Abstract
Epidemiologic data support an inverse association between green tea intake and breast cancer risk and numerous experimental studies have demonstrated the anti-tumor effects of its main component, epigallocatechin gallate (EGCG). We conducted a phase IB dose escalation trial in women with a history of stage I-III hormone receptor-negative breast cancer of an oral green tea extract, Polyphenon E (Poly E) 400mg, 600mg, 800mg bid or matching placebo for 6 months. The primary endpoint was to determine the maximum tolerated dose (MTD), defined as the dose that causes 25% dose limiting toxicity (DLT, grade≥2). Assignment to dose level was based upon an adaptive design, the continual reassessment method. A mammogram and random core biopsy of the contralateral breast were obtained at baseline and 6 months and serial blood/urine collections every 2 months for biomarker analyses. Forty women were randomized: 10 to placebo, 30 to Poly E (16 at 400mg, 11 at 600mg, 3 at 800mg). There was 1 DLT at 400mg (grade 3 rectal bleeding), 3 DLTs at 600mg (grade 2 weight gain, grade 3 indigestion and insomnia), and 1 DLT at 800mg (grade 3 liver function abnormality). The DLT rate at 600mg was 27% (3/11). Pharmacologic levels of total urinary tea polyphenols were achieved with all three dose levels of Poly E. Using a novel phase I trial design, we determined the MTD for Poly E to be 600mg bid. This study highlights the importance of assessing toxicity for any chemopreventive agent being developed for chronic use in healthy individuals.
Keywords: green tea, chemoprevention, breast cancer, biomarkers
Introduction
Chemoprevention with selective estrogen receptor modulators (SERMs), tamoxifen (1) and raloxifene (2), or the aromatase inhibitor (AI), exemestane (3), reduces breast cancer incidence among high-risk women, however, uptake has been poor in the prevention setting. In addition, these agents have no effect on the incidence of hormone receptor (HR)-negative cancers, which account for about a third of all breast carcinomas and are associated with a poorer prognosis. The high costs of large-scale chemoprevention studies have prompted the search for intermediate markers of cancer development. A greater emphasis has been placed on developing novel clinical trial designs which use surrogate endpoint biomarkers in lieu of cancer occurrence in order to improve the efficiency and reduce the cost of chemoprevention trials. Therefore, priorities in breast cancer chemoprevention include developing safe and tolerable agents that are effective against HR-negative breast cancer and validating intermediate biomarkers which correlate with breast cancer risk.
Breast cancer is the most common cancer among women worldwide, with a six-fold variation in incidence between high-risk regions (e.g., North America and Europe) and low-risk regions (e.g., Asia) (4). This geographic variation is often attributed to the Asian diet, which is rich in soy-based products and antioxidant-containing foods and beverages, such as green tea. A recent meta-analysis, encompassing 5,617 breast cancer cases, reported an inverse association between green tea consumption and breast cancer incidence (RR 0.81; 95% CI, 0.75-0.88) among case-control studies (5). In two Japanese cohort studies (6, 7), green tea intake among breast cancer patients was associated with a decrease in risk of recurrence (pooled RR 0.73; 95% CI, 0.56-0.96). A population-based, case-control study of Asian American women found that green tea intake was associated with decreased breast cancer risk with >85.7 ml/day (OR 0.53, 95% CI 0.35-0.78) (8). Interestingly, the protective effect of green tea was observed only among individuals who possessed at least one low-activity polymorphism in catechol-O-methyltransferase (COMT), which is involved in the methylation of green tea constituents (9).
The most abundant and possibly most potent polyphenol in green tea is epigallocatechin-3-gallate (EGCG) (10). EGCG has anti-oxidative, anti-inflammatory, anti-proliferative, and anti-angiogenic properties that are relevant for cancer prevention (11). In vitro studies demonstrated cytotoxic effects of EGCG on breast cancer cells regardless of estrogen receptor status (12). A major challenge is translating these preclinical findings into human intervention trials.
Polyphenon E (Poly E) is a well-defined pharmaceutical-grade decaffeinated green tea catechin mixture, including epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG), and most abundantly, ~65% EGCG (13). Each capsule contains 200mg of EGCG, which is equivalent to about 2-3 cups of brewed green tea. In phase I pharmacokinetic trials of Poly E 200-800mg daily (equivalent to 2-12 cups of green tea without caffeine) given as a single-dose or 4-week administration in healthy individuals, high plasma EGCG levels were achieved (14, 15). All adverse events were rated as mild, including nausea, abdominal pain, heartburn, excess gas, headache, dizziness, and muscle pain (15).
We conducted a phase IB randomized, double-blinded, placebo-controlled, dose escalation trial of a 6-month intervention of Poly E in women with a history of HR-negative breast cancer. Women with HR-negative breast cancer provide a relevant population for conducting secondary prevention research, since they are not candidates for adjuvant hormonal therapy and may be particularly motivated to participate in chemoprevention trials. The primary objective of this dose-finding study was to determine the maximum tolerated dose (MTD) of Poly E given over a 6-month period. Secondary objectives were to determine the dose-related biologic effects of Poly E on intermediate biomarkers correlated with breast cancer risk, including breast tissue-based biomarkers, mammographic density, and serum hormone levels, as well as COMT genotype and urinary tea polyphenols.
Subjects and Methods
Subjects
Women were enrolled at four sites: Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY; University of Texas, M.D. Anderson Cancer Center, Houston, TX; Memorial Sloan-Kettering Cancer Center, New York, NY; Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, TX. The study was approved by the institutional review boards at each participating site. All participants provided written informed consent in English or Spanish. The study was registered prior to initiating enrollment (http://clinicaltrials.gov, NCT00516243).
Eligible participants were women age 21 to 65 years with a history of histologically-confirmed resected stage I-III estrogen receptor (ER)-negative and progesterone receptor (PR)-negative (defined as <10% ER and PR expression) breast carcinoma without evidence of disease at trial entry and a minimum of six months since completion of breast surgery, adjuvant chemotherapy (including trastuzumab) and radiation therapy. Other eligibility criteria included an Eastern Cooperative Group performance status of 0-1, at least one intact breast (no radiation therapy, mastectomy, or breast implant), and normal organ and marrow function, including total bilirubin and transaminases within normal institutional limits.
Exclusion criteria included bilateral breast cancer or metastatic disease, history of gastrointestinal (GI) bleeding, uncontrolled or significant co-morbid illness, and current use of hormone replacement therapy, tamoxifen, or raloxifene. Both premenopausal and postmenopausal women were included in this study. Postmenopausal status was defined as the absence of menses for >12 months, history of bilateral oophorectomy, or serum follicle-stimulating hormone (FSH) >20 mIU/ml.
