Abstract
Background and Aims
Metabolic syndrome (MetS) is common among kidney transplant patients. We studied the relationship between MetS, vitamin D deficiency/insufficiency and hypoadiponectinemia early post-transplantation and their impact on clinical outcomes.
Methods
Seventy-four previously non-diabetic kidney transplant patients were enrolled in a prospective cohort study between February and November 2008. Participants underwent a 2-hours oral glucose tolerance test (OGTT) and had their plasma levels of 25-hydroxyvitamin D (25[OH]D), adiponectin, insulin, intact parathyroid hormone and lipids measured at 11 weeks after transplantation. Clinical events including cardiovascular events, new onset diabetes after transplantation, acute rejection, graft loss and death were recorded during the follow-up to December 2012.
Results
Thirty-four study patients (45.9%) had MetS. Patients with MetS had lower plasma concentrations of 25[OH]D (20.5±7.2 vs. 24.8±11.1 ng/ml, p=0.049) and adiponectin (8.2±4.5 vs. 14.6±8.0 μg/ml, p<0.0001) early on, and higher composite clinical event rate (61.8% vs. 27.5%, p=0.003) during the follow-up. Multivariate analysis showed that the presence of MetS early after transplantation was independently associated with 25[OH]D insufficiency/deficiency (OR 14.0, 95% CI 1.8, 107.5, p=0.011), depressed plasma adiponectin levels (β -6.39, r2 0.195, p<0.0001) and increased risk for clinical events (OR 5.6, 95% CI 1.9, 16.5, p=0.002).
Conclusion
Kidney transplants patients with MetS early after transplantation had lower levels of 25[OH]D and adiponectin, and unfavorable clinical outcomes.
Keywords: adiponectin, clinical outcomes, kidney transplantation, metabolic syndrome, vitamin D deficiency
Introduction
Metabolic syndrome (MetS) is a constellation of metabolic and non-metabolic abnormalities frequently seen among kidney transplant patients (1, 2). The presence of MetS is associated with inferior transplant outcomes including increased risks of new onset diabetes mellitus after transplantation (NODAT) and cardiovascular events (CVEs), and diminished kidney transplant and patient survival (1-4).
Vitamin D deficiency and insufficiency are common in both the general and kidney transplant population and are associated with an increased risk of diabetes mellitus and cardiovascular diseases and MetS (5-8). Adiponectin is a protein secreted exclusively by adipocytes and plays an important role in modulating insulin sensitivity, thus the risk of type 2 diabetes (T2D) (9). Low levels of adiponectin have been also associated with glucose intolerance, T2D, early CVEs and MetS (10-14). It is not known whether there is any relationship between vitamin D deficiency/insufficiency and reduced adiponectin levels. We performed a prospective cohort study to elucidate the relationship between vitamin D deficiency/insufficiency, hypoadponectinemia, and MetS early after kidney transplantation, as well as their impact on overall clinical outcomes among previously non-diabetic kidney transplant patients.
Materials and Methods
Between February and November 2008, de novo kidney transplant patients without a history of diabetes prior to transplantation were enrolled in a prospective cohort study. Study participants underwent a protocol driven 2-hours oral glucose tolerance test (OGTT) between week 10-14 post-transplantation (15). Fasting blood plasma was collected and stored at -80°C. The local Institutional Review Board approved the study.
Anthropometric parameters, baseline demographic and clinical information obtained at the time that the OGTT was performed include height, body weight and waist circumference, body mass index, blood pressure readings and the numbers of anti-hypertensive medications, age, gender, racial/ethnicity, renal diagnosis, family history of T2D, previous history of cardiovascular disease, the use of dialysis modalities and the duration of dialysis prior to transplantation, type of kidney transplant, number of kidney transplant, status of hepatitis C serology, the presence of delayed graft function, maintenance immunosuppression regimens and cumulative doses of corticosteroids and calcinuerin inhibitors (CNIs) up to the time of OGTT. During subsequent follow-up to December 31, 2012, the clinical events including the occurrence of CVEs, NODAT, acute rejection (AR), and kidney graft loss (including death) were documented, and final transplant function, expressed as estimated glomerular filtration rate (eGFR) using abbreviated Modification of Diet in Renal Disease (aMDRD) formula, were recorded.
