reported after AHCT | ||
| CR | 81(43%) | 47(25%) |
| PR | 73(39%) | 81(43%) |
| MR | 6(3%) | 11(6%) |
| NR/SD | 20(11%) | 30(16%) |
| Progression | 5(3%) | 18(10%) |
| Missing | 2(1%) | – |
| Maintenance therapy | ||
| None | – | 143(71) |
| Imid (lenalidomide, thalidomide) |
– | 22(11) |
| Interferon/interleukin-2 | – | 14(7) |
| Steroid | – | 10(5) |
| Bortezomib | – | 9(5) |
| Others (cyclophosphamide, melphalan) |
– | 3(2) |
| Median follow-up of survivors, (range), mo |
– | 47(3-97) |
CR indicates complete response; PR, partial response; MR, minimal response; NR, no response; SD, stable disease; TBI, total body irradiation.
Peripheral blood progenitor cell grafts were collected before AHCT1 in all patients, and no use of remobilized grafts after AHCT1 was reported. High-dose melphalan was used as a preparative regimen in 84%, whereas the use of total body irradiation–based regimens was minimal (3%). The number of reported salvage AHCT2 increased within the time frame of the collected data: 18 transplantations each were reported during the years 1995 to 2000 and 2001 to 2002, 35 transplantations occurred during 2003 to 2004, 53 transplantations were recorded during 2005 to 2006, and 63 transplantations occurred during 2007 to 2008.
Safety and Relapse
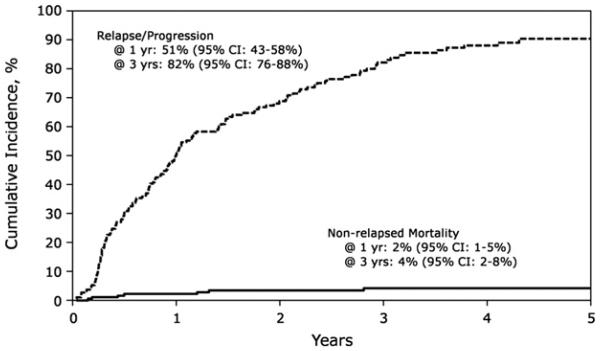
Figure 1 shows the cumulative incidence of NRM and relapse/progression. The incidence of NRM after AHCT2 was 2% (95% confidence interval [CI], 1%-5%) at 1 year and 4% (95% CI, 2%-8%) at 3 years, respectively. Of the 187 patients, 10 died from NRM, including infection (n = 4), organ failure (n = 4), and second malignancy (n = 2). The two reported second malignancies were breast cancer and myelodysplastic syndrome. Engraftment rate of neutrophils (absolute neutrophil count ≥500/mm3 for 3 subsequent days) and platelets (platelet count ≥20,000/mm3 for 7 subsequent days without platelet transfusion) at 28 days was 96% (range, 93% to 98%) and 88% (range, 83% to 92%), respectively.
Figure 1.
Cumulative incidence of relapse/progression and NRM after salvage AHCT2.
Cumulative incidence of relapse/progression after AHCT2 is shown in Figure 1. Incidence of relapse/progression was 51% (95% CI, 43%-58%) at 1 year, 82% (95% CI, 76%-88%) at 3 years, and 91% (95% CI, 85%-95%) at 5 years. In multivariate analysis (Table 2), a longer interval from AHCT1 to initial relapse (≥36 months) was associated with a lower risk of relapse/progression after AHCT2 (relative risk, .63; 95% CI, .49-.97). In individuals with a greater than 36-month interval between AHCT1 and initial relapse/progression, the incidence of relapse/progression was 41% (95% CI, 25%-57%) at 1 year, 68% (95% CI, 51%-82%) at 3 years, and 81% (95% CI, 65%-93%) at 5 years after AHCT2.
Table 2.
Multivariate Analysis of Risk Factors for Relapse/Progression, Treatment Failure (Inverse of PFS), and OS
| Outcome | n | HR | 95% CI | P Value |
|---|---|---|---|---|
| Relapse/progression | ||||
| Time from AHCT1 to REL | ||||
| ≥36 mo | 36 | 1 | ||
| <36 mo | 151 | 1.58 | (1.03-3.41) | .036 |
| Treatment failure/PFS | ||||
| Time from AHCT1 to REL | ||||
| ≥36 mo | 36 | 1 | ||
| <36 mo | 151 | 1.52 | (1.01-2.30) | .045 |
| Overall mortality/survival | ||||
| Time from AHCT1 to REL | ||||
| ≥36 mo | 36 | 1 | ||
| <36 mo | 151 | 1.91 | (1.12-3.28) | .019 |
| Year of AHCT2 | ||||
| 1995-2004 | 100 | 1 | ||
| 2005-2008 | 87 | .61 | (.40-.94) | .026 |
HR indicates hazard ratio; REL, Relapse.
