Abstract
Limited studies indicate a possible association of 5′-UTR thymidylate synthase enhancer region polymorphism and treatment outcome in patients medicated with 5-fluorouracil (5-FU). The study was designed to verify the relationship in patients with colorectal cancer (CRC), a Polish population that received 5-FU-based adjuvant chemotherapy. The study analyzed 145 Astler-Coller B2 and C CRC patients. Genotyping for a variable number of tandem repeats and G to C single-nucleotide polymorphism in the 5′-UTR of the thymidylate synthase (TS) gene was carried out. TS genotypes were classified into high expression (high TS) and low expression types (low TS). High TS was found in 22.8% of patients. The right-side tumors were more frequently associated with high TS than the left-side tumors (p=0.024). High TS was only found in 9.3% of rectal tumors, but in 29.7% of colon cancers (p=0.0042). Disease-free survival after 20 months (DFS 20) was longer in subjects with low TS than in high TS (p=0.043). Patients who underwent chemotherapy had longer DFS 20 in the low TS than in the high TS subgroup (p=0.051). The low TS was found to be an independent good prognostic factor for DFS 20 in the whole group as well as in the subgroup treated with chemotherapy (p=0.024 and p=0.034, respectively). Patients with low TS did not show any differences in DFS 20 whether they were treated with adjuvant chemotherapy or not. Proximal CRC tumors are characterized by higher TS expression genotypes than distal tumors, and are at significantly greater risk of early recurrence during the first 20 months after surgery.
Introduction
Colorectal cancer (CRC) belongs to one of the most common human malignancies and the stage of the disease decides the mode of treatment. Surgery is essential for the localized form, and chemotherapy is indicated in stage III (node positive) patients, although some stage II (node negative) patients may also benefit from its application (Quasar Collaborative Group et al., 2007; Wolpin and Mayer, 2008; Midgley et al., 2009). In both adjuvant and palliative settings, 5-fluorouracil (5-FU) plays a central role in chemotherapy regimens. However, only about one-third of the patients treated with adjuvant chemotherapy benefit from it. Thus, there is an urgent need to identify markers of tumor sensitivity to proposed chemotherapy.
Thymidylate synthase (TS) is the enzyme that catalyzes the reductive methylation of deoxyuridine monophosphate to form deoxythymidine monophosphate and dihydrofolate (Carreras and Santi, 1995). This reaction provides a de novo source of thymidylate, an essential precursor for DNA biosynthesis (Carreras and Santi, 1995). TS functions as an RNA binding protein that, at the translational level, regulates the expression of its own mRNA translation and other cellular mRNAs, including that of p53 (Ju et al., 1999; Xi et al., 2006). Recently, it has been found that TS may act as an oncogene as well (Rahman et al., 2004). TS is the target enzyme for 5-FU (Danenberg, 1977). Several studies have demonstrated that TS tissue levels may modulate prognosis, irrespectively of 5-FU and the response to 5-FU medication, with high expression levels generally associated with a poor response, especially in advanced disease (Leichman et al., 1997). Yet, the predictive role of the TS level for 5-FU sensitivity in adjuvant treatment is controversial. In a recent meta-analysis by Popat et al. (2004), 13 studies consisting of 887 patients with metastatic CRC and 7 studies involving 2610 patients with localized CRC were analyzed. The authors showed that tumors expressing high levels of TS seemed to have poor overall survival (OS) compared with tumors expressing low TS levels (Popat et al., 2004). TS gene expression is modulated by functional, significant germ-line polymorphisms in the 5′-UTR thymidylate synthase enhancer region (TSER), and 3′-UTR (TS 1494del6b) of the gene (Horie et al., 1995; Mandola et al., 2003; Lurje et al., 2009). A variable number of tandem repeats (VNTR) in the promoter region of TS (TSER), mainly 2 (2R) and 3 repeats (3R), and cytosine versus guanine single-nucleotide polymorphism (SNP) can influence the efficiency of translation (Mandola et al., 2003; Lurje et al., 2009). The presence of a G to C SNP within the second repeat of the 3R allele (3G/3C) alters mRNA stability, and therefore enzyme activity (Mandola et al., 2003; Lurje et al., 2009). Thus, TS polymorphisms in tumor cells might influence the clinical outcome under 5-FU treatment through the altered TS activity. TS genotypes of patients with CRC were classified into high expression types (high TS): 2R/3G, 3C/3G, 3G/3G and low expression types (low TS): 2R/2R, 2R/3C, 3C/3C (Kawakami and Watanabe, 2003).