Metabolic syndrome (MetS) was defined according to the criteria established by the National Cholesterol Education Expert Panel III (NCEP-III) and present if a participant had at least three of following five components: fasting glucose levels of 100 mg/dl or greater, fasting triglyceride values of 150 mg/dl or greater, HDL cholesterol (HDL-C) levels of less than 40 mg/dl for males or 50 mg/dl for females, blood pressure equal or greater than 130/85 mmHg or on antihypertensive medications, and waist circumference equal or greater than 40 and 35 inches for males and females, respectively (16).
The measurement of 25-hydroxylvitamin D (25[OH]D), adiponectin, intact parathyroid hormone (iPTH), and insulin was carried out in fasting plasma through the chemistry laboratory of Michigan Diabetes Research and Training Center (MDRTC) (see acknowledgment) using commercialized kits. In brief, radioimmunoassay (RIA) technique was implemented for 25[OH]D (DiaSorin, Stillwater, Minnesota, USA), adiponectin (Millipore, Billerica, Massachusetts, USA) and insulin (Linco Research Inc. St. Charles, Missouri, USA), and chemiluminescent enzyme-labeled immunometric assay for iPTH (Siemens Healthcare Diagnostics Inc. Tarrytown, New York, USA). Insulin resistance (IR) was calculated using the homeostasis model assessment (HOMA) as HOMA-IR value ([fasting glucose level in mg/dl × fasting insulin level in μU/l]/405) (17).
Continuous and categorical variables were compared using student t-test and chi-square test, respectively. Multivariate regression analyses were utilized to test association between the presence of MetS and various measured parameters including plasma 25[OH]D and adiponectin levels among others, and to correlate them with observed clinical outcomes (composite). All statistical analyses were performed using SAS 9.3. Statistical significance was set at p≤0.05.
Results
There were 74 study participants with a median follow-up of 4.27 years (3.99, 4.46). The mean age of was 46±16 years, fifty (67.6%) were male patients and 22 (29.7%) of African American race. The prevalence of MetS was 45.9% (n=34) at a median time of 79 days (71, 89) post-transplantation.Table 1 displayed the demographic and baseline characteristics of study participants with and without MetS. Participants with MetS had higher BMI (29.5±5.7 vs. 26.2±4.8, p=0.01), were less likely to have received a living donor kidney transplant (38.2% vs. 60.0%, p=0.06), and received slightly higher cumulative doses of CNIs (CsA: 41221±13665 vs. 35513±11142 mg, p=0.07; Tacro: 743±250 vs. 436±66 mg, p=0.05). The two groups of participants were otherwise similar with regard to age, gender, racial identity, the numbers of transplant, family history of T2D, positive hepatitis serology, smoking history, renal disease diagnosis, dialysis modalities and duration, fasting glucose levels prior to transplantation, the use of different calcineurin inhibitors, cumulative doses of corticosteroids, and the presence of delayed graft function,
Table 1. Demographic and baseline parameters.