PFS and OS
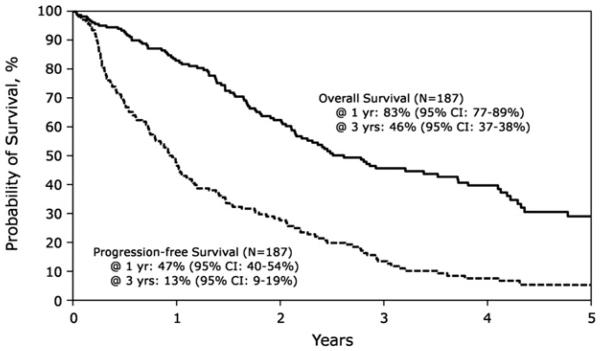
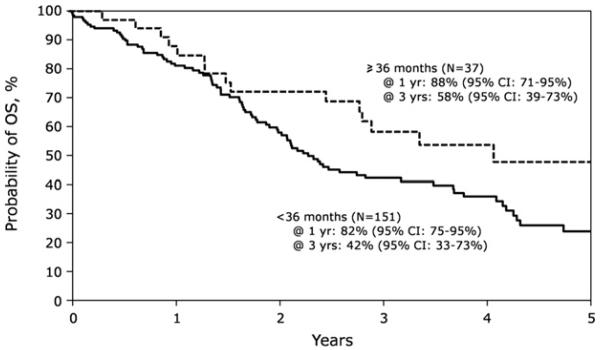
The 1-, 3-, and 5-year PFS after AHCT2 was 47% (95% CI 40% to 54%), 13% (95% CI 9% to 19%), and 5% (95% CI 2% to 11%), respectively. The OS at 1 year was 83% (95% CI, 77%-89%), whereas it was 46% (95% CI, 37%-54%) and 29% (95% CI, 21%-38%) at 3 and 5 years, respectively (Figure 2). In multivariate analysis, a longer interval from AHCT1 to relapse/ progression (≥36 months) was associated with superior PFS and OS (Figure 3). For those relapsing ≥36 months after AHCT1, the PFS at 1, 3, and 5 years was 59% (95% CI, 41%-74%), 26% (95% CI, 13%-41%), and 13% (95% CI, 4%-28%), respectively. Corresponding OS was 88% (95% CI, 71%-95%) at 1 year, 58% (95% CI, 39%-73%) at 3 years, and 48% (95% CI, 28%-65%) at 5 years. OS stratified by the time from AHCT1 to relapse/ progression is shown in Figure 3. AHCT2 performed after 2004 was associated with superior survival (relative risk, .61; 95% CI, .4-.94).
Figure 2.
Probability of PFS and OS after AHCT2.
Figure 3.
Probability of OS after AHCT2 stratified by time to relapse/ progression from AHCT1.
DISCUSSION
AHCT is used as an up front or salvage treatment for patients with MM [17]. AHCT2 has also been used to salvage patients relapsing after an initial (up front) AHCT [18]. Current National Comprehensive Cancer Network guidelines recommend that AHCT-eligible patients with MM undergo leukopheresis with the intent to collect enough peripheral blood progenitor cell to undergo two AHCTs, in which case the second graft can be cryopreserved for use as salvage AHCT2 [17].
There is considerable heterogeneity in practice patterns, and no current standard of care exists as to whether or when to implement ACHT2 for relapsed/progressive myeloma. ACHT2 is a distinct treatment strategy compared with tandem AHCT, which is defined as two planned cycles of high-dose therapy with peripheral blood progenitor cell support in which a second AHCT is performed within 180 days of the first, with the objective of increasing the likelihood of a complete or very good partial remission [19]. Inconsistent results have been reported by the studies randomizing patients to single versus tandem AHCT [19-22]. The most recent National Comprehensive Cancer Network guidelines are not prescriptive regarding tandem transplantation [17].
