Abstract
Cannabinoid compounds affect synaptic activity and plasticity in numerous brain areas by activating CB1 receptors (CB1). In hippocampus, varying results have been obtained on the extent and site of cannabinoid actions on excitatory transmission, ranging from no effect to complete obliteration of synaptic responses. Here we used the rat hippocampal slice preparation to study and compare the effect of various synthetic and endogenous CB1 ligands on excitatory synaptic transmission. The full CB1 agonist WIN55212-2 (WIN2) greatly decreased excitatory synaptic transmission by 62%. The effect of WIN-2 was concentration dependent (EC50 of 200 nM) and completely prevented by CB1 antagonists. The nondegradable partial CB1 agonist R1-methanandamide (mAEA) decreased transmission by 25% and the endocannabinoids 2-arachid-onylglycerol (2-AG) and anandamide (AEA) had no significant effect. The action of AEA was improved by inhibiting its degradation but not its transport. The effect of 2-AG was enhanced upon inhibition of COX-2 but remained unchanged with blockade of monoacylglycerol lipase (MAGL). The observed effects were prevented by CB1 antagonists regardless of the ligand used, and paired-pulse paradigms pointed to presynaptic mechanisms of cannabinoid action. Our results show that cannabinoid effects on neuronal activity differ widely according to the CB1 ligand used. We observed large differences between full (synthetic) and partial (endogenous) CB1 agonists in altering synaptic transmission, notably because of the involvement of active degradation mechanisms.
Keywords: CB1, anandamide, 2-arachidonoylglycerol, degradation, hippocampus
Cannabinoid ligands have psychoactive properties and alter many central physiological processes such as cognition, appetite, and nociception (Iversen, 2003). Cannabinoid central effects are mediated principally via the specific receptor CB1 (Freund et al., 2003), one of the most abundantly expressed of the neuronal receptors, with a high distribution observed in hippocampus (Herkenham et al., 1991). CB1 ligands include a wide range of compounds with varying affinity and efficacy, and selective CB1 antagonists such as rimonabant (SR141716; SR1) have enhanced the range of functional investigations. The discovery of specific cannabinoid receptors led to the isolation of endogenously formed ligands synthesized and degraded by brain neurons, principally the arachidonate derivatives arachidonylethanolamide (anandamide; AEA) and 2-arachidonoylglycerol (2-AG; Devane et al., 1992; Di Marzo et al., 1994; Mechoulam et al., 1995; Stella et al., 1997). Exogenous AEA and 2-AG usually mimic the effects obtained with synthetic cannabinoids such as WIN2, but with lower efficacy (Felder and Glass, 1998; Luk et al., 2004).
Cannabinoid ligands decrease synaptic activity in neuronal preparations (Davies et al., 2002; Freund et al., 2003), and the diminution of inhibitory transmission via decreased release of γ-aminobutyric acid (GABA) is well established. However, studies on the CB1 modulation of hippocampal excitatory transmission have been controversial, and varying results have been obtained on the extent of cannabinoid effects, ranging from no effect to complete obliteration by WIN2 (Ameri et al., 1999; Ohno-Shosaku et al., 2002) and from little or no effect to 30–40% decrease by 2-AG and AEA (Stella et al., 1997; Ameri et al., 1999; Lees and Dougalis, 2004; Straiker and Mackie, 2005; Yang et al., 2008). Although the different CB1 ligands studied (synthetic ligands such as the aminoalkylindole WIN2 vs. endogenous ligands such as 2-AG and AEA) have different potencies at CB1, the varying effects obtained with the same ligand appear unreliable.
Active mechanisms of degradation in brain slices most likely play a role in limiting the effects of endogenous forms of cannabinoids on mature neurons, but the involvement of specific degradation processes remains to be determined. AEA is actively transported into the cytosol prior to degradation by fatty acid amidohydrolase (FAAH), and selective inhibitors of FAAH and AEA transport have been characterized (Piomelli, 2003; Fowler, 2007). 2-AG is degraded by a monoacylglycerol lipase (MAGL), but there are no selective MAGL inhibitors readily available (Blankman et al., 2007), although the compounds URB602 and N-arachidonoyl maleimide (NAM) have been shown to inhibit MAGL and augment endogenous levels of 2-AG (Saario et al., 2005; King et al., 2007). In addition to MAGL, cyclooxygenase-2 (COX-2) has recently been indicated to degrade 2-AG actively (Kozak et al., 2004), and COX-2 inhibition has been shown to potentiate endocannabinoid signaling at hippocampal CB1 (Kim and Alger, 2004; Slanina and Schweitzer, 2005; Yang et al., 2008). Here we used the adult rat hippocampal slice preparation to perform a detailed study and compare the effect of 2-AG and AEA applied alone or in combination with inhibitors of degradation, as well as the nondegradable mAEA and WIN2.
MATERIALS AND METHODS
Slice Preparation
All experimental protocols were consistent with guidelines issued by the National Institutes of Health and approved by our Institutional Animal Care and Use Committee. Rat hippocampal slices were prepared from male Sprague-Dawley rats (100–170 g) anesthetized with halothane (3%) and decapitated as described previously (Schweitzer et al., 1993). In brief, the brain was rapidly removed and placed into ice-cold artificial cerebrospinal fluid (ACSF) gassed with 95% O2-5% CO2. Transverse slices 350 μm thick were cut and incubated in an interface configuration for 30 min, then completely submerged and continuously superfused at a constant flow rate of 2–3 ml/ min with warm (32°C), gassed ACSF of the following composition in mM: NaCl, 130; KCl, 3.5; NaH2PO4, 1.25; MgSO4, 1.5; CaCl2, 2.0; NaHCO3, 24; glucose, 10. The inner recording chamber had a total volume of 0.8 ml, and 90% replacement of the chamber solution could be obtained within 1 min at the superfusion rate used. Drugs were added to the ACSF from stock solutions to obtain known concentrations.