The present study was designed to investigate whether 5-TSER polymorphisms (TSER 2R/3R and TSER 3G/3C) are associated with disease-free survival (DFS) and OS in patients with CRC who received 5-FU-based adjuvant chemotherapy.
Materials and Methods
Participants and study design
The study included 145 patients, aged 63 years (range 28–81 years) with Astler-Coller B2 and C CRC, who underwent radical resection (R0) at the Department of Surgery of the Pomeranian Medical University, Szczecin, Poland. The clinicopathological characteristics of patients are summarized in Table 1; 117 (59 stage B2 and 58 stage C Astler-Coller). Patients received 5-FU-based adjuvant chemotherapy according to the Mayo regimen (FA 20 mg/m2, 5-FU 425 mg/m2/day, on days 1–5 every 28 days, six cycles). For stage B, chemotherapy was applied only for high risk patients (obstruction, T4 tumor, perforation). Twenty-two patients with stage B2, and six with stage C Astler-Coller CRC were not treated with adjuvant chemotherapy (patients with stage C did not receive adjuvant chemotherapy due to contraindications). Chemotherapy and survival details were obtained from the hospital medical case histories. Patients with rectal cancer also received preoperative radiotherapy (5×5 Gy).
Table 1.
Patient Characteristics
| |
|
TS expression type |
||
|---|---|---|---|---|
| n | Low (3C/3C, 2R/3C, 2R/2R) n (%) | High (3G/3G, 3G/3C, 2R/3G) n (%) | p | |
| Sex | ||||
| Male | 80 | 59 (73.8) | 21 (26.2) | 0.18 |
| Female | 65 | 54 (83.1) | 11 (16.9) | |
| Astler-Coller | ||||
| B2 | 81 | 65 (80.2) | 16 (19.8) | 0.45 |
| C | 64 | 48 (75.0) | 16 (25.0) | |
| Grade | ||||
| 1/2 | 102 | 81 (79.4) | 21 (20.6) | 0.42 |
| 3 | 10 | 9 (90.0) | 1 (10.0) | |
| Tumor location | ||||
| Right-side tumor | 33 | 21 (63.6) | 12 (36.4) | 0.024 |
| Left-side tumor | 112 | 92 (82.1) | 20 (17.9) | |
| Tumor location | ||||
| Rectum | 54 | 49 (90.7) | 5 (9.3) | 0.0042 |
| Colon | 91 | 64 (70.3) | 27 (29.7) | |
| Chemotherapy | ||||
| Yes | 117 | 92 (78.6) | 25 (21.4) | 0.68 |
| No | 28 | 21 (75.0) | 7 (25.0) | |
| Age | ||||
| <63 | 70 | 56 (80.0) | 14 (20.0) | 0.56 |
| ≥63 | 75 | 57 (76.0) | 18 (24.0) | |
TS, thymidylate synthase.
The time from surgery until the time of death due to cancer or to the last known follow-up was regarded as OS, and the time until the first appearance of metastasis or local recurrence was regarded as DFS.
The project was approved by the Ethics Committee of Pomeranian Medical University, and all patients signed informed consent.