Notably, many third-party payers, including Centers for Medicare and Medicaid Services in the United States, reimburse for a single AHCT only (National coverage determination for stem cell transplantation, Centers for Medicare and Medicaid Services, Manual section number 110.8.1. http://www.cms.gov/medicare-coverage-database. Accessed 4/30/ 2012).
Our data provide an estimate of the effectiveness of AHCT2 as salvage therapy in a select group of patients and suggest that it is safe with a relatively low NRM (4%). NRM rates are similar to up front AHCT in reported studies [20,23,24] and previously reported rates after second salvage transplantation [9-15]. About 29% of patients were alive at 5 years, and 25% of these pretreated patients achieved a complete remission after AHCT2. Thus, AHCT2 at first relapse/progression may harness the advantage of durable remission and preserves the option of using other therapies for subsequent relapses/progressions.
We summarize in Table 3 some of the comparable published retrospective analyses of salvage autologous transplantation in patients with MM [9,10,12,14,15]. The most consistent finding among these studies is that a longer progression-free interval after AHCT1 predicts improved survival after AHCT2. In our analysis, patients progressing later than 36 months after AHCT1 had a median OS after AHCT2 of 49 months (95% CI, 34-108) compared with a median OS of 28 months (95% CI, 24-42) in patients who had a shorter progression-free interval after AHCT1. Other published retrospective studies summarized in Table 3 have correlated better outcomes in patients who relapse/progress more than 2 years after ACHT19,10,12,15. It may be that patients who relapse/progress within 2 to 3 years of AHCT1 would be better served by participation in clinical trials rather than a second high-dose melphalan AHCT, although confirmation of this hypothesis would require a randomized study.
Table 3.
Comparison of Our Data with the Major Studies That Evaluated Outcome of Second Salvage AHCT for Myeloma
| CIBMTR (our data) | Toronto/Princess Margaret [10] |
MD Anderson [14] | University of Pennsylvania [12] |
S. Texas VA [9] | Germany [15] | |
|---|---|---|---|---|---|---|
| Year published | 2011 | 2011 | 2009 | 2009 | 2011 | |
| Number of patients | 187 | 81 | 44 | 41 | 25 | 55 |
| Years inclusive | 1995-2008 | 1992-2009 | 1992-2008 | 1998-2007 | 1999-2007 | 1993-2008 |
| Interval between ASCT1 and ASCT2 (range) |
32 (6-122) | NR | 30 (2-78) | 37 (3-91) | 39 (4-74) | |
| Post-ASCT outcomes | ||||||
| CR | 25% | 7.7% | 20% | 9% | ||
| CR/VGPR | 11% | 16%(6/38 cases) | 9% | |||
| VGPR | 39.7% | |||||
| PR | 43% | 50% | 79% | 44% | 56% | |
| ORR | 97% (day 100) | 90% | 55% (21/38 cases) | 64% | 85% | |
| NRM | 4% (5 yr) | 2.6% (100 days) | 2% (100 days) | 7% (100 d) | 8% | 5% (100 d) |
| Median PFS post AHCT2, mo | 11.2 | 16.4 | 12.3 | 8.5 | 12 | EFS: 14 |
| Median OS post AHCT2, mo | 30 | 53 | 31.7 | 20.7 | 19 | 52 |
| Multivariate analysis | Improved OS if interval between AHCT1 relapse ≥36 mo and for AHCT2 after 2004 |
Improved PFS and OS if interval between AHCT1 and AHCT2 >24 mo |
Worse OS associated with AA race, shorter TTP after AHCT1, IgG, and increased number of prior therapies |
Worse outcomes if >4 prior therapies, TTP after AHCT1 ≥12 mo |
NR | Improved outcomes if remission >12 mo after AHCT1 |
VA indicates Veterans Administration; CR, complete response; VGPR, very good partial response; PR, partial response; EFS, event-free survival; AA, African American; TTP, time to progression; IgG, immunoglobulin G; NR, not recorded.
Aiming to understand the optimal timing of a second autologous transplantation, researchers with the European Group for Blood and Marrow Transplantation looked at 7,452 patients, of which 2,655 had an up front planned AHCT2, and 4,797 had unplanned AHCT2 in the years between 1993 and 2002. They found superior outcomes when AHCT2 was performed before relapse (within 6 to 12 months of AHCT1) [25]. In this study, the median OS (from AHCT1) of the unplanned AHCT2 was 51 months. This is not to be confused with our OS of 30 months from AHCT2. This is one of the largest retrospective studies addressing the timing of a second transplantation, but it was performed before the advent of novel agents and aims to answer a different question from that proposed here.