Recordings
Extracellular recordings were performed with an Axo-clamp 2B preamplifier using pClamp acquisition software (Axon Instruments, Foster City, CA). The Schaffer collateral pathway was stimulated at 0.033 Hz with a bipolar tungsten electrode, and extracellular fields of excitatory postsynaptic potentials (fEPSPs) were recorded with a glass electrode (filled with 3 M NaCl or ACSF) placed in CA1 stratum radiatum at least 500 μm from the stimulation site. Stability of fEPSPs was established by stimulating at 40% of maximal fEPSP amplitude (determined with an input/output relationship) for at least 20 min prior to beginning experiments. The synaptic responses were quantified by averaging two consecutive responses (30 sec apart, i.e., 1 data point/min) and calculating the fEPSP slopes with pClamp software (Axon Instruments). We examined paired-pulse facilitation (PPF) using 100-msec interpulse intervals and calculated the PPF ratio as the second fEPSP slope over that of the first fEPSP. Intracellular current-clamp recordings were performed with an Axoclamp 2B in bridge mode, using sharp glass micropipettes filled with 3 M KCl (impedance range of 60–90 MΩ) to penetrate CA1 pyramidal neurons. We evoked excitatory postsynaptic potentials (EPSPs) by stimulating the Schaffer collateral pathway in the presence of 30 μM bicuculline to block GABAA receptors. Current and voltage records were acquired by digital-to-analog sampling and acquisition software and measured with analysis software (Axon Instruments). Data are presented as mean ± SEM. For statistics, data were subjected to one-sample and unpaired t-test analysis, with P < 0.05 considered significant.
Drugs
Drugs were dissolved in dimethylsulfoxide (0.1–0.2% final concentration), which alone did not affect the measured parameters (n = 6). We purchased AEA, 2-AG, mAEA [R(+)-arachidonyl-1′-hydroxy-2′-propylamide], WIN2 ([(3R)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo(1,2, 3-de)-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone, mono-methanesulfonate), URB597 (3′-carbamoyl-biphenyl-3-y-cyclo-hexylcarbamate), URB602 [(1,1′-biphenyl)-3-yl-carbamic acid,, cyclohexyl ester], AM404 [N-(4-hydroxyphenyl)-5Z,8Z, 11Z, 14Z-eicosatetrenamide], AM374 (palmitylsulphonyl fluoride), AM251 [1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide], NAM (N-arachidonyl maleimide), and NS398 (N-[2-(cyclohexyloxy)-4-nitro-phenyl]-methanesulfonamide) from Cayman Chemicals (Ann Arbor, MI) and all other chemicals from Sigma-Aldrich (St. Louis, MO). We obtained SR1 [(N-piperidin-l-yl)-5-(4-chloro-phenyl)-l-(2,4-dichlorophenyl)-4-methyl-lH-pyrazole-3-carbox-amide] from the National Institute of Mental Health’s Chemical Synthesis and Drug Supply Program.
RESULTS
Modulation of Basal Transmission by Endogenous Forms of CB1 Ligands
We first assessed the effect elicited by superfusion of the endogenous CB1 ligands AEA and 2-AG on hippocampal basal synaptic transmission. After establishing a stable fEPSP recording for at least 20 min, we added 30 μM AEA in the superfusate. A small decrease of the fEPSP slope developed 7–8 min after the start of application and reached a maximum effect after about 18 min of application (Fig. 1A, D). To ensure that the full effect was reached, we monitored AEA effects on basal transmission for 40 min. We observed a decrease of fEPSPs to 93% ± 3% of control (predrug) value, an effect not statistically different from control (P > 0.05; n = 7). We obtained similar results with 2-AG. Upon application of 30 μM 2-AG, fEPSPs decreased to 94% ± 4% of control (n = 8; Fig. 1D), an effect that was not statistically significant. Thus, exogenous application of the endogenous forms of CB1 ligand had a small and non-significant effect on hippocampal excitatory transmission.
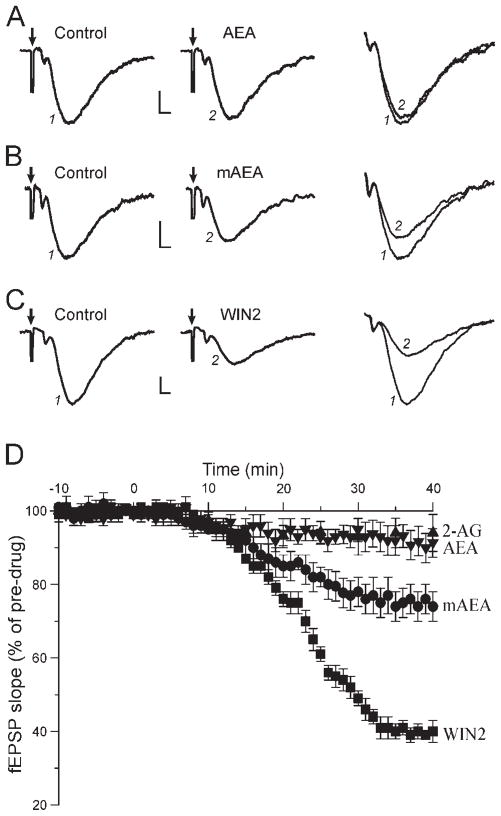
Fig. 1.
Cannabinoids differentially decrease excitatory synaptic transmission. Representative recordings showing fEPSPs elicited before (control) and during superfusion of various CB1 ligands applied for 35–40 min. The delivery of a single electric stimulation to evoke synaptic responses produced an artifact (arrow); traces (identified by numbers) are magnified and superimposed at right; calibration for all panels is 0.2 mV, 2 msec. A: Superfusion of 30 μM AEA had little effect on synaptic transmission. B: The nondegradable mAEA (10 μM) decreased fEPSPs by 27%. C: The synthetic WIN2 (1 μM) decreased excitatory transmission by 60%. D: Average effect of the cannabinoids on fEPSPs overtime. The CB1 agonists were applied at t = 0. AEA and 2-AG had little effect on excitatory transmission, whereas mAEA decreased fEPSPs by 25%. WIN2 had a large effect and decreased synaptic responses by 60%. The effect of the different drugs developed slowly: maximal effect was obtained about 20 min after the start of application for AEA and 2-AG, 30 min for mAEA, and 35 min for WIN2.
Because endogenous forms of CB1 ligands are actively degraded in biological tissue, we tested the non-degradable mAEA. Superfusion of 10 μM mAEA elicited a significant decrease of fEPSPs that began 6–7 min after the start of application and took 30 min to develop fully and reach a steady level at 75% ± 4% of control (n = 10; Fig. 1B, D). We also tested concentrations of 15 μM (n = 3) and 20 μM (n = 3), which decreased fEPSPs to 78% ± 6% and 73% ± 7% of control, respectively. Thus, the maximal effect of mAEA was reached at a concentration of 10 μM. In the presence of the CB1 antagonist AM251 (1 μM), mAEA did not decrease fEPSPs, which remained at 98% ± 3% of pre-mAEA level (n = 4). The marked effect of mAEA compared with AEA on excitatory transmission suggests that active degradation mechanisms limit the action of endogenous forms of CB1 agonists.