Genotyping
Genomic DNA was extracted from 0.2 mL of K3EDTA-anticoagulated blood with a QIAamp DNA Mini Kit (Qiagen) and from serum using the Sherlock AX kit (A&A Biotechnology). Genotyping for the VNTR/SNP polymorphism in the 5′-UTR of the TS gene was carried out based on the Kawakami and Watanabe method (Kawakami and Watanabe, 2003). Polymerase chain reaction (PCR) of the polymorphic region in the promoter of TS was performed using previously published primers: forward 5′-AGGCGCGCGGAAGGGGTCCT-3′ and reverse 5′-TCCGAGCCGGCCACAGGCAT-3′. The reaction was carried out in a total volume of 20 μL containing 40 ng of template DNA, 4 pM of each primer, 1× PCR buffer (Qiagen), 1× Q-Solution (Qiagen), 1.5 mM MgCl2 (Qiagen), 200 μM each dNTP (MBI Fermentas), and 2.5 U of HotStarTaq polymerase (Qiagen). The amplification was performed with initial denaturation at 94°C for 15 min, and then 34 cycles of denaturation at 94°C for 20 s, annealing at 57°C for 40 s, and extension at 72°C for 40 s. The final 72°C incubation was extended by 8 min. The double repeat (2R) of the promoter region resulted in a 116-bp PCR product and the triple repeat (3R) in a 141-bp product. The amplified fragments were separated by electrophoresis in a 3% agarose gel stained with ethidium bromide. The G>C substitution within the triple repeat was determined by restriction fragment length polymorphism (RFLP). For the RFLP assays, a 16-μL aliquot of PCR product (which showed genotypes with the 3R allele) was incubated at 37°C for 18 h with 10 U of HaeIII enzyme. The restriction fragments sizes were 66, 37, 28, and 10 bp for the 3G allele and 94, 37, and 10 bp for the 3C allele. The fragments were separated by electrophoresis in a 4% agarose gel stained with ethidium bromide. The results were recorded by photographing the gels in UV light.
Statistical analysis
The analysis was performed using STATISTICA software (StatSoft, Inc., 2008, version 8.0). Nominal variables were tested using chi-squared analysis. Risk factors for OS and DFS were determined by univariate and multivariate Cox proportional hazards regression analysis. The results of Cox regression are expressed as a hazard ratio (HR). The differences between univariate variables were illustrated by Kaplan–Meier plots. A p-value of less than 0.05 was considered significant.
Results
The clinicopathological characteristics of 145 patients and the distribution of 5′-TSER polymorphisms are presented in Table 1. The high expression TS polymorphisms (3G/3G, 3G/3C, 2R/3G) were found in 22.1% of patients and the low TS polymorphisms (3C/3C, 2R/3C, 2R/2R) in 77.9% of patients. Tumors originating from the proximal colon (right side) were more frequently associated with high TS (36.4%) than the left-side tumors (17.9%) (p=0.024). High TS was only found in 9.3% of rectal tumors, but in 29.7% of colon cancers (p=0.0042). No significant differences in the frequencies of 5′-TSER genotypes were associated with sex, Astler-Coller stage, age, grade, or introduced chemotherapy.
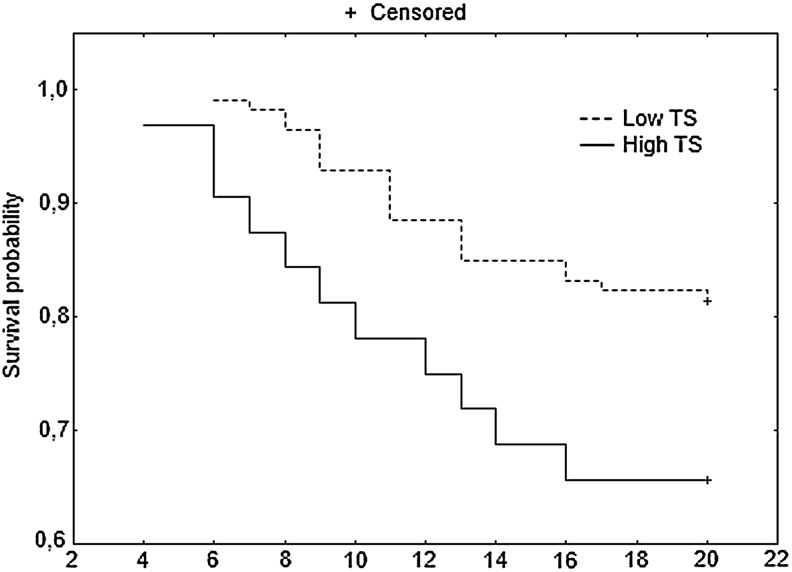
In the group of patients with B2 tumors, DFS detected 20, 36, and 60 months after the potentially curative resection was longer than in patients with Astler-Coller group C tumors. The 20-month DFS was significantly longer in patients with high TS polymorphisms (p=0.043, HR 2.13) than those with low TS expression (Fig. 1 and Table 2). Patients with colon tumors had better DFS at 60 months (DFS 60) than subjects with rectal tumors (p=0.0088) (Table 2).