Participants had to abstain from all tea consumption (including herbs, vitamin and mineral supplements that contain tea compounds) and limit total daily caffeine consumption to <375 mg for 30 days prior to the baseline evaluation and during the 6-month study intervention.
Trial Design and Intervention
The trial was a randomized, double-blinded, placebo-controlled, dose escalation study in which participants received either Poly E delivering 400mg, 600mg, or 800mg of EGCG (2-4 capsules) twice daily (total of 800mg, 1200mg, or 1600mg EGCG daily) with food or matching placebo for 6 months (10 subjects in the placebo arm, 30 subjects in the study arm with participant assignment to dose level as per the adaptive study design). The Poly E oral capsules and matching placebo were supplied by the National Cancer Institute (NCI), Division of Cancer Prevention (DCP) under an IND. Participants and investigators were blinded to Poly E or placebo, but not to dose level.
The primary objective of this phase I dose escalation study was to determine the maximum tolerated dose (MTD) of Poly E in this study population. All participants were evaluable for toxicity from the time of their first dose of study drug. Safety was assessed by monitoring routine clinical and laboratory parameters at baseline, every two weeks during the first month, then monthly for the duration of the study. The starting dose of Poly E 400mg bid was chosen based on previous clinical safety data (14, 15). The MTD was defined as a dose that causes a dose-limiting toxicity (DLT) in approximately 25% of participants during the 6-month intervention. A DLT was initially defined according to the NCI Common Terminology Criteria for Adverse Events (Version 3.0) as any grade 2 or higher toxicity. After the first two grade 2 toxicities occurred during the trial, the protocol was amended to define the DLT as any grade 2 or higher toxicity which was at least possibly related to study drug, persisted for at least one week or required stopping the study drug. A lower threshold for a DLT was used compared to cancer treatment trials as Poly E is an agent being developed for chemoprevention where prolonged use can be anticipated.
The time-to-event continual reassessment method (TITE-CRM) is a novel statistical methodology that considers long-term toxicity in dose escalation, allows staggered participant entry, and on average allocates more participants at the correct MTD (16). According to the TITE-CRM, the decision for escalation was made after every 5 subjects treated with Poly E were evaluated for toxicity at a single dose. Dose escalation took place with 5 non-toxic observations. Once a DLT was observed, the dose-toxicity curves were re-assessed and the next group of 5 were given a dose according to the TITE-CRM. Once a DLT occurred at any time in the 6-month window in any subject, new subjects were considered at the most updated MTD estimate. A participant who developed a DLT was taken off of study drug, but was encouraged to complete the 6-month evaluations. There were no intra-participant dose modifications.
Correlative Studies
Secondary objectives of this study were to investigate the dose-related biologic effects of Poly E. Participants underwent a digital mammogram and random core biopsy of the contralateral breast at baseline and after the 6-month intervention for mammographic density assessment and immunohistochemical (IHC) analyses of Ki-67 (proliferation marker) and ER-α. Due to changes in breast density and breast tissue proliferation with the menstrual cycle, mammograms and breast biopsies were obtained during day 7-14 of the menstrual cycle in premenopausal women. Mammographic percent density (proportion of the breast with dense tissue) from the cranio-caudal view of the contralateral breast was assessed using semiautomated methods by the Cumulus software (17). All mammographic density readings were conducted by an investigator blinded to treatment assignment and the timing of the mammograms (baseline or 6 months). A random core biopsy from the upper outer quadrant of the contralateral breast approximately 1 cm from the areolar edge was obtained at baseline and 6 months. Tissue was placed in a vial of 10% formalin for a maximum of 24 hours prior to embedding and processing. IHC analysis of Ki-67 (Clones MIB-1 and M7240 mouse monoclonal, 1:50; Dako, Carpinteria, CA) and ER-α (Clones 1D5 and M7047 mouse monoclonal, 1:60; Dako, Carpinteria, CA) was performed on paraffin-embedded sections. Quantitative changes of the expression of markers based upon percentage of positive cells were scored in a blinded fashion.
Blood samples were collected at baseline, 2, 4, and 6 months for measurement of serum hormone levels: estradiol, testosterone, insulin-like growth factor-1 (IGF-1), IGF binding protein-3 (IGFBP-3), and sex hormone binding globulin (SHBG). Radioimmunoassays were used to measure serum estradiol and testosterone levels (Diagnostic Systems Laboratories). The lowest limit of detection for estradiol and testosterone were 5.0 pg/ml and 20 ng/dl, respectively. Assays for IGF-1, IGFBP-3, and SHBG were immunometric using enzyme/substrate for detection (Diagnostic Systems Laboratories).
Spot urine samples were collected every 2 months during the 6-month intervention. Five metabolites of tea catechins (EGC, EC, methylepigallocatechin [4’-MeEGC], 5-(3’,4’,5’-trihydroxyphenyl)-γ-valerolactone [M4], and 5-(3’,4’-dihydroxyphenyl)-γ-valerolactone [M6]) were assayed by high performance liquid chromatography, which allows the determination of free and conjugated forms of tea catechins in urine, as previously described (18). The lower limit of detection for each catechin was 0.02 μM. For each subject, we summed the EGC, EC, 4’-MeEGC, M4, and M6 levels to create a total tea polyphenols index. The concentration of total tea polypenols was expressed in units of urinary creatinine by weight (μmol/g Cr) to account for varying volumes between the spot urine samples.
Genomic DNA was extracted from blood cells (10 ml/sample) taken at baseline using standard RNase/proteinase K technique and genotyping for COMT (G/A transition at codon 158, rs4680) was conducted using the Taqman 5′-Nuclease Assay (Applied Biosystems, Foster City, CA). The fluorescence profile of each well was measured in an ABI 7500HT Sequence Detection System and the results were analyzed with Sequence Detection Software (Applied Biosystems).
A questionnaire on quality of life (SF-36) was self-administered at baseline and 6 months. The short form of the Medical Outcomes Study (SF-36) is comprised of 36 items across eight scales: physical functioning, role function-physical, bodily pain, general health, vitality, social functioning, role function-emotional, and mental health (19).
Statistical Considerations
Before the actual implementation of the trial, computer simulations were used to evaluate the operating characteristics of the TITE-CRM approach to dose assignment (Table 1). A robust dose-toxicity model was calibrated according to Cheung and Chappell (20). Overall, the design would be able to identify the correct MTD with a probability over 0.60 when the neighboring doses had DLT rates of 10% and 35%, respectively. If the neighboring doses had DLT rates significantly different from the target of 25%, the probability of correct selection of the MTD would be higher. The TITE-CRM has been shown to generally yield a high probability of selecting the correct MTD even with a small sample size. Only the 30 participants receiving active study drug participated in the dose escalation study. In principle, as few as 12 participants could be recruited with the TITE-CRM, but having at least 10 participants at the MTD would avoid too much variability in the comparisons to placebo. Assuming a 10% drop-out rate, 36 of the 40 participants would be evaluable for the long-term toxicity and efficacy endpoints. This sample size would ensure that estimates of any binary variable, including incidence of toxicity, would have a 95% confidence interval of width < 0.36. All participants who received any study drug were included in the report of toxicities. The percentage of participants with each toxicity was compared between the intervention groups using a Fisher's exact test of proportions at a two-sided 0.05 level of significance.