In our multivariate analyses, disease status and chemotherapy sensitivity before AHCT2 did not affect outcomes, because at the time of AHCT2, 46% of patients were in less than a partial remission. Given that the cohort spans 15 years, these data were largely accumulated before the relatively recent era of maintenance therapy after AHCT1. Recently published, high-quality, prospective data demonstrate improvement in PFS and OS when lenalidomide is initiated after AHCT1 [26,27]. Given that the use of novel agents as maintenance therapy improve PFS after AHCT1, it is likely that in the modern era, a greater proportion of relapses/progressions after AHCT1 may be beyond the 36-month interval identified. It is unclear whether the same benefits of AHCT2 would be preserved in patients who receive maintenance after AHCT1. Survival has improved since 2004, although NRM and PFS after AHCT2 were not different. We hypothesize that the improved OS seen in patients who underwent transplantation after 2004 is due to the use of novel agents in treatment of relapse/progression after AHCT2.
MM is as twice as common among African Americans than white Americans [28]. In the US population, about 13% of individuals identify themselves in census rolls as African American/black [29]. The percentage of African Americans who underwent AHCT2 in our study is 12%—which is less than what might be expected. Previous analyses have shown that African Americans are less likely to have access to AHCT1 as a treatment for MM [30], although the outcome after AHCT is similar among blacks and whites [31]. The reasons for this disparity are unclear and probably multifactorial.
Limitations inherent to our analyses include its retrospective nature and incomplete data on maintenance therapy and on modern prognostic factors, such as cytogenetics and International Staging System (ISS) stage. These limitations are because the reported time period predates newer maintenance therapies, ISS staging, and risk stratification. Information about new cytogenetic or molecular characteristics of disease at the time of AHCT2 was unavailable. Retrospective data collection also meant that the dataset includes only patients who actually received an AHCT2. We were not able to determine the characteristics of patients who were excluded on the basis of nonavailability of a graft or other factors such as rapid relapse/progression, poor performance status, age, insurance status, comorbidities, or patient/provider preference. This may account for the relatively low numbers of overall AHCT2 recorded by the registry, and it limits the applicability of the findings compared with prospective data. All AHCT2 were performed using hematopoietic cells collected and stored before AHCT1 and is consistent with current clinical practice recommendations.
These data provide support for the use of late second AHCT in patients with relapsed/progressive MM. We also underscore the need for randomized studies looking at therapies after relapse/progression comparing available options, including AHCT2, chemotherapy combinations, and allogeneic transplantations. One particular unmet need is identifying investigational strategies in patients who have early progression after AHCT1: Do these individuals benefit from newer induction or conditioning regimens? An ongoing phase III clinical trial (myeloma X) in the United Kingdom enrolls patients who relapse/progress at least 18 months after AHCT1 to receive reinduction with bortezomib, doxorubicin, and dexamethasone and randomizes them to AHCT2 versus low-dose maintenance cyclophosphamide (see www.clinicaltrials.gov, NCT00747877). Prospective clinical trials like these are needed to define the risk-to-benefit ratio as well as the placement and sequencing of AHCT2 relative to other therapies.
ACKNOWLEDGMENTS
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Financial disclosure: The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/ DHHS); two grants, N00014-06-1-0704 and N00014-08-1-0058, from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc.
Footnotes
This study was presented as an “oral abstract” at the 44th annual meeting of the American Society of Hematology, San Diego, CA, December 10-13, 2011.
Conflict of interest statement: There are no conflicts of interest to report.