Modulation of Basal Transmission by WIN2
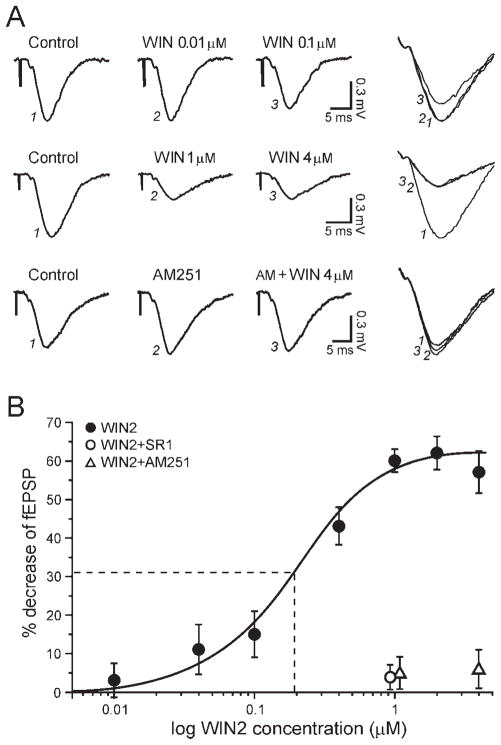
Synthetic CB1 ligands have been available for many years, and the compound WIN2 is one of the most potent and widely used CB1 agonists. Upon application of 1 μM WIN2, fEPSPs started to decrease 8 min after beginning the application, and the effect was fully developed after 33 min of superfusion to reach a steady value of 40% ± 3% of control (n = 12; Fig. 1C, D). The site and extent of WIN2 action on hippocampal excitatory transmission have been controversial, and recently published data remain conflicting (Takahashi and Castillo, 2006; Nemeth et al., 2008). We therefore established a dose-response relationship of the WIN2 effect on hippocampal excitatory transmission. The WIN2-elicited decrease of excitatory transmission was concentration dependent (Fig. 2). We observed no effect of 0.01 μM WIN2 (97% ± 4% of control, n = 4), whereas fEPSPs were decreased to 89% ± 7% of control with 0.04 μM WIN2 (n = 4), to 85% ± 6% with 0.1 μM (n = 4), to 57% ± 5% with 0.4 μM (n = 5), to 40% ± 3% with 1 μM (n = 12), to 38% ± 4% with 2 μM (n = 6), and to 43% ± 5% with 4 μM (n = 4). We used a sigmoidal (logistic) fit to analyze the dose-response relationship (Fig. 2B). The threshold response of WIN2 was just below 0.01 μM, and the maximal effect was obtained at 1 μM, with an apparent EC50 of 0.2 μM.
Fig. 2.
The effect of WIN2 is concentration dependent and solely involves CB1. A: fEPSP recordings obtained after a 35-min exposure to various concentrations of WIN2. Top: A WIN2 concentration of 0.01 μM did not alter excitatory transmission, but a clear effect was observed with 0.1 μM. Middle: The maximal effect was obtained with 1 μM WIN2, insofar as increasing the dose to 4 μM did not further alter fEPSPs. Bottom: The CB1 antagonist AM251 completely prevented the effect of the highest concentration of WIN2, confirming the sole involvement of CB1. B: Dose-response curve (logistic fit) of the WIN2 effect. WIN2 decreased fEPSPs by a maximum of 62%, with an apparent EC50 of 0.2 μM (dashed line). WIN2 did not affect fEPSPs in the presence of SR1 (open circle) or AM251 (open triangles).
We then verified the involvement of CB1 in the effect of WIN2 by using CB1 antagonists. It has been reported that SR1 may antagonize CB1 as well as non-CB1 receptors to prevent WIN2 actions on hippocampal excitatory transmission, whereas AM251 may be more selective and antagonizes only CB1 in hippocampus (Hájos and Freund, 2002). We therefore tested both SR1 and AM251. In slices pretreated with 1 μM SR1, the superfusion of 1 μM WIN2 did not significantly alter fEPSPs, which remained at 96% ± 3% of control 40 min after the start of WIN2 application (n = 5; Fig. 2B). Similarly, 1 μM WIN2 did not significantly alter fEPSPs in slices pretreated with 1 μM AM251 (95% ± 4%, n = 4; Fig. 2B). We also tested a concentration of 4 μM WIN2 in the presence of AM251. Again, AM251 fully prevented the effect of WIN2, which nonsignificantly decreased fEPSPs to 94% ± 5% of pre-WIN2 value (n = 4; Fig. 2). We conclude that the decrease of synaptic transmission elicited by WIN2 is solely mediated by activation of CB1.
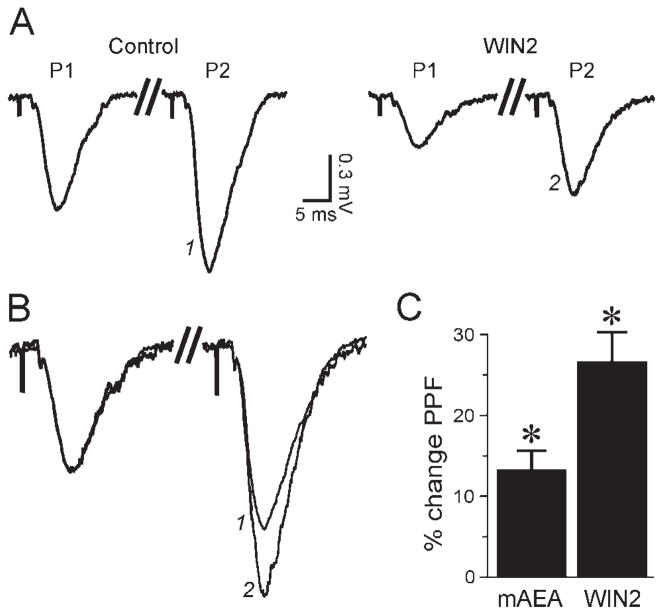
To determine whether mAEA and WIN2 acted at a presynaptic site, we used a paired-pulse facilitation (PPF) protocol consisting of two stimuli of the same intensity delivered 100 msec apart. At this time interval, the second fEPSP (P2) is larger than the first fEPSP (P1), and an increase of the PPF ratio (P2/P1) reflects a decrease of transmitter release. The PPF protocol revealed that WIN2 (1 μM) significantly increased the PPF ratio by 27% ± 3% (n = 12; Fig. 3), from 1.60 ± 0.05 in control conditions to 2.02 ± 0.05 in the presence of WIN2. Application of mAEA (10 μM) also significantly increased the PPF ratio by 13% ± 2% (n = 10; Fig. 3C), from 1.63 ± 0.06 in control to 1.85 ± 0.07 in mAEA. These data confirm that WIN2 and mAEA decreased excitatory transmission by decreasing transmitter release at a presynaptic site.