Table 1.
A priori operating characteristics under each scene based upon 5000 computer simulations.
| Dose | 400 mg | 600 mg | 800 mg | Average #DLT |
|---|---|---|---|---|
| Scene 1: 400 mg is the MTD | ||||
| DLT rate | 25% | 35% | 35% | |
| P (Select) | 0.73 | 0.23 | 0.04 | 8.5 |
| Average #allocation | 20 | 8 | 2 | |
| Scene 2: 600 mg is the MTD | ||||
| DLT rate | 10% | 25% | 35% | |
| P (Select) | 0.13 | 0.64 | 0.22 | 6.6 |
| Average #allocation | 10 | 14 | 6 | |
| Scene 3: 800 mg is the MTD | ||||
| DLT rate | 5% | 10% | 25% | |
| P (Select) | 0.00 | 0.19 | 0.80 | 4.7 |
| Average #allocation | 6 | 10 | 14 | |
DLT, Dose-limiting toxicity. Average #allocation, average number of participants allocated to each dose level. Numbers in bold indicate the pre-specified target DLT rate (25%) and probability of selecting that dose as the correct maximum tolerated dose (MTD).
Of the 40 participants, 28 completed the drug intervention (8 placebo, 20 Poly E) and 34 completed their 6-month assessments (8 placebo, 26 Poly E) and were included in the secondary endpoint analyses. There were missing data for some of the endpoints due to inability to collect some of the specimens or inadequate samples for biomarker analysis. The missing rates of the submitted biospecimens were 1.4% for serum/urine biomarkers, 2.5% for COMT genotyping, 9% for mammographic density readings, and 12% for IHC tissue biomarkers. Descriptive statistics were performed on each of the biomarker endpoints within each intervention group. Due to skewness in the distributions of these variables, Wilcoxon signed rank tests were used to compare between-group and within-group differences for the Poly E and placebo groups for each of the outcome measures using a continuous scale. We also calculated the mean absolute change and percent change from baseline for each group to account for differences in baseline measures. Between-group comparison for continuous data was performed using repeated-measure analyses of variance (ANOVA) with a time-interaction term using treatment and dose levels as main effects. These secondary endpoints were not corrected for multiple comparisons due to their exploratory nature. All analyses were two-sided and performed using SAS software version 9.1.3 (SAS Institute, Cary, NC).
Results
Participant Characteristics
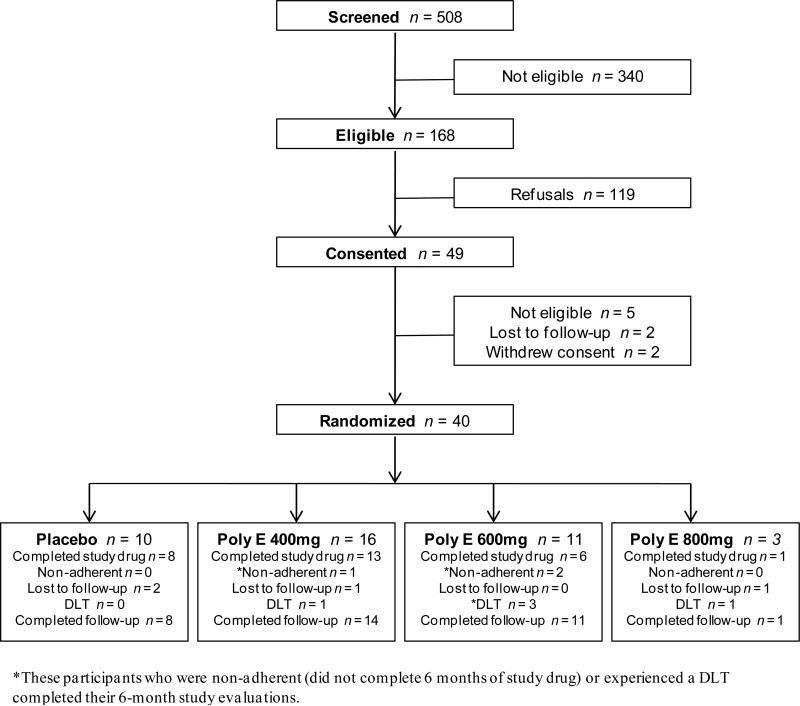
Among 508 women screened, 168 met initial eligibility criteria (Figure 1). From July 2007 to August 2009, 40 participants were randomized 3:1 to Poly E (n = 30; 16 at 400mg, 11 at 600mg, 3 at 800mg) or placebo (n = 10). Baseline characteristics are presented in Table 2. Median age was 52 years (range 36-64). The study population was diverse by race/ethnicity (42% minorities), 75% were postmenopausal, and 74% had a body mass index (BMI) of 25 kg/m2 or greater. Over three-quarters (78%) of participants had stage I or II breast cancer and the median time since diagnosis was 33 months (range 10-170). Twenty-eight (70%) women completed the 6-month drug intervention, 3 (8%) were non-adherent (did not complete the 6-month drug intervention, 4 (10%) were lost to follow-up, and 5 (12.5%) developed a DLT (Figure 1). A total of 34 out of 40 (85%) participants had 6-month biomarker data available for analysis.
Figure 1.
Flow diagram for subjects who were accrued into the study. DLT, dose limiting toxicity.
Table 2.