FIG. 1.
Disease-free survival (DFS) at 20 months for patients with Astler-Coller B2 and C stages of colorectal cancer (CRC).
Table 2.
Univariate Analysis of Survival for all Patients
| |
DFS 20 |
DFS 36 |
DFS 60 |
|||
|---|---|---|---|---|---|---|
| pa | HR | pa | HR | pa | HR | |
| Sex | ||||||
| Male | 0.39 | 1.37 | 0.69 | 1.12 | 0.94 | 0.98 |
| Female | 1 | 1 | 1 | |||
| Astler-Coller | ||||||
| B2 | 0.0027 | 0.32 | 0.0065 | 0.45 | 0.0004 | 0.41 |
| C | 1 | 1 | 1 | |||
| Grade | ||||||
| 1/2 | 0.50 | 0.66 | 0.92 | 1.07 | 0.19 | 0.58 |
| 3 | 1 | 1 | 1 | |||
| Tumor location | ||||||
| Right-side tumor | 0.96 | 0.98 | 0.34 | 0.69 | 0.12 | 0.59 |
| Left-side tumor | 1 | 1 | 1 | |||
| Tumor location | ||||||
| Rectum | 0.66 | 1.17 | 0.22 | 1.43 | 0.0088 | 1.92 |
| Colon | 1 | 1 | 1 | |||
| Chemotherapy | ||||||
| Yes | 0.92 | 1.04 | 0.65 | 1.19 | 0.53 | 1.23 |
| No | 1 | 1 | 1 | |||
| Age | ||||||
| <63 | 0.36 | 0.72 | 0.74 | 0.91 | 0.66 | 1.11 |
| ≥63 | 1 | 1 | 1 | |||
| 5′-TSER TS | ||||||
| High TS 3G/3G, 3G/3C, 2R/3G | 0.043 | 2.13 | 0.17 | 1.57 | 0.22 | 1.42 |
| Low TS 3C/3C, 2R/3C, 2R/2R | 1 | 1 | 1 | |||
Test long-rank.
DFS 20, DFS 36, DFS 60, disease-free survival at 20, 36, and 60 months; HR, hazard ratio.
Patients with stage B2 and C CRC who underwent adjuvant chemotherapy had better DFS 20 in the subgroup with low versus high TS (Table 3) (p=0.051). In the 36 and 60 months after the surgical treatment, a trend of longer DFS for the low TS subgroup was still observed, but was not statistically significant. DFS 60 in patients who underwent adjuvant chemotherapy was influenced by tumor location, with better prognosis for colon cancers than rectal tumors (p=0.01), and longer DFS 60 for proximal tumors than distal tumors (p=0.033).
Table 3.
Univariate Analysis of Survival for Patients Treated with Adjuvant Chemotherapy
| |
DFS 20 |
DFS 36 |
DFS 60 |
|||
|---|---|---|---|---|---|---|
| pa | HR | pa | HR | pa | HR | |
| Sex | ||||||
| Male | 0.70 | 1.17 | 0.89 | 0.96 | 0.61 | 0.87 |
| Female | 1 | 1 | 1 | |||
| Astler-Coller | ||||||
| B2 | 0.0035 | 0.26 | 0.0068 | 0.40 | 0.00069 | 0.38 |
| C | 1 | 1 | 1 | |||
| Grade | ||||||
| ½ | 0.49 | 0.65 | 0.85 | 1.12 | 0.23 | 0.61 |
| 3 | 1 | 1 | 1 | |||
| Tumor location | ||||||
| Right-side tumor | 0.58 | 0.76 | 0.18 | 0.55 | 0.033 | 0.42 |
| Left-side tumor | 1 | 1 | 1 | |||
| Tumor location | ||||||
| Rectum | 0.55 | 1.26 | 0.25 | 1.45 | 0.01 | 2.02 |
| Colon | 1 | 1 | 1 | |||
| Age | ||||||
| <63 | 0.20 | 0.60 | 0.59 | 0.84 | 0.83 | 1.06 |
| ≥63 | 1 | 1 | 1 | |||
| 5′-TSER TS | ||||||
| High TS 3G/3G, 3G/3C, 2R/3G | 0.051 | 2.23 | 0.14 | 1.69 | 0.23 | 1.47 |
| Low TS 3C/3C, 2R/3C, 2R/2R | 1 | 1 | 1 | |||
Test long-rank.