Fig. 3.
The cannabinoid-mediated effect is presynaptic. A: With the PPF paradigm (two pulses of the same intensity separated by 100 msec), the second fEPSP (P2) is larger than the first fEPSP (P1). WIN2 (1 μM, applied for 35 min) decreased P1 and P2, but the PPF ratio (P2/P1) was increased. Recording truncated between P1 and P2 (double slash) for magnification. B: Recordings from A superimposed and manually scaled to the same P1 amplitude (aligned on pre-synaptic volley) to magnify the PPF ratio. For equivalent P1 amplitudes, P2 (identified with numbers) was much larger in the presence of WIN2. C: Graph average showing the PPF change relative to control condition: 10 μM mAEA and 1 μM WIN2 increased PPF by 13% and 27%, respectively. Asterisk denotes statistical significance relative to predrug level.
Intracellular Recordings
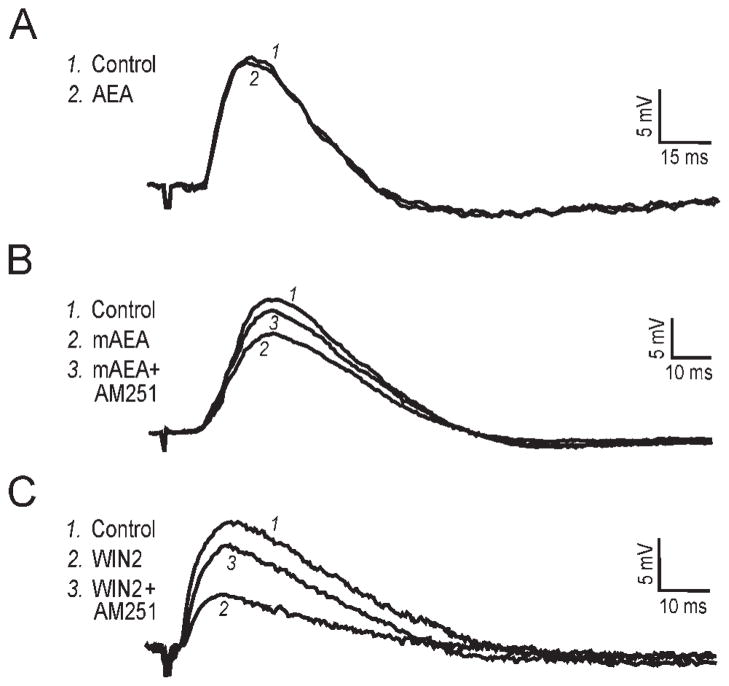
We performed intracellular recordings using sharp electrodes to verify the effects of WIN2, mAEA, and AEA on excitatory postsynaptic potentials (EPSPs) in CA1 pyramidal neurons. All EPSP data reported so far in the literature have been obtained using the whole-cell configuration, which could introduce artifacts because of the washout of the cytosol. We therefore used the “sharp” (high-resistance) electrode technique to ensure long recording times that cannot always be obtained with whole-cell recordings. The average resting membrane potential (RMP) of our neuronal sample (n = 11) was −70 ± 1 mV. EPSPs were elicited by stimulating the Schaffer collaterals, and 30 μM bicuculline was included in the superfusate to block GABAA-mediated transmission. Superfusion of 30 μM AEA did not affect the EPSP amplitude, which remained at 97% of the control value (n = 4; Fig. 4A). The nondegradable mAEA (10 μM) decreased EPSPs to 79% of control, with recovery to 93% upon subsequent addition of 1 μM AM251 (n = 3; Fig. 4B). WIN2 (1 μM) decreased EPSPs to 47% of control, and the CB1 antagonist AM251 (1 μM) reversed this effect to 82% of control (n = 4; Fig. 4C). Thus, the results we obtained with intracellular recordings using sharp electrodes were similar to those we obtained on fEPSPs using extracellular recordings.
Fig. 4.
Intracellular recordings using sharp electrodes. Recordings performed in 30 μM bicuculline to block GABAA transmission. A: Superimposed EPSPs recorded before and after a 30-min exposure to 30 μM AEA. AEA did not affect the EPSP amplitude in this neuron held at −75 mV. RMP was −71 mV. B: In this neuron held at −78 mV, 10 μM mAEA applied for 30 min decreased the EPSP to 77% of control, with recovery to 92% of control upon addition of the CB1 antagonist AM251 (1 μM; applied for 40 min). RMP was −70 mV. C: The superfusion of 1 μM WIN2 (applied for 35 min) largely decreased the EPSP to 44% of control in this neuron held at −79 mV, and subsequent addition of AM251 (40 min application) reversed the WIN2 effect to 82% of control. RMP was −72 mV.
Inhibitors of AEA Degradation
Because we obtained an effect with the nondegradable mAEA compared with AEA, we attempted to improve the action of AEA by pretreating the slices with inhibitors of its endogenous mechanisms of inactivation. We used URB597 as well as AM374 to block FAAH and AM404 to block the AEA transporter. We first tested whether URB597, AM374, or AM404 applied alone (25 min) would affect synaptic transmission. The FAAH inhibitors URB597 (0.1 μM) or AM374 (0.1 μM) did not significantly alter synaptic responses, with fEPSPs remaining at 100% ± 2% and 97% ± 3% of control, respectively (n = 6 each). Similarly, the transport inhibitor AM404 (10 μM) did not significantly affect fEPSPs, which remained at 99% ± 2% of predrug level (n = 10). Thus, these inhibitors superfused alone had no effect on synaptic transmission.