Participant characteristics
| Characteristics | Poly E (n = 30) | Placebo (n = 10) | Total (n = 40) |
|---|---|---|---|
| Age, years | |||
| Median | 52 | 53.5 | 52 |
| Range | 36-64 | 40-59 | 36-64 |
| Menopausal status, n (%) | |||
| Premenopausal | 7 (23) | 3 (30) | 10 (25) |
| Postmenopausal | 23 (77) | 7 (70) | 30 (75) |
| Race, n (%) | |||
| White | 18 (60) | 5 (50) | 23 (58) |
| Hispanic | 6 (20) | 3 (30) | 9 (22) |
| Black | 5 (17) | 2 (20) | 7 (18) |
| Asian | 1 (3) | 0 | 1 (2) |
| Body mass index, kg/m2 | |||
| Median | 28.2 | 28.7 | 28.6 |
| Range | 21.1-40.7 | 22.6-37.3 | 21.1-40.7 |
| Breast cancer stage at diagnosis, n (%) | |||
| I | 14 (47) | 2 (20) | 16 (40) |
| II | 9 (30) | 6 (60) | 15 (38) |
| III | 7 (23) | 2 (20) | 9 (22) |
| Breast cancer treatments, n (%) | |||
| Chemotherapy | 29 (97) | 10 (100) | 39 (98) |
| Trastuzumab | 5 (17) | 2 (20) | 7 (18) |
| Radiation therapy | 23 (77) | 8 (80) | 31 (78) |
| Months since breast cancer diagnosis | |||
| Median | 31.5 | 37 | 33 |
| Range | 10-170 | 15-73 | 10-170 |
Toxicities
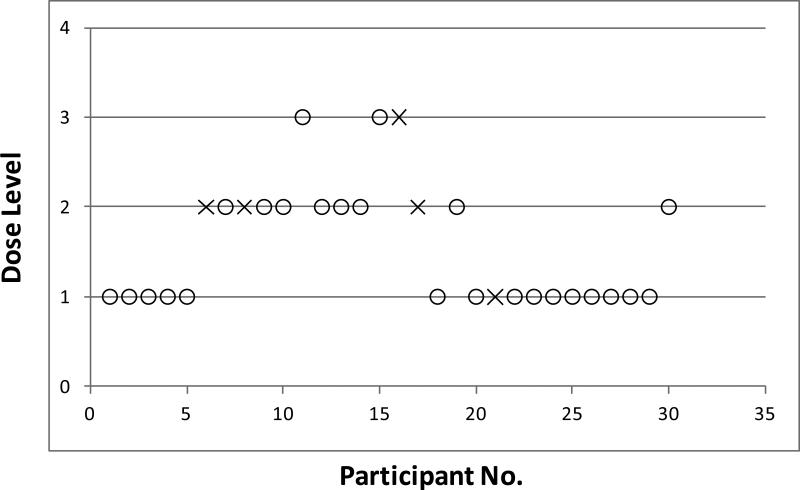
Figure 2 shows the course of participant flow, including dose escalation and de-escalation in the study. The figure illustrates that after 5 non-toxic observations at the first dose level (Poly E 400mg bid or total of 800mg EGCG daily), dose escalation occurred. Initial escalation to dose level 3 (Poly E 800mg bid or total of 1600mg EGCG daily) occurred before the two DLTs occurred at dose level 2 (Poly E 600mg bid or total of 1200mg EGCG daily). However, additional participants were assigned to the lower dose groups because the study design is able to account for late toxicities. In total, there were 5 DLTs during the trial (in sequential order): grade 2 weight gain (day 138 on study drug) and grade 3 indigestion (day 40) at dose level 2; grade 3 ALT elevation (day 91) at dose level 3; grade 3 insomnia (day 6) at dose level 2; and grade 3 rectal bleeding (day 18) at dose level 1. The episode of grade 3 rectal bleeding occurred in a woman with pre-existing diverticulosis and required hospitalization. After this serious adverse event, the protocol was amended to exclude women with a prior history of a GI bleed. All DLTs occurred in participants receiving active Poly E. The frequency of DLTs was 6.25% (1/16) for dose level 1, 27% (3/11) for dose level 2, and 33% (1/3) for dose level 3. These DLT rates are similar to what was predicted with the computer simulations (Table 1). Based upon these findings, dose level 2 (600mg bid) was defined as the MTD for Poly E, because it was the closest to the prespecified toxicity level of 25%.
Figure 2.
Participant flow throughout the course of the trial. Participants who received active Poly E (n = 30) are presented in chronological order from left to right with dose levels on the vertical axis (1=400mg bid, 2=600mg bid, 3=800mg bid). Open circles indicate participants who did not develop a dose-limiting toxicity (DLT) and crosses indicate participants who developed a DLT. Note the dose escalations and de-escalations that occurred with ongoing data collection on DLTs. The first participant at dose level 3 was enrolled before the two participants at dose level 2 developed a DLT.
Additional toxicities are summarized in Table 3. The toxicity profile of Poly E was consistent with the published literature, with the most common adverse events (mainly grade 1) being GI in nature. There were a handful of grade 2 toxicities in the Poly E and placebo arms that did not meet criteria for a DLT based upon investigator assessment, since they did not require stopping study drug. The toxicities did not differ significantly by Poly E dose level (data not shown) or compared to placebo.
Table 3.
Adverse events by treatment group. Only toxicities that were possibly related to study drug with at least a 5% incidence rate are listed.
| Any Toxicity n (%) | Poly E (n = 30) | Placebo (n = 10) | P-value* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Total n (%) | Grade 1 | Grade 2 | Grade 3 | Total n (%) | ||
| Gastrointestinal | |||||||||
| Nausea | 8 | 0 | 0 | 8 (27) | 2 | 0 | 0 | 2 (20) | 0.31 |
| Diarrhea | 2 | 1 | 0 | 3 (10) | 1 | 1 | 0 | 2 (20) | 0.17 |
| Constipation | 3 | 0 | 0 | 3 (10) | 0 | 0 | 0 | 0 | 0.41 |
| Indigestion | 9 | 0 | 1^ | 10 (33) | 2 | 0 | 0 | 2 (20) | 0.20 |
| Abdominal pain | 1 | 0 | 0 | 1 (3) | 2 | 0 | 0 | 2 (20) | 0.14 |
| Flatulence | 0 | 1 | 0 | 1 (3) | 1 | 0 | 0 | 1 (10) | 0.19 |
| GI bleed | 0 | 0 | 1^ | 1 (3) | 0 | 0 | 0 | 0 | 0.75 |
| Weight gain | 0 | 1^ | 0 | 1 (3) | 0 | 0 | 0 | 0 | 0.75 |
| Cardiopulmonary | |||||||||
| Palpitations | 1 | 0 | 0 | 1 (3) | 1 | 0 | 0 | 1 (10) | 0.38 |
| Dyspnea | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 (10) | 0.25 |
| Cough | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 (10) | 0.25 |
| Metabolic/Hematologic | |||||||||
| Transaminitis | 2 | 0 | 1^ | 3 (10) | 0 | 0 | 0 | 0 | 0.41 |
| Hyperbilirubinemia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 (10) | 0.25 |
| High alkaline phos | 2 | 0 | 0 | 2 (7) | 0 | 0 | 0 | 0 | 0.56 |
| High lipase | 1 | 1 | 0 | 2 (7) | 0 | 0 | 0 | 0 | 0.56 |
| Hyperuricemia | 1 | 0 | 0 | 1 (3) | 1 | 0 | 0 | 1 (10) | 0.38 |
| Proteinuria | 3 | 0 | 0 | 3 (10) | 1 | 0 | 0 | 1 (10) | 0.44 |
| Anemia | 2 | 0 | 0 | 2 (7) | 0 | 0 | 0 | 0 | 0.56 |
| Neurologic | |||||||||
| Headache | 0 | 2 | 0 | 2 (7) | 2 | 0 | 0 | 2 (20) | 0.11 |
| Confusion | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 (10) | 0.25 |
| Insomnia | 3 | 0 | 1^ | 4 (13) | 0 | 0 | 0 | 0 | 0.30 |
| Endocrine | |||||||||
| Irregular menses | 1 | 0 | 0 | 1 (3) | 1 | 0 | 0 | 1 (10) | 0.38 |
| Hot flashes | 0 | 1 | 0 | 1 (3) | 0 | 0 | 0 | 0 | 0.75 |
| Flushing | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 (10) | 0.25 |
| Vaginal symptoms | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 (10) | 0.25 |
Comparison using Fisher's exact test.