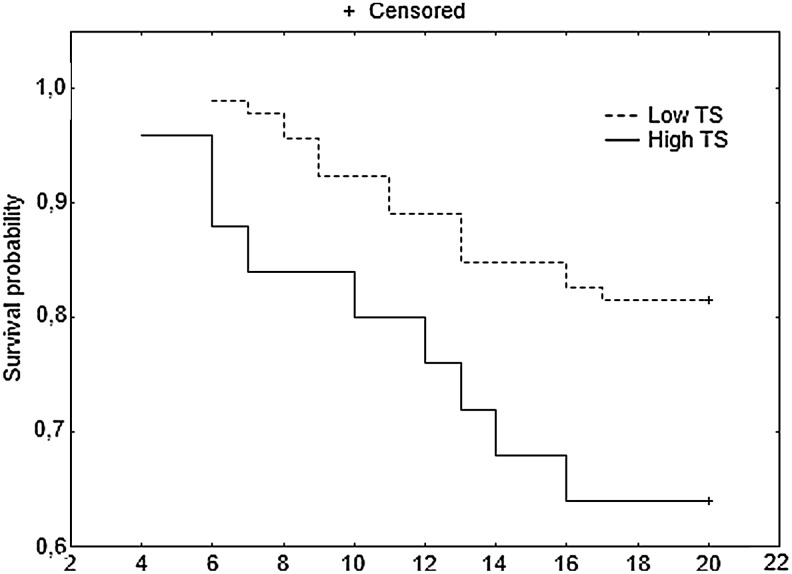
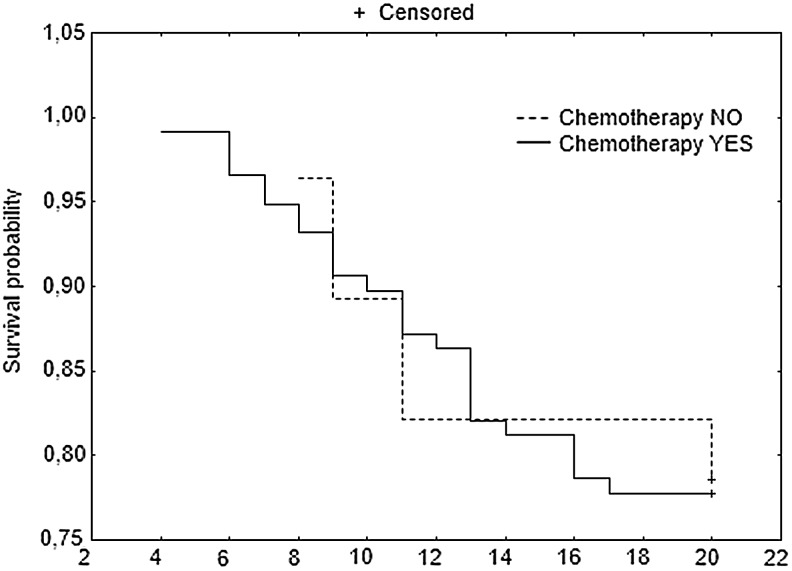
Furthermore, patients with stage B2 and C CRC who underwent adjuvant chemotherapy had better DFS 20 in the subgroup with low versus high TS (Fig. 2). When B2 and C groups were analyzed separately, no significant differences in DFS 20 were found between patients with low versus high TS, but a trend toward longer DFS 20 in patients with low TS was observed (data not shown). When patients with B2 and C tumors with low TS were grouped together, no differences in DFS 20 were found between the subgroups that received and did not receive adjuvant therapy (Fig. 3). A similar analysis for the B2 and C groups with high TS could not be performed due to the small number of patients.
FIG. 2.
DFS at 20 months for patients with stage B2 and C CRC who underwent adjuvant chemotherapy.
FIG. 3.
DFS at 20 months for patients with stage B2 and C CRC with low thymidylate synthase (TS) polymorphisms.