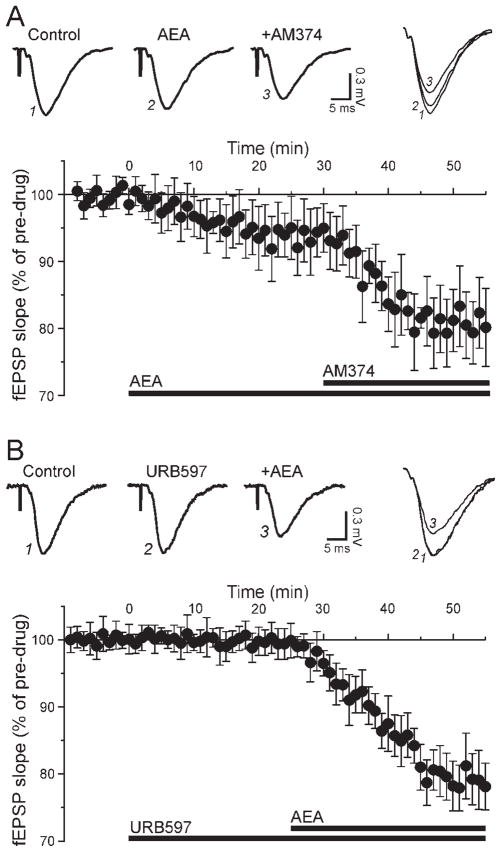
To determine whether an active degradation mechanism played a role to limit the effect of superfused AEA on synaptic transmission, we first applied 30 μM AEA and added 0.1 μM AM374 in the continued presence of AEA (Fig. 5A). AEA alone decreased fEPSPs to 94% ± 4%, and subsequent addition of 0.1 μM AM374 further decreased fEPSPs to 81% ± 5% (n = 4; Fig. 5A). We reversed the sequence of drug application by pretreating the slices with AM374 and subsequent adding AEA in the continued presence of AM374. We obtained similar results with this protocol: in the presence of 0.1 μM AM374, superfusion of 30 μM AEA reduced fEPSPs to 83% ± 4% of control (n = 5; data not shown). We pooled the results obtained with the two protocols (AEA + AM374 and AM374 + AEA) and determined that AEA significantly decreased excitatory transmission to 82% ± 3% of control in the presence of AM374 (n = 9). To verify the FAAH involvement further, we assessed the effect of another inhibitor, URB597. Addition of 30 μM AEA in the presence of 0.1 μM URB597 significantly decreased fEPSPs to 79% ± 4% of pre-URB values (n = 6; Fig. 5B). Delivery of the PPF paradigm indicated that URB597 alone did not alter PPF (102% ± 3% of control), but AEA significantly increased PPF to 116% ± 5% in the presence of the FAAH inhibitor (n = 6; not shown), suggesting a pre-synaptic site of AEA action.
Fig. 5.
Inhibition of FAAH enhances the effect of AEA. A: Inhibition of FAAH with AM374. Top: The superfusion of 30 μM AEA decreased the fEPSP to 92% of control, and addition of 0.1 μM AM374 in the continued presence of AEA further decreased the fEPSP to 73% of control. Bottom: Averaged time course of AEA followed by AM374 (drug application indicated with bars). The inhibition of FAAH potentiated the effect of AEA. B: Inhibition of FAAH with URB597. Top: In this experiment, the FAAH inhibitor URB597 was applied first and AEA subsequently. URB597 (0.1 μM) alone had no effect on fEPSPs, but further addition of 30 μM AEA decreased fEPSPs to 74% of control. Bottom: With FAAH blocked, AEA elicited a marked decrease of excitatory transmission.
We next assessed a possible participation of the AEA transporter by pretreating the slices with AM404. In the presence of 10 μM AM404, superfusion of 30 μM AEA nonsignificantly reduced fEPSPs to 95% ± 5% of pre-AM404 level (n = 5), an effect comparable to that of AEA alone. The combined inhibition of the transporter and FAAH has been reported to enhance the neuroprotective activity of endocannabinoids in hippocampal slice cultures (Karanian et al., 2005). We therefore determined the effect of AEA in conjunction with the combined blockade of FAAH and the transporter. In the presence of 0.1 μM URB597, 30 μM AEA, and 10 μM AM404, fEPSPs were decreased to 76% ± 5% of predrug level, a value not significantly different from that obtained with AEA in the presence of only URB597. Overall, inhibition of AEA transport did not improve AEA effects, but inhibition of FAAH enhanced the AEA effect to a level comparable to that of mAEA.
Inhibitors of 2-AG Degradation
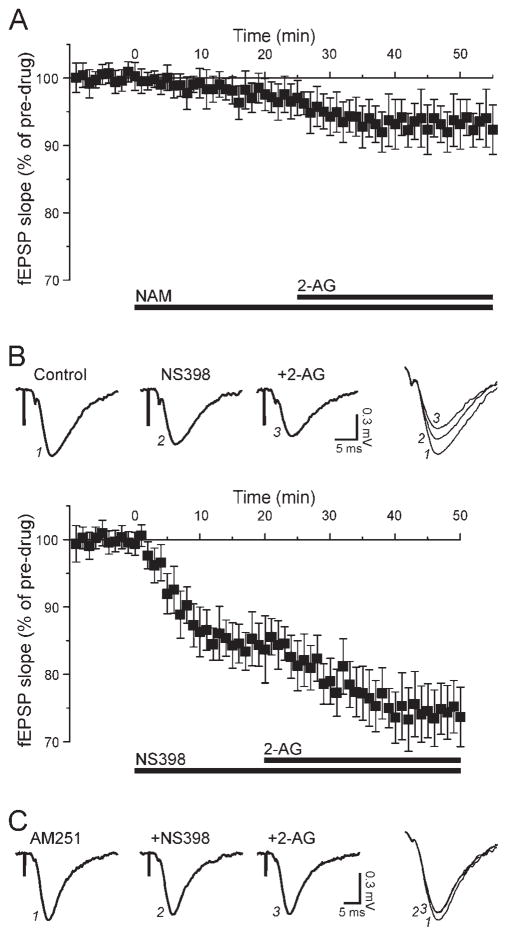
The CB1 ligand 2-AG is believed to play a primary role in hippocampus, and the most often described route of degradation for this molecule involves MAGL. We attempted to determine the participation of this enzyme in modulating excitatory transmission by using the inhibitors URB602 and NAM. Application of 100 μM URB602 alone for 25 min elicited a small decrease of fEPSPs to 96% ± 4% of control (n = 4), an effect that was not statistically significant. Addition of 2-AG for 30 min in the continued presence of URB602 further decreased fEPSPs to 89% ± 5% of pre-URB values (n = 4; not shown), a value that was not significantly different from values obtained with 2-AG alone. We then assessed the effect of NAM, a compound that has a relative selectivity for MAGL. Superfusion of 20 μM NAM alone for 25 min did not affect excitatory transmission, which remained at 98% ± 2% of control (n = 6; Fig. 6A). Subsequent application of 30 μM 2-AG in the continued presence of NAM slightly decreased fEPSPs to 93% ± 4% of pre-NAM values (n = 6; Fig. 6A), an effect similar to that obtained with 2-AG alone. Thus, we were unable to potentiate the effect of 2-AG by treating hippocampal slices with MAGL inhibitors, in contrast to the additional effect obtained with AEA in the presence of FAAH inhibitors.
Fig. 6.