Indicates a dose-limiting toxicity (DLT).
Biomarker Analyses
Secondary biomarker endpoints are summarized in Table 4. No significant trends were observed with the serial blood/urine biomarkers over time (data not shown), therefore, only the baseline and 6 month data are presented. Baseline and 6 month biomarker data were available for 26 participants in the Poly E group and 8 in the placebo group. The Poly E-treated group had a greater increase in mean total urinary tea polyphenols compared to placebo (+152.1 ±186.4 vs. +11.8 ±13.7 μmol/g Cr, p=0.001). However, the levels of urinary tea polyphenols did not differ significantly by dose level of Poly E (data not shown). Due to small numbers, the three dose levels of Poly E were combined for all biomarker analyses. There was about a 70% reduction in serum estradiol levels (p=0.05) and a significant decrease in SHBG (p=0.03) at 6 months compared to baseline in the Poly E group. However, these changes did not differ significantly compared to the placebo group, with the magnitude of changes in the placebo arm being greater but not statistically significant due to smaller numbers. Serum IGFBP-3 showed a greater mean increase for those on Poly E relative to placebo, but again this did not reach statistical significance (+462 ±921 vs. +250 ±782 ng/ml, p=0.53). The IGF-1/IGFBP-3 ratio decreased by 31% in the Poly E group compared to a 2.5% reduction among those who received placebo (p=0.26).
Table 4.
Secondary biomarker endpoints
| Biomarker | Poly E (n = 26) | Placebo (n = 8) | P-value2 | ||
|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | ||
| Total urinary tea polyphenols, μmol/g Cr | |||||
| Mean (SD) | 16.8 (29.2) | 163.8 (176.8) | 8.0 (7.0) | 19.9 (18.6) | |
| Mean absolute change from baseline (SD) | 152.1 (186.4) | 11.8 (13.7) | 0.001 | ||
| Percent change from baseline | 905 | 148 | |||
| P-value1 | <.001 | 0.05 | |||
| Estradiol, pg/ml | |||||
| Mean (SD) | 19.4 (39.5) | 6.3 (13.3) | 20.0 (35.1) | 3.4 (2.6) | |
| Mean absolute change from baseline (SD) | −13.6 (34.2) | −19.7 (37.7) | 0.98 | ||
| Percent change from baseline | −70.1 | −98.5 | |||
| P-value1 | 0.05 | 0.18 | |||
| Testosterone, ng/dl | |||||
| Mean (SD) | 18.9 (14.3) | 16.9 (9.7) | 22.0 (17.1) | 17.5 (11.9) | |
| Mean absolute change from baseline (SD) | −2.3 (9.5) | −5.1 (8.6) | 0.58 | ||
| Percent change from baseline | −12.2 | −23.2 | |||
| P-value1 | 0.29 | 0.14 | |||
| SHBG, nmol/l | |||||
| Mean (SD) | 53.1 (22.2) | 43.9 (18.0) | 68.4 (40.1) | 52.2 (14.9) | |
| Mean absolute change from baseline (SD) | −7.2 (15.9) | −16.5 | (30.5) | 0.81 | |
| Percent change from baseline | −13.5 | −24.1 | |||
| P-value1 | 0.03 | 0.17 | |||
| IGF-1, ng/ml | |||||
| Mean (SD) | 157.3 (48.1) | 147.8 (43.2) | 159.9 (45.0) | 170.8 (35.1) | |
| Mean absolute change from baseline (SD) | 0.3 (34.7) | 2.7 (36.8) | 0.89 | ||
| Percent change from baseline | 0.2 | 1.7 | |||
| P-value1 | 0.97 | 0.84 | |||
| IGFBP-3, ng/ml | |||||
| Mean (SD) | 4077 (1200) | 4383 (1003) | 4122 (947) | 4506 (1257) | |
| Mean absolute change from baseline (SD) | 462 (921) | 250 (782) | 0.91 | ||
| Percent change from baseline | 11.3 | 6.1 | |||
| P-value1 | 0.02 | 0.40 | |||
| IGF-1/IGFBP-3 | |||||
| Mean (SD) | 0.048 (0.056) | 0.034 (0.008) | 0.040 (0.011) | 0.040 (0.012) | |
| Mean absolute change from baseline (SD) | −0.015 (0.062) | −0.001 (0.006) | 0.25 | ||
| Percent change from baseline | −31.3 | −2.5 | |||
| P-value1 | 0.24 | 0.77 | |||
| Ki-67, % | |||||
| Mean (SD) | 1.5 (1.4) | 2.5 (2.8) | 0.8 (1.1) | 1.3 (1.7) | |
| Mean absolute change from baseline (SD) | 1.0 (3.1) | 0.5 (2.4) | 0.76 | ||
| Percent change from baseline | 66.7 | 62.5 | |||
| P-value1 | 0.19 | 0.58 | |||
| ER, % | |||||
| Mean (SD) | 32.0 (17.6) | 27.6 (14.8) | 26.2 (16.2) | 15.9 (14.4) | |
| Mean absolute change from baseline (SD) | −4.4 (14.9) | −10.2 (21.4) | 0.76 | ||
| Percent change from baseline | −13.8 | −38.9 | |||
| P-value1 | 0.23 | 0.25 | |||
| MD, % | |||||
| Mean (SD) | 16.4 (15.6) | 17.1 (16.1) | 15.5 (10.4) | 16.6 (12.1) | |
| Mean absolute change from baseline (SD) | 0.7 (5.6) | 1.1 (4.0) | 0.73 | ||
| Percent change from baseline | 4.3 | 7.1 | |||
| P-value1 | 0.58 | 0.46 | |||
Comparing follow-up to baseline by paired t-test.
Comparing the absolute change from baseline in the Poly E vs. placebo groups using Wilcoxon rank sum test.