| Variables | Metabolic Syndrome | p | |
|---|---|---|---|
|
| |||
| Yes (34) | No (40) | ||
| Age, years (SD) | 47.5 (13.9) | 44.1 (15.0) | 0.32 |
| Gender male, n (%) | 24 (70.6) | 26 (65.0) | 0.61 |
| Race AA, n (%) | 9 (26.4) | 13 (32.5) | 0.57 |
| BMI, kg/m2 (SD) | 29.5 (5.7) | 26.2 (4.8) | 0.01 |
| Living donor transplant, n (%) | 13 (38.2) | 24 (60.0) | 0.06 |
| First transplant, n (%) | 29 (85.3) | 36 (90.0) | 0.72 |
| Positive HCV serology, n (%) | 3 (8.8) | 3 (7.5) | 1.00 |
| Family history for T2D, n (%) | 10 (29.4) | 7 (17.5) | 0.22 |
| Smoking history, n (%) | 0.89 | ||
| Never | 21 (61.8) | 24 (60.0) | |
| Former | 10 (29.4) | 11 (27.5) | |
| Current | 3 (8.8) | 5 (12.5) | |
| Renal diagnosis, n (%) | 0.49 | ||
| APKD | 5 (15.2) | 5 (12.5) | |
| Glomerular disease | 17 (51.5) | 14 (35.0) | |
| Hypertension | 6 (17.7) | 9 (22.5) | |
| Others | 6 (18.2) | 12 (30.0) | |
| Dialysis modalities, n (%) | 0.65 | ||
| None | 7 (20.6) | 12 (30.0) | |
| HD | 5 (14.7) | 5 (12.5) | |
| PD | 22 (64.7) | 23 (57.5) | |
| Dialysis duration, years (SD) | 3.9 (3.0) | 2.9 (2.5) | 0.17 |
| Fasting glucose at transplant, mg/dl (SD) | 86.2 (9.0) | 84.6 (8.7) | 0.46 |
| CNIs: CsA, n (%) | 30 (88.3) | 33 (82.5) | 0.64 |
| Cumulative CNI doses, mg (SD) | |||
| CsA | 41221 (13665) | 35513 (11142) | 0.07 |
| Tacro | 753 (64) | 436 (66) | 0.06 |
| Cumulative prednisone doses, mg (SD) | 2521 (466) | 2428 (286) | 0.30 |
| Delayed graft function, n (%) | 5 (14.7) | 5 (12.5) | 0.78 |
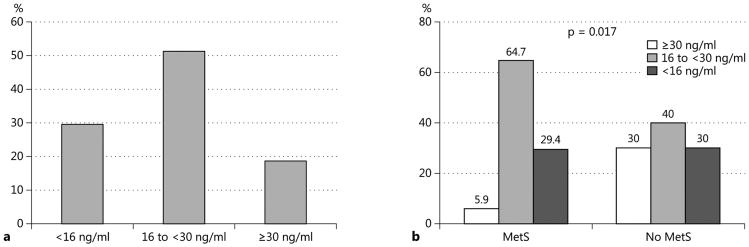
The results of the levels of 25[OH]D during the third post-transplant months are as follows: 60 (81.1%) had inadequate levels: 38 (51.4%) insufficient (16 to <30 ng/ml) and 22 (29.7%) deficient (<16 ng/ml) (Figure 1a) (18). There was a significant inverse correlation between 25[OH]D and iPTH levels (Pearson correlation coefficients -0.3408, r2 0.116, p<0.0001). Compared to patients without MetS, patients with MetS displayed significantly lower levels of 25[OH]D (20.5±7.2, vs. 24.8±11.1 ng/ml, p=0.049) with more patients having deficient/insufficient levels of 25[OH]D (64.7% vs. 40.0%, p=0.017) (Figure 1b). Stepwise multivariate logistical regression analysis demonstrated that, in addition to the independent negative association of MetS with 25[OH]D deficiency/insufficiency (OR 14.0, 95% CI 1.8, 107.5, p=0.011), a history of pre-transplant dialysis and/or a longer duration of dialysis were also independently associated with 25[OH]D deficiency/insufficiency (OR 5.2, 95% CI 1.2, 21.9, p=0.026, and/or OR 3.3, 95% CI 1.3, 8.1, p=0.010, respectively). We explored further the relationship between five elements of MetS and 25[OH]D status. None of the individual component criterion of MetS but a history of dialysis and/or the longer duration of dialysis were associated with 25[OH]D deficiency/insufficiency (data not shown).
Fig. 1.
25[OH]D status: among study cohort (a) and according to the presence or not of MetS (b).