Inhibition of COX-2, but not MAGL, improves the effect of 2-AG. A: Inhibition of MAGL with NAM. The superfusion of 20 μM NAM (t = 0, bar) did not significantly affect excitatory transmission, and further addition of 30 μM 2-AG (t = 25, bar) elicited an effect comparable to that of 2-AG alone. B: Inhibition of COX-2 with NS398. Top: The selective COX-2 inhibitor NS398 (10 μM) decreased the fEPSPs by 15%, and the subsequent application of 30 μM 2-AG in the continued presence of NS398 decreased fEPSPs by an additional 13%. Bottom: NS398 alone elicited a marked decrease of excitatory transmission and also facilitated the effect of exogenous 2-AG. C: Representative recordings depicting the effect of NS398 and 2-AG in slices pretreated with AM251. With CB1 blocked, NS398 elicited a limited decrease of the fEPSP (about 10%), and subsequent addition of 2-AG did not further alter the synaptic response.
COX-2 has emerged as a putative route to degrade endogenous cannabinoids, especially 2-AG, and inhibition of this enzyme potentiates CB1-mediated effects on hippocampal synaptic transmission. We investigated a role of COX-2 in 2-AG degradation by using the selective inhibitor NS398. Superfusion of 10 μM NS-398 alone significantly decreased fEPSPs to 85% ± 4% of control, consistent with our previous findings (Slanina and Schweitzer, 2005). Addition of 30 μM 2-AG in the continued presence of NS398 further decreased fEPSPs to 74% ± 5% of pre-NS398 values (n = 6; Fig. 6B). We investigated the involvement of CB1 by first treating the slices with AM251 and subsequently applying NS398 and 2-AG. In the presence of 1 μM AM251, the application of 10 μM NS398 nonsignificantly decreased fEPSPs to 93% ± 4% of pre-NS398 level. Further addition of 30 μM 2-AG in the continued presence of AM251 and NS398 did not alter fEPSPs, which remained at 92% ± 5% of pre-NS398 level (n = 4; Fig. 6C). Thus, the non-CB1-mediated component can be attributed to inhibition of COX-2 by NS398.
Summary
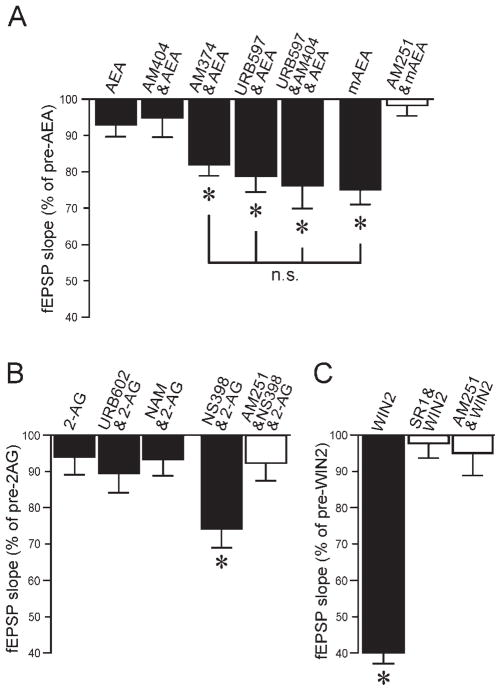
The effects of the various CB1 ligands tested on excitatory synaptic transmission are summarized and compared in Figure 7. AEA alone had little effect, and active transport did not appear to play a role. In the presence of a FAAH inhibitor, however, AEA decreased excitatory transmission to a level comparable to that with the nondegradable mAEA (Fig. 7A). The effect of 2-AG alone on basal transmission did not reach significance and was comparable to the effect of AEA alone. Inhibition of MAGL did not improve the effect of 2-AG, whereas inhibition of COX-2 potentiated the decrease of fEPSPs by 2-AG (Fig. 7B). These results suggest that degradation processes quickly inactivate endogenous forms of CB1 ligands when they penetrate into the slice. Finally, the effect of WIN2 on excitatory transmission (Fig. 7C) was by far the largest and sets this CB1 ligand apart from the endogenous cannabinoids.
Fig. 7.
Summary of cannabinoid effects on excitatory transmission. A: AEA alone or in the presence of the transport inhibitor AM404 did not significantly affect fEPSPs. Inhibition of FAAH with AM374 or URB597 potentiated the effect of AEA to a level similar to the effect of mAEA. The effect of mAEA was completely prevented by the CB1 antagonist AM251. Asterisks denote statistical significance compared with pre-AEA values (n.s., no statistical significance). B: The cannabinoid 2-AG did not significantly alter fEPSPs whether applied alone or in the presence of the MAGL inhibitors URB602 or NAM. However, the combined application of 2-AG and the COX-2 inhibitor NS398 significantly decreased fEPSPs, an effect largely prevented by AM251. C: WIN2 had a very large effect in decreasing synaptic transmission. The effect of WIN2 was completely prevented by the CB1 antagonists SR1 or AM251. Asterisks denote statistical significance relative to control (no drug) values.
DISCUSSION
Cannabinoids Act at CB1 To Affect Excitatory Transmission Differentially
The reported actions of WIN2 on excitatory transmission in rat hippocampal neurons using the slice preparation have been inconsistent, ranging from no effect (Al Hayani and Davies, 2000) to a 50–60% decrease (Misner and Sullivan, 1999; Hoffman et al., 2003; Lees and Dougalis, 2004; Kawamura et al., 2006; Kang-Park et al., 2007) to a 100% decrease (Ameri et al., 1999). In our hands, WIN2 decreased synaptic responses by 60%, in agreement with the majority of these studies. We also determined that WIN2 diminished excitatory transmission in a concentration-dependent manner, with an apparent EC50 of 0.20 μM, a value similar to the EC50 of 0.14 μM and 0.24 μM reported for the WIN2-elicited decrease of inhibitory transmission in hippocampal slices (Hoffman and Lupica, 2000; Hájos and Freund, 2002). Thus there does not appear to be a differential sensitivity to CB1 activation between excitatory and inhibitory transmission in adult hippocampus. Our result, however, differs from the EC50 value of 2.01 μM reported by Hájos and Freund (2002) for the WIN2 decrease of excitatory transmission, but in their study they determined that WIN2 acted at a non-CB1 receptor, a finding in contradiction to our data and those of others (Kawamura et al., 2006; Takahashi and Castillo, 2006).