Abbreviations: SHBG (sex hormone binding globulin), IGF-1 (insulin-like growth factor-1), IGFBP-3 (IGF binding protein-3), ER (estrogen receptor), MD (mammographic density)
In terms of target tissue effects, no significant changes in the Ki-67 proliferation index or mammographic density were seen after 6 months of Poly E compared to placebo. These results did not differ when stratified by menopausal status (data not shown). Of note, mean baseline Ki-67 levels in the random core breast biopsies of the women in the Poly E and placebo arms were low (1.5 ±1.4% and 0.8 ±1.1%, respectively). There was a favorable but nonsignificant decrease in mean ER-α expression with Poly E and placebo (−4.4 ±14.9%, p=0.23 vs. −10.2 ±21.4%, p=0.25). Supplementary Figure 1 provides a graphical representation of these results.
The genotypic distribution of COMT among study subjects was: GG, 28%; GA, 54%; and AA, 18%. In the Poly E-treated group, mean urinary total tea polyphenol levels did not differ significantly among those who carried at least one high-activity COMT allele (GG and GA genotype) compared to individuals possessing the homozygous variant genotype (AA). Finally, no significant change in quality of life as assessed by the SF-36 was observed in either treatment group (data not shown).
Discussion
This study represents a novel approach to conducting a phase I trial evaluating long-term safety and determining the optimal dose of a potential chemopreventive agent, Polyphenon E. We encountered 5 DLTs during the trial: 2 occurring within the first month on study drug and the others up to nearly 5 months on study drug. Other toxicities were generally mild and did not differ significantly compared to placebo. Although there were transient increases in liver function tests and pancreatic enzymes, there was no evidence of clinically significant liver or pancreatic disease.
The TITE-CRM is a novel statistical methodology that considers long-term (e.g., 6-month) toxicity in dose escalation, while allowing staggered participant entry. Therefore, the trial was conducted in a continuous fashion without accrual suspension. The TITE-CRM on average allocates more participants at the correct MTD, thus enhancing comparison between the placebo and the MTD on other secondary endpoints. However, in our study more women were assigned to the 400mg dose (n = 16) rather than 600mg dose (n = 11). The main advantage of the TITE-CRM is that it allows for the evaluation of long-term toxicities, unlike a conventional dose escalation method with one month of observation which may underestimate late toxicities. Thus, by allowing additional participants to be enrolled at lower dose levels, the TITE-CRM will account for late toxicities that occur after months of treatment, as well as acute toxicity that may appear in the first month. With this trial, we were able to demonstrate that an adaptive method of dose escalation that has been used extensively in cancer treatment trials may also be useful in an early phase chemoprevention trial. An alternative to defining the optimal dose by the MTD (dose with a 25% DLT rate) is to determine the minimal effective dose. One could argue that since there was no difference in urinary tea catechin levels and biomarker effects at the three dose levels of Poly E, the dose of 400mg bid (total of 800mg EGCG daily) may be preferable with a DLT rate of 6%.
This study highlights the importance of assessing long-term toxicity for any chemopreventive agent being developed for chronic use in healthy individuals. The toxicities that we observed with Poly E were consistent with the published literature. For example, we had a case of grade 3 rectal bleeding and liver function abnormalities. A 9-month study in Beagle dogs demonstrated significant GI toxicity and mortality when Poly E was administered in the fasting compared to the fed state (21). Therefore, Poly E was administered within one hour after a substantial meal in this trial to minimize GI toxicities. A recent review reported 34 cases of hepatitis following consumption of green tea supplements used as weight loss products (22). In a phase I dose escalation trial of Poly E 400mg to 2000mg twice daily in patients with chronic lymphocytic leukemia, 33% developed grade I transaminitis (23). Although the pervading public perception is that dietary supplements are generally safe, these toxicities need to be taken into account when weighing the risk:benefit ratio of any chemopreventive agent.
Since individual dietary components have not been successful in preventing cancer (24-28), perhaps using a polyphenolic mixture may be more effective. In a mouse model of lung carcinogenesis, the mixture of catechins with Poly E had more anti-tumor activity than EGCG alone (29). Bioavailability is another common issue with dietary supplements and often the doses used in preclinical studies are not always achievable in humans. Previous studies have found low bioavailability of tea catechins (15, 30). However, when large pharmacological doses of polyphenols are orally administered, peak plasma EGCG concentrations of 5-7 μM are observed in humans, compared to 0.5 μM with green tea consumption (11). We tested pharmacologic doses of Poly E (400-800mg twice daily or a total of 800-1600mg of EGCG daily) with the EGCG content equivalent to 8-24 cups of brewed green tea daily. We demonstrated high levels of urinary EGC and related metabolites (>150 μmol/g Cr) at these doses, compared to individuals who drink upwards of 4-5 cups of green tea daily with urinary metabolites in the 50-100 μmol/g Cr range (18).
For the secondary exploratory analyses, we focused on systemic biomarkers which have been correlated with breast cancer risk, such as circulating sex steroid hormones (31) and IGF axis markers (32). Observational studies have correlated these biomarkers of breast cancer risk with green tea intake. In a cross-sectional study from Japan, higher serum IGF-1 levels, which have been hypothesized to promote rather than prevent cancer growth, were positively associated with green tea consumption (33). Green tea intake has also been correlated with lower circulating estrogen levels in premenopausal and postmenopausal women (34, 35). Proposed mechanisms include tea polyphenols preventing binding of estrogen to its receptor in breast cancer cells (36) and inhibition of aromatase activity (37, 38). In our trial, the Poly E intervention resulted in favorable but not statistically significant changes in serum estradiol and IGF-1/IGFBP-3 ratio. Since we did not adjust for multiple comparisons, we should interpret these trends in biomarker changes with caution given the small sample size. Our results are consistent with a recently published trial of 103 postmenopausal women randomized to a 2-month intervention of placebo vs. Poly E 400mg or 800mg daily (39). Administration of Poly E did not produce consistent patterns of changes in estradiol, testosterone, SHBG, IGF-1, and IGFBP-3. Other explanations for the negative biomarker results include the relatively short-term drug intervention or Poly E mediating its effects via alternative pathways.
The most well-documented modifiable biomarkers of breast cancer risk include mammographic density (40), Ki-67 (41) and ER (42) expression in benign breast tissue. One study demonstrated that daily green tea drinkers had significantly lower percent mammographic density (19.5%) than non-tea drinkers (21.7%, P=0.002) (43). We did not observe a significant change in breast density or the Ki-67 proliferation index after 6 months of Poly E. Of note, the yield of epithelial cells from the random core biopsies and low baseline levels of Ki-67 staining in benign breast tissue may have limited our ability to detect change over time with this drug intervention. In addition, the 1-month washout period for tea consumption may have been insufficient to change baseline breast density measurements among regular green tea drinkers. ER expression in benign breast epithelium increases with age, postmenopausal status, and increasing morphologic abnormality, supporting a positive correlation with breast cancer risk (42, 44, 45). We observed a nonsignificant decrease in mean ER-α expression in the Poly E and placebo groups. We did not measure catechin levels at the tissue level and a potential reason for these negative results may be due to low achievable tissue concentrations of these polyphenols. A recent trial of Poly E 800mg daily for 3-6 weeks in prostate cancer patients demonstrated low bioaccumulation of green tea polyphenols in prostate tissue (46). Therefore, bioavailability at the tissue level may have influenced the effects of Poly E on breast tissue-based biomarkers.