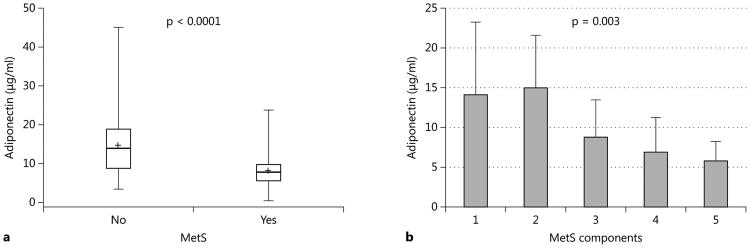
Adiponectin, an adipose-tissue derived plasma protein, has been implicated in the homeostasis of glucose metabolism and insulin resistance (10, 11). We measured plasma adiponectin levels among the study participants. Patients with MetS had significantly lower levels of plasma adiponectin (8.2±4.5 μg/ml) compared to patients without MetS (14.6±8.0 μg/ml) (p<0.0001) (Figure 2a). Stepwise multivariate regression analysis confirmed a statistically significant negative association between either the presence of MetS or the numbers of MetS components, and plasma adiponectin concentration (β estimate -6.39 and -3.27, r2 0.195 and 0.163, p<0.0001 and p=0.0004, respectively) (Figure 2b). Other variables associated with low plasma adiponectin concentration included male gender and African American race (β estimate -4.29 and -3.25, r2 0.273 and 0.315, p=0.007 and 0.042, respectively). Among the five components of MetS, only HDL-C levels and waist circumference were individually correlated with the plasma concentration of adiponectin (β estimate and r2: 0.154 and 0.164 for HDL-C with p=0.0004, -0.305 and 0.232 for waist circumference with p=0.014, respectively). However, there was no association between the plasma levels of 25[OH]D and those of adiponectin. The use of immunosuppressive drugs, neither the type of CNIs nor the cumulative doses of CNIs and corticosteroids, had no effect on the plasma concentration of adiponectin (data not shown).
Fig. 2.
Plasma adiponectin levels: between patients with and without MetS (a) and among patients with varying MetS components (b).
Insulin resistance (IR), measured as HOMA-IR, is considered the hallmark of MetS (19). We found HOMA-IR higher among the participants with than without MetS (7.2±5.8 vs. 5.7±5.8) though the difference was not statistically significant in either univariate or multivariate analysis (p=0.28 and p=0.11, respectively). Other laboratory parameters were shown in Table 2. Although there was no difference in the levels of glycosylated hemoglobin, the 2 hours post challenge glucose levels were considerably higher among the study participants with than without MetS (133.5±45.0 vs. 112.9±35.1 mg/dl, β estimate 18.63, r2 0.037, p=0.056).
Table 2. Clinical information and laboratory parameters at the time of OGTT.
| Metabolic syndrome | p | ||
|---|---|---|---|
|
| |||
| Yes (34) | No (40) | ||
| Winter season, n (&percnt);) | 11 (32.4) | 8 (20.0) | 0.23 |
| Vitamin D supplementation, n (%) | 4 (11.8) | 2 (5.0) | 0.40 |
| Calcium supplementation, n (%) | 1 (2.9) | 6 (15.0) | 0.12 |
| 25[OH]D, ng/ml (SD) | 20.5 (7.2) | 24.8 (11.1) | 0.049 |
| iPTH, pg/ml (SD) | 148.4 (186) | 95.5 (56.2) | 0.12 |
| Serum calcium, mg/dl (SD) | 9.6 (0.6) | 9.6 (0.7) | 0.90 |
| Serum adiponectin, μg/ml (SD) | 8.2 (4.5) | 14.6 (8.0) | <0.0001 |
| Fasting glucose, mg/dl (SD) | 100.7 (13.7) | 91.8 (11.2) | 0.004 |
| Fasting insulin, μU/ml (SD) | 29.1 (23.3) | 24.6 (22.8) | 0.40 |
| HOMA-IR, (SD) | 7.2 (5.8) | 5.7 (5.8) | 0.28 |
| 2-hours OGTT glucose, mg/dl (SD) | 133.5 (45.0) | 112.9 (35.1) | 0.03 |
| Glycated hemoglobin 1c, A1c (%) | 5.5 (0.6) | 5.4 (0.5) | 0.25 |
| Uric acid, mg/dl (SD) | 7.1 (1.2) | 6.2 (1.6) | 0.01 |
| Use of allopurinol, n (%) | 5 (14.7) | 5 (12.5) | 0.78 |
| Triglyceride, mg/dl (SD) | 224.8 (94.9) | 127.3 (49.6) | <0.001 |
| HDL cholesterol, mg/dl (SD) | 45.4 (13.5) | 57.0 (16.8) | 0.002 |
| LDL cholesterol, mg/dl (SD) | 131.1 (35.0) | 117.1 (44.5) | 0.13 |