The site of WIN2 action on hippocampal excitatory transmission remains somewhat controversial. It has been reported that, in the presence of SR1 (but not AM251) or in mice deficient for CB1, WIN2 can still decrease glutamatergic transmission (Hájos and Freund, 2002). This result has been contradicted in studies also using SR1 and AM251 as well as CB1-deficient mice (Ohno-Shosaku et al., 2002; Marsicano et al., 2003; Kawamura et al., 2006; Takahashi and Castillo, 2006). Our data show that the action of WIN2 was completely abolished by SR1 or AM251 and therefore do not support the existence of a non-CB1 receptor to mediate WIN2 effects on excitatory transmission. A more recent study indicated that micromolar concentrations of WIN2 can still decrease glutamatergic transmission in the presence of AM251 (Nemeth et al., 2008), pointing at a direct effect of WIN2 on presynaptic calcium channels. However, our data with AM251 and those of others (Kawamura et al., 2006; Takahashi and Castillo, 2006) again point to a sole CB1 site of action at WIN2 concentrations of up to 5 μM, in contradiction to the occurrence of a CB1-independent mechanism of WIN2.
Slow Penetration of Cannabinoid Substances Into Brain Slices
The use of brain slices is essential to assess the effects of cannabinoids on native neurons and on populations of neurons. We observed that the effects elicited by CB1 ligands on synaptic transmission were very slow to develop and reached a maximum only after 20–35 min of application. This slowly developing CB1 effect is similar to the time courses of 25–40 min reported in other studies using superfusion of cannabinoids onto slices (Ameri et al., 1999; Lees and Dougalis, 2004; Hoffman et al., 2005; Takahashi and Castillo, 2006), although in a recent study a maximal effect was obtained in 6–8 min (Nemeth et al., 2008). Time courses usually obtained in slice studies are in sharp contrast to time courses seen using isolated neurons such as cultured or dissociated cells, with which a CB1 effect may be observed within 1 min (Shen et al., 1996; Ohno-Shosaku et al., 2002; Straiker and Mackie, 2005). Because cannabinoid agonists are highly lipophilic, the lengthy time-to-peak observed in slice studies most likely is due to poor penetration of these substances into brain tissue (see also Brown et al., 2004).
Our results with AEA and 2-AG are in agreement with other studies conducted in rat slices in which it was shown that up to 30 μM AEA or 2-AG had little or no effect on excitatory synaptic transmission (Stella et al., 1997; Lees and Dougalis, 2004; Yang et al., 2008). In cultured hippocampal neurons, however, 2-AG or AEA applied alone potently decreases excitatory transmission (Straiker and Mackie, 2005; Hashimotodani et al., 2007). The absence of an effect of 2-AG or AEA when applied onto slices could arise from poor penetration of the lipids into the slice. On the other hand, WIN2 is the most lipophilic cannabinoid compound and was indeed the slowest to reach maximal effect in our experiments. However, our EC50 of 200 nM for WIN2 is in a range comparable to the EC50 of 60 nM reported for cultured hippocampal neurons (Ohno-Shosaku et al., 2002), suggesting that poor penetration principally delays the peak effect and does not grossly affect the maximal effect in the slice preparation compared with isolated (cultured) neurons. Note that, because slowly developing effects require long recording times, our use of extracellular recording techniques or intracellular techniques with sharp pipettes is suitable to obtain extensive recording times and ascertain cannabinoid effects on native neurons. It is likely that degradation mechanisms inactivating AEA and 2-AG are much more active in the brain slice compared with isolated neurons, where cannabinoids can readily access CB1, and the inferior effect of mAEA compared with WIN2 may be accounted for by its partial agonism and lower efficacy at CB1 (Felder and Glass, 1998; Luk et al., 2004).
Inactivation Mechanisms
Superfusion of 2-AG or AEA had little effect, but the nondegradable mAEA decreased synaptic responses by 25%, suggesting that active degradation mechanisms preempt the action of AEA and perhaps 2-AG. AEA is transported into neurons prior to enzymatic inactivation (Piomelli, 2003), and blockade of the transporter with AM404 could slow the elimination of AEA and enhance its physiological response. Moreover, AM404 alone has been shown to diminish evoked synaptic responses in CA1 hippocampus (Wilson and Nicoll, 2001; Hájos et al., 2004; Lees and Dougalis, 2004). We found that inhibition of uptake did not facilitate the action of AEA or improve the AEA effect observed upon inhibition of FAAH. Alternatively, the slowing of AEA degradation induced by inhibition of the transporter may be insufficient to alter excitatory transmission. AEA is degraded by FAAH found in the cytoplasm of pyramidal neurons. In our study, the inhibition of FAAH with URB597 or AM374 improved the effect of AEA on basal transmission to a level close to that obtained with the nonde-gradable mAEA, pointing to a primary role of FAAH in limiting or preventing AEA effects.
The application of 2-AG alone decreases excitatory transmission in cultured hippocampal neurons (Straiker and Mackie, 2005; Hashimotodani et al., 2007), whereas it has little or no effect when superfused onto hippocampal slice (Stella et al., 1997; Lees and Dougalis, 2004; Yang et al., 2008; this study), suggesting that active degradation mechanisms prevent the action of 2-AG in the slice. MAGL is the primary route of 2-AG degradation, and blockade of MAGL with URB602 alters inhibitory transmission in hippocampal slices and elevates 2-AG content in hippocampal cultures (Makara et al., 2005; King et al., 2007). In addition, NAM has been described as a relatively selective MAGL inhibitor that does not affect FAAH (Blankman et al., 2007). Under our experimental conditions, pretreatment of the slices with URB602 or NAM did not improve the effect of 2-AG, nor did the MAGL inhibitors alter synaptic transmission when applied alone. Thus the role of MAGL on excitatory synaptic transmission in adult hippocampal tissue remains to be firmly established.
COX-2 degrades 2-AG (Kozak et al., 2004), and inhibition of this enzyme has been shown to cause a decrease of synaptic transmission in hippocampal neurons (Kim and Alger, 2004; Slanina and Schweitzer, 2005; Hashimotodani et al., 2007). Our present data confirm that the selective COX-2 inhibitor NS398 applied alone decreased basal excitatory transmission in part via CB1. Superfusion of 2-AG in the presence of NS398 further decreased excitatory transmission to an extent similar to that obtained with mAEA or AEA together with an FAAH inhibitor. The overall effect elicited by NS398 and 2-AG was largely prevented by the CB1 antagonist AM251, indicating that the observed effect was mediated principally via CB1. We observed the non-CB1 component only upon application of NS398, and inhibition of COX-2 has recently been shown to elicit non-CB1 effects on neuronal activity by preventing the formation of oxygenated metabolites of 2-AG that increase synaptic transmission (Sang et al., 2007; Yang et al., 2008). We conclude that, in hippocampal slices of adult rat, the endogenous forms of CB1 ligands can decrease CA1 basal excitatory transmission by 20–25% when applied in the presence of inhibitors of degradation.