Astler-Coller stage B2 was an independent, significant factor for better DFS 20, 36, and 60 (p=0.016, p=0.0055, and p=0.0033, respectively) in all B2+C patients (n=145). The low TS polymorphism type was an independent, significant factor for better DFS 20 in all B2+C patients (n=145, p=0.024) (Table 4) and in the patient group treated with adjuvant chemotherapy (p=0.034) (Table 5).
Table 4.
Multivariate Analysis of Survival for All Patients
| |
DFS 20 |
DFS 36 |
DFS 60 |
|||
|---|---|---|---|---|---|---|
| pa | HR | pa | HR | pa | HR | |
| Sex | ||||||
| Male | 0.19 | 1.83 | 0.63 | 1.19 | 0.93 | 0.98 |
| Female | 1 | |||||
| Astler-Coller | ||||||
| B2 | 0.016 | 0.20 | 0.0055 | 0.36 | 0.0033 | 0.40 |
| C | 1 | |||||
| Grade | ||||||
| ½ | 0.72 | 0.78 | 0.58 | 1.43 | 0.56 | 0.77 |
| 3 | 1 | |||||
| Tumor location | ||||||
| Right-side tumor | 0.50 | 0.63 | 0.34 | 0.57 | 0.52 | 0.73 |
| Left-side tumor | 1 | |||||
| Tumor location | ||||||
| Rectum | 0.61 | 1.29 | 0.22 | 1.59 | 0.058 | 1.88 |
| Colon | 1 | |||||
| Chemotherapy | ||||||
| Yes | 0.15 | 0.43 | 0.75 | 0.85 | 0.82 | 0.91 |
| No | 1 | |||||
| Age | ||||||
| <63 | 0.30 | 0.63 | 0.95 | 1.02 | 0.86 | 1.06 |
| ≥63 | 1 | |||||
| 5′-TSER TS | ||||||
| High TS 3G/3G, 3G/3C, 2R/3G | 0.024 | 2.91 | 0.12 | 1.86 | 0.14 | 1.70 |
| Low TS 3C/3C, 2R/3C, 2R/2R | 1 | |||||
Test long-rank.
Table 5.
Multivariate Analysis of Survival for Patients Treated with Adjuvant Chemotherapy
| |
DFS 20 |
DFS 36 |
DFS 60 |
|||
|---|---|---|---|---|---|---|
| pa | HR | pa | HR | pa | HR | |
| Sex | ||||||
| Male | 0.50 | 1.38 | 0.97 | 1.01 | 0.75 | 0.90 |
| Female | 1 | |||||
| Astler-Coller | ||||||
| B2 | 0.0027 | 0.14 | 0.0037 | 0.31 | 0.0037 | 0.37 |
| C | 1 | |||||
| Grade | ||||||
| ½ | 0.69 | 0.75 | 0.54 | 1.48 | 0.60 | 0.79 |
| 3 | 1 | |||||
| Tumor location | ||||||
| Right-side tumor | 0.58 | 0.63 | 0.30 | 0.50 | 0.31 | 0.55 |
| Left-side tumor | 1 | |||||
| Tumor location | ||||||
| Rectum | 0.64 | 1.30 | 0.32 | 1.52 | 0.078 | 1.91 |
| Colon | 1 | |||||
| Age | ||||||
| <63 | 0.23 | 0.55 | 0.89 | 1.05 | 1.04 | 0.90 |
| ≥63 | 1 | |||||
| 5′-TSER TS | ||||||
| High TS 3G/3G, 3G/3C, 2R/3G | 0.034 | 2.87 | 0.18 | 1.76 | 0.34 | 1.44 |
| Low TS 3C/3C, 2R/3C, 2R/2R | 1 | |||||
Test long-rank.
Statistical analysis of OS at 20 months was not possible due to a small number of patients. Any analyzed parameter had an impact on OS at 36 months. The OS at 60 months (OS 60) was influenced only by tumor location with better prognosis for colon cancers than rectal tumors (p=0.018) (Table 6). Female sex and colon location of tumors were significant and independent good prognostic factors for OS 60 (p=0.044 and p=0.006, respectively) (Table 7). In the subgroup treated with chemotherapy, solely the colon location was an independent favorable prognostic factor for OS 60 (p=0.037) (data not shown).