Bioavailability of green tea is also influenced by host-related factors, such as genetic polymorphisms which modulate the metabolism of tea polyphenols. A single G to A transition at codon 158 of COMT results in an amino acid change causing a 3- to 4-fold decrease in enzymatic activity (47). Data from the Shanghai Cohort Study showed that individuals who were homozygous for the low-activity associated COMT genotype (AA) had significantly lower urinary levels of tea polyphenol metabolites relative to those who had at least one high-activity allele (18). We did not observe a significant association between COMT genotype and urinary tea polyphenols among Poly E-treated women. However, our sample size was likely too small to show an association given the 18% incidence of the low-activity (AA) genotype.
We demonstrated the feasibility of enrolling breast cancer survivors in an early phase chemoprevention trial with frequent study visits and involving invasive procedures (e.g., core breast biopsy). Secondary prevention trials in breast cancer survivors evaluating the contralateral breast for surrogate endpoint biomarkers is a useful clinical model for testing novel chemopreventive agents (48). These women have a risk of developing contralateral breast primaries of 0.5-1% per year (49). One study demonstrated a high concordance of 70% among women diagnosed with an ER-negative primary breast cancer having an ER-negative contralateral breast cancer (50). Therefore, this serves as a relevant clinical model for testing chemopreventive agents targeting ER-negative breast cancer.
Strengths of this study include the novel adaptive study design for assessing long-term toxicity of a potential chemopreventive agent. The placebo group provided the background rate of lower grade toxicities, as well as important reference levels for all biomarkers. We had relatively good participant retention with 85% completing the 6-month evaluations. The main weakness is the relatively small sample size for assessing secondary biomarker endpoints. Future studies using this clinical model will need to account for rates of missing data due to inadequate samples for biomarker analysis, particularly for the tissue biomarkers. Our goal was to obtain preliminary data on the biological effects of Poly E, which may elucidate potential mechanisms of action that would inform future clinical efficacy trials. Ongoing trials of green tea in breast cancer include a study in 50 women with newly diagnosed ductal carcinoma in situ given Poly E 600mg daily for 4-6 weeks prior to surgical resection (http://clinicaltrials.gov, NCT01060345) and a placebo-controlled trial of a 1-year intervention of a green tea extract (800mg EGCG daily) in postmenopausal women with high mammographic density (NCT00917735). These trials include biomarker endpoints, such as Ki-67 and mammographic density.
In conclusion, using a novel clinical trial design for phase I testing which evaluates long-term toxicity, we determined the MTD for Polyphenon E to be 600mg bid (total of 1200mg EGCG daily), which will serve as the upper safety limit in future long-term intervention trials. We also demonstrated the bioavailability of Poly E at pharmacologic levels and the feasibility of conducting an early phase chemoprevention trial in a highly-motivated group of women with hormone receptor-negative breast cancer. However, in order to conduct more efficient chemoprevention studies, we need to validate surrogate endpoint biomarkers for short-term breast cancer risk assessment. In general, the public perception is that dietary supplements are safe and therefore may gain wider acceptance in the prevention setting compared to pharmacologic drugs. These agents need to be rigorously tested and future studies should evaluate the clinical efficacy of Poly E on biomarkers of breast cancer risk.
Supplementary Material
Acknowledgments
Grant Support: The study was supported by the National Cancer Institute (grant N01-CN-35159), the National Institute of Environmental Health Sciences (grant ES009089) and the American Cancer Society (grant ACS MRSG-08-021-01-CNE).
Footnotes
Disclosure of Potential Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 2.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 3.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 5.Ogunleye AA, Xue F, Michels KB. Green tea consumption and breast cancer risk or recurrence: a meta-analysis. Breast Cancer Res Treat. 2010;119(2):477–84. doi: 10.1007/s10549-009-0415-0. [DOI] [PubMed] [Google Scholar]
- 6.Nakachi K, Suemasu K, Suga K, Takeo T, Imai K, Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn J Cancer Res. 1998;89(3):254–61. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue M, Tajima K, Mizutani M, Iwata H, Iwase T, Miura S, et al. Regular consumption of green tea and the risk of breast cancer recurrence: follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer Lett. 2001;167(2):175–82. doi: 10.1016/s0304-3835(01)00486-4. [DOI] [PubMed] [Google Scholar]
- 8.Wu AH, Yu MC, Tseng CC, Hankin J, Pike MC. Green tea and risk of breast cancer in Asian Americans. Int J Cancer. 2003;106(4):574–9. doi: 10.1002/ijc.11259. [DOI] [PubMed] [Google Scholar]
- 9.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63(21):7526–9. [PubMed] [Google Scholar]
- 10.Yang CS. Inhibition of carcinogenesis by tea. Nature. 1997;389(6647):134–5. doi: 10.1038/38154. [DOI] [PubMed] [Google Scholar]
- 11.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9(6):429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuart EC, Scandlyn MJ, Rosengren RJ. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sci. 2006;79(25):2329–36. doi: 10.1016/j.lfs.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 13.Chang PY, Mirsalis J, Riccio ES, Bakke JP, Lee PS, Shimon J, et al. Genotoxicity and toxicity of the potential cancer-preventive agent polyphenon E. Environ Mol Mutagen. 2003;41(1):43–54. doi: 10.1002/em.10129. [DOI] [PubMed] [Google Scholar]
- 14.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10(1):53–8. [PubMed] [Google Scholar]
- 15.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9(9):3312–9. [PubMed] [Google Scholar]
- 16.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56(4):1177–82. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- 17.Byng JW, Yaffe MJ, Lockwood GA, Little LE, Tritchler DL, Boyd NF. Automated analysis of mammographic densities and breast carcinoma risk. Cancer. 1997;80(1):66–74. doi: 10.1002/(sici)1097-0142(19970701)80:1<66::aid-cncr9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Inoue-Choi M, Yuan JM, Yang CS, Van Den Berg DJ, Lee MJ, Gao YT, et al. Genetic Association Between the COMT Genotype and Urinary Levels of Tea Polyphenols and Their Metabolites among Daily Green Tea Drinkers. Int J Mol Epidemiol Genet. 2010;1(2):114–123. [PMC free article] [PubMed] [Google Scholar]