| Use of statins, n (%) | 5 (14.7) | 5 (12.5) | 0.78 |
| Systolic BP, mmHg (SD) | 132.2 (17.5) | 134.6 (16.4) | 0.55 |
| Diastolic BP, mmHg (SD) | 76.0 (9.2) | 77.3 (9.2) | 0.54 |
| Pulse pressure, mmHg (SD) | 56.2 (16.3) | 57.3 (15.8) | 0.78 |
| Hypertension drugs, n (SD) | 1.8 (0.9) | 1.6 (0.8) | 0.29 |
During the follow-up, 10 patients developed NODAT, 7 patients suffered CVEs, 20 patients had AR and 8 kidney grafts were lost including 2 deaths with graft function. Table 3 displayed the distribution of events between patients with and without MetS. Due to limited sample size with small numbers of individual events, we combined all patients with any event and used this as composite end-point. Patients with MetS at 11 weeks were more likely to have an event during subsequent follow-up than patients without MetS (61.8% vs. 27.5%, p=0.003). Multivariate logistic regression analysis confirmed independent association of MetS with subsequent development of an event (OR 5.6, 95% CI 1.9, 16.5, p=0.002). The plasma concentration of 25[OH]D and adiponectin, on the other hand, were not independently associated with future event risk (data not shown). Renal function was not statistically different between the groups.
Table 3. Clinical outcomes.
| Metabolic syndrome | p | ||
|---|---|---|---|
|
| |||
| Yes (34) | No (40) | ||
| CVE, n (%) | 0.24 | ||
| MI | 2 (5.9) | 0 (0.0) | |
| Angina | 2 (5.9) | 1 (2.5) | |
| CHF | 1 (2.9) | 1 (2.5) | |
| NODAT, n (%) | 8 (25.3) | 2 (5.0) | 0.04 |
| Acute rejection, n (%) | 12 (35.3) | 8 (20.0) | 0.14 |
| Graft loss, n (%) | 6 (17.6) | 2 (5.0) | 0.08 |
| Patients with any event*, n (%) | 21 (61.8) | 11 (27.5) | 0.003 |
| eGFR, ml/min (SD) | 53.9 (21.2) | 57.1 (18.7) | 0.52 |
Some patients had more than one event.
Discussion
We have previously shown that MetS is highly prevalent early after kidney transplantation among non-diabetic patients and was associated with abnormal glucose homeostasis (15). In the current study, we show that patients with MetS early post-transplantation have significantly lower plasma levels of 25[OH]D and adiponectin. However, it's the presence of MetS, but not the lower levels of 25[OH]D and adiponectin, that was independently associated with the risk of future clinical events.
MetS has been associated with increased risk of diabetes mellitus and cardiovascular events in general population. The pathogenic mechanism underlying such association includes insulin resistance due to likely the presence of low grade chronic inflammation, deficiency in certain nutritional and/or hormonal elements, such as vitamin D and adiponectin among them (8, 11, 19, 21, 22). Inadequate blood levels of vitamin D are common in general population and present among 80 to 97% of stable kidney transplant patients (5, 7, 11). Vitamin D deficiency not only cause impairment in calcium and bone metabolism, but also increased risks of many chronic medical conditions in general population (20, 21, 23). In subjects with MetS, lower levels of vitamin D predicted all-cause and cardiovascular disease mortality (24). Although our study did not find independent negative association of vitamin deficiency/insufficiency with negative clinical outcomes which could be related to the small sample size with limited follow-up, based on the magnitude of vitamin D deficiency, its association with MetS and its many putative salutary effects, the role of its supplementation should be investigated in kidney transplant patient population.