Table 6.
Univariate Analysis of Survival of all Patients
| |
OS 36 |
OS 60 |
||
|---|---|---|---|---|
| pa | HR | pa | HR | |
| Sex | ||||
| Male | 0.14 | 2.66 | 0.17 | 1.86 |
| Female | 1 | 1 | ||
| Astler-Coller | ||||
| B2 | 0.35 | 0.59 | 0.24 | 0.61 |
| C | 1 | 1 | ||
| Grade | ||||
| ½ | 0.10 | 0.26 | 0.19 | 0.43 |
| 3 | 1 | 1 | ||
| Tumor location | ||||
| Right-side tumor | 0.54 | 1.45 | 0.89 | 0.93 |
| Left-side tumor | 1 | 1 | ||
| Tumor location | ||||
| Rectum | 0.46 | 1.51 | 0.018 | 2.74 |
| Colon | 1 | 1 | ||
| Chemotherapy | ||||
| Yes | 0.73 | 0.79 | 0.79 | 0.87 |
| No | 1 | 1 | ||
| Age | ||||
| <63 | 0.43 | 0.64 | 0.71 | 0.85 |
| ≥63 | 1 | 1 | ||
| 5′-TSER TS | ||||
| High TS 3G/3G, 3G/3C, 2R/3G | 0.93 | 1.06 | 0.88 | 1.08 |
| Low TS 3C/3C, 2R/3C, 2R/2R | 1 | 1 | ||
Test long-rank.
OS 36 and OS 60, overall survival at 36 and 60 months.
Table 7.
Multivariate Analysis of Survival of All Patients
| |
OS 60 |
|
|---|---|---|
| pa | HR | |
| Sex | ||
| Male | 0.044 | 3.27 |
| Female | 1 | |
| Astler-Coller | ||
| B2 | 0.11 | 0.40 |
| C | 1 | |
| Grade | ||
| ½ | 0.30 | 0.49 |
| 3 | 1 | |
| Tumor location | ||
| Right-side tumor | 0.46 | 2.12 |
| Left-side tumor | 1 | |
| Tumor location | ||
| Rectum | 0.006 | 9.52 |
| Colon | 1 | |
| Chemotherapy | ||
| Yes | 0.17 | 0.35 |
| No | 1 | |
| Age | ||
| <63 | 0.29 | 0.57 |
| ≥63 | 1 | |
| 5′-TSER TS | ||
| High TS 3G/3G, 3G/3C, 2R/3G | 0.41 | 1.76 |
| Low TS 3C/3C, 2R/3C, 2R/2R | 1 | |
Test long-rank.
Discussion
In the present study, patients were stratified into two groups according to TS gene polymorphisms associated with high or low TS expression, as indicated by Kawakami and Watanabe (2003). In our study, the following distribution of the 5′-TSER polymorphisms was documented: 22.8% of polymorphisms were associated with high TS expression (3G/3G, 3G/3C, 2R/3G; high TS) and 77.2% were associated with low TS expression (3C/3C, 2R/3C, 2R/2R; low TS). The frequency of these alleles differs with ethnicity. Homozygous triple repeat subjects were twice as common in Chinese subjects (67%) than in Caucasian subjects (38%) (Marsh et al., 1999). In the white population, 2R/3R heterozygosity occurs in ∼50% of the population and each of the two homozygous genotypes is found in ∼25% of the population (Marsh et al., 2001). The frequency of the 3C allele among all 3R alleles showed a variation of 56%, 47%, 28%, and 37% for whites, Hispanics, African–Americans, and Chinese, respectively (Lurje et al., 2009).
Tumors originating from the proximal colon in respect to the splenic flexure (proximal CRC) were significantly more frequently associated with high TS than tumors originating from the distal colon in respect to the splenic flexure. In addition, rectal tumors were associated with high TS in only 9.3% of cases, whereas colon tumors were associated with high TS in 29.7% of patients. Similar results were reported by Fernández-Contreras et al. (2009). In a previous study (Sulzyc-Bielicka et al., 2009), we found that proximal CRC was characterized by a higher TS protein expression, as detected by immunohistochemistry, than distal tumors. Elsaleh et al. (2000) found a striking survival benefit after adjuvant 5-FU-based therapy in patients with Dukes C CRC who had right-side tumors, especially in women.