- 19.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 20.Cheung YK, Chappell R. A simple technique to evaluate model sensitivity in the continual reassessment method. Biometrics. 2002;58(3):671–4. doi: 10.1111/j.0006-341x.2002.00671.x. [DOI] [PubMed] [Google Scholar]
- 21.Kapetanovic IM, Crowell JA, Krishnaraj R, Zakharov A, Lindeblad M, Lyubimov A. Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs. Toxicology. 2009;260(1-3):28–36. doi: 10.1016/j.tox.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65(4):331–41. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 23.Shanafelt TD, Call TG, Zent CS, LaPlant B, Bowen DA, Roos M, et al. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J Clin Oncol. 2009;27(23):3808–14. doi: 10.1200/JCO.2008.21.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 25.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88(21):1560–70. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 26.Omenn GS, Goodman G, Thornquist M, Grizzle J, Rosenstock L, Barnhart S, et al. The beta-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: smokers and asbestos-exposed workers. Cancer Res. 1994;54(7 Suppl):2038s–2043s. [PubMed] [Google Scholar]
- 27.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 28.Klein EA, Thompson IM, Jr., Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306(14):1549–56. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu H, He J, Mei F, Zhang Q, Hara Y, Ryota S, et al. Lung cancer inhibitory effect of epigallocatechin-3-gallate is dependent on its presence in a complex mixture (polyphenon E). Cancer Prev Res (Phila) 2009;2(6):531–7. doi: 10.1158/1940-6207.CAPR-08-0185. [DOI] [PubMed] [Google Scholar]
- 30.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1025–32. [PubMed] [Google Scholar]
- 31.Cauley JA, Lucas FL, Kuller LH, Stone K, Browner W, Cummings SR. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1999;130(4 Pt 1):270–7. doi: 10.7326/0003-4819-130-4_part_1-199902160-00004. [DOI] [PubMed] [Google Scholar]
- 32.Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11(6):530–42. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maruyama K, Iso H, Ito Y, Watanabe Y, Inaba Y, Tajima K, et al. Associations of food and nutrient intakes with serum IGF-I, IGF-II, IGFBP-3, TGF-b1, total SOD activity and sFas levels among middle-aged Japanese: the Japan Collaborative Cohort study. Asian Pac J Cancer Prev. 2009;10(Suppl):7–22. [PubMed] [Google Scholar]
- 34.Nagata C, Kabuto M, Shimizu H. Association of coffee, green tea, and caffeine intakes with serum concentrations of estradiol and sex hormone-binding globulin in premenopausal Japanese women. Nutr Cancer. 1998;30(1):21–4. doi: 10.1080/01635589809514635. [DOI] [PubMed] [Google Scholar]
- 35.Wu AH, Arakawa K, Stanczyk FZ, Van Den Berg D, Koh WP, Yu MC. Tea and circulating estrogen levels in postmenopausal Chinese women in Singapore. Carcinogenesis. 2005;26(5):976–80. doi: 10.1093/carcin/bgi028. [DOI] [PubMed] [Google Scholar]
- 36.Farabegoli F, Barbi C, Lambertini E, Piva R. (−)-Epigallocatechin-3-gallate downregulates estrogen receptor alpha function in MCF-7 breast carcinoma cells. Cancer Detect Prev. 2007;31(6):499–504. doi: 10.1016/j.cdp.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Satoh K, Sakamoto Y, Ogata A, Nagai F, Mikuriya H, Numazawa M, et al. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol. 2002;40(7):925–33. doi: 10.1016/s0278-6915(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 38.Goodin MG, Rosengren RJ. Epigallocatechin gallate modulates CYP450 isoforms in the female Swiss-Webster mouse. Toxicol Sci. 2003;76(2):262–70. doi: 10.1093/toxsci/kfh001. [DOI] [PubMed] [Google Scholar]
- 39.Wu AH, Spicer D, Stanczyk FZ, Tseng CC, Yang CS, Pike MC. Effect of 2-Month Controlled Green Tea Intervention on Lipoprotein Cholesterol, Glucose, and Hormone Levels in Healthy Postmenopausal Women. Cancer Prev Res (Phila) 2012 doi: 10.1158/1940-6207.CAPR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103(9):744–52. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 41.Khan QJ, Kimler BF, Clark J, Metheny T, Zalles CM, Fabian CJ. Ki-67 expression in benign breast ductal cells obtained by random periareolar fine needle aspiration. Cancer Epidemiol Biomarkers Prev. 2005;14(4):786–9. doi: 10.1158/1055-9965.EPI-04-0239. [DOI] [PubMed] [Google Scholar]
- 42.Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ. Estrogen receptor expression in benign breast epithelium and breast cancer risk. J Natl Cancer Inst. 1998;90(1):37–42. doi: 10.1093/jnci/90.1.37. [DOI] [PubMed] [Google Scholar]
- 43.Wu AH, Ursin G, Koh WP, Wang R, Yuan JM, Khoo KS, et al. Green tea, soy, and mammographic density in Singapore Chinese women. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3358–65. doi: 10.1158/1055-9965.EPI-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shoker BS, Jarvis C, Sibson DR, Walker C, Sloane JP. Oestrogen receptor expression in the normal and pre-cancerous breast. J Pathol. 1999;188(3):237–44. doi: 10.1002/(SICI)1096-9896(199907)188:3<237::AID-PATH343>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Shaaban AM, Sloane JP, West CR, Foster CS. Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor-alpha and Ki-67 expression. Am J Pathol. 2002;160(2):597–604. doi: 10.1016/s0002-9440(10)64879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen MM, Ahmann FR, Nagle RB, Hsu CH, Tangrea JA, Parnes HL, et al. Randomized, Double-blind, Placebo Controlled Trial of Polyphenon E in Prostate Cancer Patients before Prostatectomy: Evaluation of Potential Chemopreventive Activities. Cancer Prev Res (Phila) 2011 doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61(18):6716–22. [PubMed] [Google Scholar]
- 48.Stearns V, Gallagher A, Kleer CG, Singh B, Freedman M, Haddad BR, et al. A pilot study to establish a clinical model to perform phase II studies of breast cancer chemopreventive agents in women at high risk with biomarkers as surrogate endpoints for activity. Clin Cancer Res. 2004;10(24):8332–40. doi: 10.1158/1078-0432.CCR-04-0297. [DOI] [PubMed] [Google Scholar]
- 49.Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 2010;28(14):2404–10. doi: 10.1200/JCO.2009.24.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swain SM, Wilson JW, Mamounas EP, Bryant J, Wickerham DL, Fisher B, et al. Estrogen receptor status of primary breast cancer is predictive of estrogen receptor status of contralateral breast cancer. J Natl Cancer Inst. 2004;96(7):516–23. doi: 10.1093/jnci/djh097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.