Adiponectin is adipokine that positively affects lipid and glucose metabolism(9). The plasma concentration of adiponectin is inversely correlated with the risk of impaired glucose tolerance and T2D, premature coronary artery disease, and MetS (10, 12-14, 25). Our study confirmed the previous findings of an independent association between hypoadiponectinemia and MetS. Furthermore, only two out 5 elements of MetS, HDL-C levels and waist circumference showed a significant correlation with the plasma concentration of adiponectin. However, we could not demonstrate a significant association between plasma concentration of adiponectin and insulin resistance as previously reported (13, 25). Furthermore, we also did not found influence of immunosuppressive drugs on plasma concentration of adiponectin.
Our study has several important limitations. First, it's an observational study which precluded us from drawing any cause-effect relationship between the presence of MetS and the observed laboratory and clinical parameters. Second, our study involved only a small number of patients with relatively younger age and short follow-up period which could explain the fewer cases of CVEs observed, and the lack of association between the lower levels of 25[OH]D and adiponectin and negative clinical outcomes. Alternatively, the lower levels of 25[OH]D and adiponectin could represent an epiphenomenon of MetS rather than potential mechanisms through which MetS exercises its negative clinical impact. Finally we measured only serum concentrations of total adiponectin which does not discriminate high from low-molecule-weight forms and thus we were unable to determine which form of adiponectin was associated with MetS.
In conclusion, non-diabetic kidney transplant recipients with MetS have low plasma concentration of 25[OH]D and adiponectin. The presence of MetS early after transplantation was associated with inferior clinical outcomes during relatively short follow-up period thereafter. Additional studies involving larger kidney transplant patient population and with longer follow up are needed to determine whether the reported association has an etiologic role and whether vitamin D supplementation or other therapeutic interventions can mitigate the high prevalence of MetS in the kidney transplant population, and counteract its negative impact on long-term clinical outcomes.
Acknowledgments
This work has been accepted for oral presentation at the American Transplant Congress 2012, June 2-6, Boston, MA, USA
Funding/financial support: Institutional discretional research fund, Department of Internal Medicine, University of Michigan
This work utilized the Chemistry Laboratory of the Michigan Diabetes Research and Training Center (MDRTC) funded by NIH5P60 DK20572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure: Authors declare no conflict of interest related to the performance of present research.
References
- 1.de Vries AP, Bakker SJ, van Son WJ, van der Heide JJ, Ploeg RJ, The HT, de Jong PE, Gans RO. Metabolic syndrome is associated with impaired long-term renal allograft function; not all component criteria contribute equally. Am J Transplant. 2004 Oct;4(10):1675–83. doi: 10.1111/j.1600-6143.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 2.Luan FL, Langewisch E, Ojo A. Metabolic syndrome and new onset diabetes after transplantation in kidney transplant recipients. Clin Transplant. 2010 Nov-Dec;24(6):778–83. doi: 10.1111/j.1399-0012.2009.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porrini E, Delgado P, Bigo C, Alvarez A, Cobo M, Checa MD, Hortal L, Fernandez A, Garcia JJ, Velazquez S, Hernandez D, Salido E, Torres A. Impact of metabolic syndrome on graft function and survival after cadaveric renal transplantation. Am J Kidney Dis. 2006 Jul;48(1):134–42. doi: 10.1053/j.ajkd.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 4.Courivaud C, Kazory A, Simula-Faivre D, Chalopin JM, Ducloux D. Metabolic syndrome and atherosclerotic events in renal transplant recipients. Transplantation. 2007 Jun 27;83(12):1577–81. doi: 10.1097/01.tp.0000266898.93894.3d. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008 Jun 23;168(12):1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 7.Stavroulopoulos A, Cassidy MJ, Porter CJ, Hosking DJ, Roe SD. Vitamin D status in renal transplant recipients. Am J Transplant. 