REFERENCES
- 1.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Moreau P, Avet-Loiseau H, Harousseau JL, Attal M. Current trends in autologous stem-cell transplantation for myeloma in the era of novel therapies. J Clin Oncol. 2011;29:1898–1906. doi: 10.1200/JCO.2010.32.5878. [DOI] [PubMed] [Google Scholar]
- 4.Koreth J, Cutler CS, Djulbegovic B, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13:183–196. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saad AA, Sharma M, Higa GM. Treatment of multiple myeloma in the targeted therapy era. Ann Pharmacother. 2009;43:329–338. doi: 10.1345/aph.1L428. [DOI] [PubMed] [Google Scholar]
- 8.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burzynski JA, Toro JJ, Patel RC, et al. Toxicity of a second autologous peripheral blood stem cell transplant in patients with relapsed or recurrent multiple myeloma. Leuk Lymph. 2009;50:1442–1447. doi: 10.1080/10428190903085936. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez-Zepeda VH, Mikhael J, Winter A, et al. Second autologous stem cell transplantation as salvage therapy for multiple myeloma: impact on progression-free and overall survival. Biol Blood Marrow Transplant. 2012;18:773–779. doi: 10.1016/j.bbmt.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 11.Mehta J, Tricot G, Jagannath S, et al. Salvage autologous or allogeneic transplantation for multiple myeloma refractory to or relapsing after a first-line autograft? Bone Marrow Transplant. 1998;21:887–892. doi: 10.1038/sj.bmt.1701208. [DOI] [PubMed] [Google Scholar]
- 12.Olin RL, Vogl DT, Porter DL, et al. Second auto-SCT is safe and effective salvage therapy for relapsed multiple myeloma. Bone Marrow Transplant. 2009;43:417–422. doi: 10.1038/bmt.2008.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qazilbash MH, Saliba R, De Lima M, et al. Second autologous or allogeneic transplantation after the failure of first autograft in patients with multiple myeloma. Cancer. 2006;106:1084–1089. doi: 10.1002/cncr.21700. [DOI] [PubMed] [Google Scholar]
- 14.Shah N, Ahmed F, Bashir Q, et al. Durable remission with salvage second autotransplants in patients with multiple myeloma. Cancer. 2012;118:3549–3555. doi: 10.1002/cncr.26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenk R, Liese V, Neubauer F, et al. Predictive factors for successful salvage high-dose therapy in patients with multiple myeloma relapsing after autologous blood stem cell transplantation. Leuk Lymph. 2011;52:1455–1462. doi: 10.3109/10428194.2011.575967. [DOI] [PubMed] [Google Scholar]
- 16.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 17.Anderson KC, Alsina M, Bensinger W, et al. Multiple myeloma. JNCCN. 2011;9:1146–1183. doi: 10.6004/jnccn.2011.0095. [DOI] [PubMed] [Google Scholar]
- 18.Larocca A, Palumbo A. Evolving paradigms in the treatment of newly diagnosed multiple myeloma. JNCCN. 2011;9:1186–1196. doi: 10.6004/jnccn.2011.0096. [DOI] [PubMed] [Google Scholar]
- 19.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 20.Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–2441. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 21.Kumar A, Kharfan-Dabaja MA, Glasmacher A, Djulbegovic B. Tandem versus single autologous hematopoietic cell transplantation for the treatment of multiple myeloma: a systematic review and meta-analysis. JNCI. 2009;101:100–106. doi: 10.1093/jnci/djn439. [DOI] [PubMed] [Google Scholar]
- 22.Sonneveld P, van der Holt B, Segeren CM, et al. Intermediate-dose melphalan compared with myeloablative treatment in multiple myeloma: long-term follow-up of the Dutch Cooperative Group HOVON 24 trial. Haematologica. 2007;92:928–935. doi: 10.3324/haematol.11168. [DOI] [PubMed] [Google Scholar]
- 23.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. [PubMed] [Google Scholar]
- 24.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 25.Morris C, Iacobelli S, Brand R, et al. Benefit and timing of second transplantations in multiple myeloma: clinical findings and methodological limitations in a European Group for Blood and Marrow Transplantation registry study. J Clin Oncol. 2004;22:1674–1681. doi: 10.1200/JCO.2004.06.144. [DOI] [PubMed] [Google Scholar]
- 26.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem--cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6:225–247. [PubMed] [Google Scholar]
- 29.Rastogi S, Johnson TD, Hoeffel EM, Drewery JMB, U.S. Census Bureau The Black Population: 2010. Available at: http://wwwcensusgov/prod/cen2010/briefs/c2010br-06pdf 2010.
- 30.Joshua TV, Rizzo JD, Zhang MJ, et al. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer. 2010;116:3469–3476. doi: 10.1002/cncr.25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hari PN, Majhail NS, Zhang MJ, et al. Race and outcomes of autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2010;16:395–402. doi: 10.1016/j.bbmt.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]