Synthetic (WIN2) vs. Endogenous (2-AG and AEA) Cannabinoid Ligands
Although active degradation mechanisms play a major role and the penetration of lipophilic CB1 ligands in biological tissue remains an important issue, the effect obtained with the endogenous forms of CB1 ligands does not compare with the effect observed with the synthetic WIN2. Thus WIN2 appears somewhat “too efficacious” at CB1 to assess the true physiological effects elicited by the endogenous CB1 ligands 2-AG or AEA. For example, the effects of WIN2 at low doses are similar to those of isolated lesions of the hippocampus, and high doses produced effects similar to lesions of both hippocampus and surrounding retrohippocampal areas (Hampson and Deadwyler, 1999). However, cofactors (or other unknown synergistic factors) yet to be uncovered could enhance endocannabinoid effects to a level similar to that of WIN2.
The various cannabinoids tested differentially affected the parameters studied. The full CB1 agonist WIN2 greatly decreased excitatory transmission, and our results are consistent with CB1 being the sole site of WIN2 action. The partial CB1 agonist mAEA had a lesser effect on basal transmission compared with WIN2, a difference that can be explained by its partial agonism and lower efficacy at CB1 compared with WIN2. AEA and 2-AG had a very limited role, because of active degradation processes. Blockade of FAAH improved the effect of AEA, and inhibition of COX-2 enhanced the effect of 2-AG. Our results suggest that the investigation of cannabinoid effects with powerful synthetic agonists may not reflect the physiological role played by the endogenous ligands, underscoring the need to assess the actions of endogenous CB1 agonists in order to determine the physiological role of the CB1 transmitter system.
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant number: DA13658; Contract grant number: AA06420.
References
- Al Hayani A, Davies SN. Cannabinoid receptor mediated inhibition of excitatory synaptic transmission in the rat hippocampal slice is developmentally regulated. Br J Pharmacol. 2000;131:663–665. doi: 10.1038/sj.bjp.0703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameri A, Wilhelm A, Simmet T. Effects of the endogeneous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices. Br J Pharmacol. 1999;126:1831–1839. doi: 10.1038/sj.bjp.0702478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SN, Pertwee RG, Riedel G. Functions of cannabinoid receptors in the hippocampus. Neuropharmacology. 2002;42:993–1007. doi: 10.1016/s0028-3908(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz J-C, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- Fowler CJ. The pharmacology of the cannabinoid system—a question of efficacy and selectivity. Mol Neurobiol. 2007;36:15–25. doi: 10.1007/s12035-007-0001-6. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Hájos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Hájos N, Kathuria S, Dinh T, Piomelli D, Freund TF. Endocannabinoid transport tightly controls 2-arachidonoyl glycerol actions in the hippocampus: effects of low temperature and the transport inhibitor AM404. Eur J Neurosci. 2004;19:2991–2996. doi: 10.1111/j.0953-816X.2004.03433.x. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life Sci. 1999;65:715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic mono-acylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABAA synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Riegel AC, Lupica CR. Functional localization of cannabinoid receptors and endogenous cannabinoid production in distinct neuron populations of the hippocampus. Eur J Neurosci. 2003;18:524–534. doi: 10.1046/j.1460-9568.2003.02773.x. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Macgill AM, Smith D, Oz M, Lupica CR. Species and strain differences in the expression of a novel glutamate-modulating cannabinoid receptor in the rodent hippocampus. Eur J Neurosci. 2005;22:2387–2391. doi: 10.1111/j.1460-9568.2005.04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Kang-Park MH, Wilson WA, Kuhn CM, Moore SD, Swartzwelder HS. Differential sensitivity of GABA A receptor-mediated IPSCs to cannabinoids in hippocampal slices from adolescent and adult rats. J Neurophysiol. 2007;98:1223–1230. doi: 10.1152/jn.00091.2007. [DOI] [PubMed] [Google Scholar]
- Karanian DA, Brown QB, Makriyannis A, Kosten TA, Bahr BA. Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. J Neurosci. 2005;25:7813–7820. doi: 10.1523/JNEUROSCI.2347-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, Astarita G, Geaga JA, Luecke H, Mor M, Tarzia G, Piomelli D. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–1365. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Prusakiewicz JJ, Marnett LJ. Oxidative metabolism of endocannabinoids by COX-2. Curr Pharm Des. 2004;10:659–667. doi: 10.2174/1381612043453081. [DOI] [PubMed] [Google Scholar]
- Lees G, Dougalis A. Differential effects of the sleep-inducing lipid oleamide and cannabinoids on the induction of long-term potentiation in the CA1 neurons of the rat hippocampus in vitro. Brain Res. 2004;997:1–14. doi: 10.1016/j.brainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Luk T, Jin W, Zvonok A, Lu D, Lin XZ, Chavkin C, Makriyannis A, Mackie K. Identification of a potent and highly efficacious, yet slowly desensitizing CB1 cannabinoid receptor agonist. Br J Pharmacol. 2004;142:495–500. doi: 10.1038/sj.bjp.0705792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makara JK, Mor M, Fegley D, Szabó SI, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, Freund TF, Piomelli D. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, Van der Stelt M, López-Rodríguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-mono-glyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth B, Ledent C, Freund TF, Hájos N. CB1 receptor-dependent and -independent inhibition of excitatory postsynaptic currents in the hippocampus by WIN 55,212–2. Neuropharmacology. 2008;54:51–57. doi: 10.1016/j.neuropharm.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Saario SM, Salo OM, Nevalainen T, Poso A, Laitinen JT, Jarvinen T, Niemi R. Characterization of the sulfhydryl-sensitive site in the enzyme responsible for hydrolysis of 2-arachidonoyl-glycerol in rat cerebellar membranes. Chem Biol. 2005;12:649–656. doi: 10.1016/j.chembiol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. COX-2 oxidative metabolite of endo-cannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. J Neurochem. 2007;102:1966–1977. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- Schweitzer P, Madamba SG, Champagnat J, Siggins GR. Somato-statin inhibition of hippocampal CA1 pyramidal neurons: mediation by arachidonic acid and its metabolites. J Neurosci. 1993;13:2033–2049. doi: 10.1523/JNEUROSCI.13-05-02033.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MX, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocam-pal cultures. J Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slanina KA, Schweitzer P. Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippocampus. Neuropharmacology. 2005;49:653–659. doi: 10.1016/j.neuropharm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi KA, Castillo PE. The CB1 cannabinoid receptor mediates glutamatergic synaptic suppression in the hippocampus. Neuroscience. 2006;139:795–802. doi: 10.1016/j.neuroscience.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhang J, Andreasson K, Chen C. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Mol Cell Neurosci. 2008;37:682–695. doi: 10.1016/j.mcn.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]