2007 Nov;7(11):2546–52. doi: 10.1111/j.1600-6143.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- 8.Kayaniyil S, Vieth R, Harris SB, Retnakaran R, Knight JA, Gerstein HC, Perkins BA, Zinman B, Hanley AJ. Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. J Clin Endocrinol Metab. 2011 Jan;96(1):168–75. doi: 10.1210/jc.2010-1439. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006 Jul;116(7):1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snijder MB, Heine RJ, Seidell JC, Bouter LM, Stehouwer CD, Nijpels G, Funahashi T, Matsuzawa Y, Shimomura I, Dekker JM. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: the hoorn study. Diabetes Care. 2006 Nov;29(11):2498–503. doi: 10.2337/dc06-0952. [DOI] [PubMed] [Google Scholar]
- 11.Tajtakova M, Petrasova D, Petrovicova J, Pytliak M, Semanova Z. Adiponectin as a biomarker of clinical manifestation of metabolic syndrome. Endocr Regul. 2006 Mar;40(1):15–9. [PubMed] [Google Scholar]
- 12.Lee MC, Lee CJ, Chou KC, Shih MH, Hsu BG. Hypoadiponectinemia correlates with metabolic syndrome in kidney transplantation patients. Transplant Proc. 2011 Sep;43(7):2601–5. doi: 10.1016/j.transproceed.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Hjelmesaeth J, Flyvbjerg A, Jenssen T, Frystyk J, Ueland T, Hagen M, Hartmann A. Hypoadiponectinemia is associated with insulin resistance and glucose intolerance after renal transplantation: impact of immunosuppressive and antihypertensive drug therapy. Clin J Am Soc Nephrol. 2006 May;1(3):575–82. doi: 10.2215/CJN.01471005. [DOI] [PubMed] [Google Scholar]
- 14.Filippi E, Sentinelli F, Romeo S, Arca M, Berni A, Tiberti C, Verrienti A, Fanelli M, Fallarino M, Sorropago G, Baroni MG. The adiponectin gene SNP+276G>T associates with early-onset coronary artery disease and with lower levels of adiponectin in younger coronary artery disease patients (age <or=50 years) J Mol Med. 2005 Sep;83(9):711–9. doi: 10.1007/s00109-005-0667-z. [DOI] [PubMed] [Google Scholar]
- 15.Luan FL, Stuckey LJ, Ojo AO. Abnormal glucose metabolism and metabolic syndrome in non-diabetic kidney transplant recipients early after transplantation. Transplantation. 2010 Apr 27;89(8):1034–9. doi: 10.1097/TP.0b013e3181d05a90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–421. [PubMed] [Google Scholar]
- 17.Albareda M, Rodriguez-Espinosa J, Murugo M, de Leiva A, Corcoy R. Assessment of insulin sensitivity and beta-cell function from measurements in the fasting state and during an oral glucose tolerance test. Diabetologia. 2000 Dec;43(12):1507–11. doi: 10.1007/s001250051561. [DOI] [PubMed] [Google Scholar]
- 18.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003 Oct;42(4 Suppl 3):S1–201. [PubMed] [Google Scholar]
- 19.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 20.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Grantham N, Ebeling PR, Daly RM. Serum 25-Hydroxyvitamin D, Calcium Intake, and Risk of Type 2 Diabetes After 5 Years: Results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle study) Diabetes Care. 2011 May;34(5):1133–8. doi: 10.2337/dc10-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devaraj S, Jialal G, Cook T, Siegel D, Jialal I. Low vitamin D levels in Northern American adults with the metabolic syndrome. Horm Metab Res. 2011 Jan;43(1):72–4. doi: 10.1055/s-0030-1268485. [DOI] [PubMed] [Google Scholar]
- 22.Ingelsson E, Hulthe J, Lind L. Inflammatory markers in relation to insulin resistance and the metabolic syndrome. Eur J Clin Invest. 2008 Jul;38(7):502–9. doi: 10.1111/j.1365-2362.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 23.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008 Aug 11;168(15):1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas GN, B OH, Bosch JA, Pilz S, Loerbroks A, Kleber ME, Fischer JE, Grammer TB, Bohm BO, Marz W. Vitamin D Levels Predict All-Cause and Cardiovascular Disease Mortality in Subjects With the Metabolic Syndrome: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Diabetes Care. 2012 Mar 7; doi: 10.2337/dc11-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung J, McQuillan BM, Thompson PL, Beilby JP. Circulating adiponectin levels associate with inflammatory markers, insulin resistance and metabolic syndrome independent of obesity. Int J Obes (Lond) 2008 May;32(5):772–9. doi: 10.1038/sj.ijo.0803793. [DOI] [PubMed] [Google Scholar]