The general finding of this study is that DFS 20 was significantly better for tumors with low TS polymorphisms than for high TS polymorphisms. In addition, all patients with B2 and C Astler-Coller CRC who underwent adjuvant chemotherapy had significantly better DFS 20 in the low TS subgroup compared to the high TS subgroup. The low TS polymorphism type was an independent and significant good prognostic factor for DFS 20 in the whole analyzed group and in the subgroup treated with chemotherapy. Therefore, the high TS polymorphism type may be a risk factor for early recurrence of CRC during the first 20 months after operation. The observations of the present study revealed that the colon location and female sex were independent, and good prognostic factors for OS 60. These findings are in agreement with other studies that show lower survival rates in patients with distal tumor locations than those with proximal tumors (Laurie et al., 1989).
There were no differences in DFS 20 for patients with low TS treated with adjuvant chemotherapy compared to those not medicated. This observation suggests that patients with low TS may not benefit from 5-FU-based chemotherapy. In the study conducted by Allegra et al. (2003), no interaction between TS expression measured by immunohistochemistry in primary CRC tumors and chemotherapy outcome was shown. In contrast, Edler et al. (2002) on the basis of immunohistochemical assessment of TS in CRC, suggested a worse outcome in subjects with low TS treated with 5-FU than those not treated with chemotherapy.
Our results based on TS polymorphisms are consistent with those from immunohistochemical assessment of TS protein expression, and show that the high TS polymorphism leads to early cancer recurrence, as measured by DFS 20. In immunohistochemical studies, patients with high TS protein expression either do not develop recurrences or they tend to develop them rather quickly (Edler et al., 2002; Kornmann et al., 2002). Patients with low TS expression tend to have later recurrences, but they are more frequent (Kornmann et al., 2002). Low TS expression may be associated with a low spontaneous recurrence rate and longer survival, also indicating less benefit from adjuvant 5-FU treatment (Edler et al., 2002).
Differences in outcome between the investigated groups seem to disappear with time. This may be a consequence of our second line of treatment of a recurrent disease, that is, surgery or other types of chemotherapy both aiming at improving the chances of OS. A relatively small number of patients or too short a time of observation could also influence our observations.
High TS levels (mRNA, protein, enzyme activity) in advanced disease seem to predict nonresponsiveness to 5-FU and a worse prognosis (Leichman et al., 1997; Paradiso et al., 2000; Etienne et al., 2002; Ichikawa et al., 2003; Popat et al., 2004). For adjuvant treatment, it is controversial whether TS levels measured in primary CRC predict clinical benefit from 5-FU-based treatment. In several studies, a high TS level was associated with poor postoperative outcome, independent of the Duke's stage (Kralovánszky et al., 2002; Formentini et al., 2004), and leading to poor DFS and OS in an adjuvant setting (Cascinu et al., 2001; Kralovánszky et al., 2002; Allegra et al., 2003; Popat et al., 2004; Broll et al., 2005). In contrast, other reports indicated that high TS is predictive of 5-FU treatment and patients with a high TS level may benefit from adjuvant 5-FU therapy (Edler et al., 2002; Kornmann et al., 2003). Allegra et al. (2003) in a study on 706 patients with Dukes B and C CRC, found no predictive value of TS protein expression determined by immunohistochemistry in primary tumors, but showed a good prognostic impact of low TS protein expression on survival. The impact of TS gene polymorphisms on prognosis and 5-FU-based chemotherapy in CRC patients was reviewed by Lurje et al. (2009). The largest study concerning adjuvant 5-FU was conducted by Iacopetta et al. (2001) and showed better survival in the low TS polymorphism subset receiving adjuvant 5-FU compared to those not receiving adjuvant chemotherapy. Tsuji et al. (2003) found no survival differences among patients who received adjuvant 5-FU after curative CRC surgery, when stratified by TS gene polymorphism. Similar observations were also reported by Prall et al. (2007). Kawakami and Watanabe (2003) documented a negative predictive effect of the C/G SNP for adjuvant 5-FU chemotherapy, but Prall et al. (2007) reported no predictive value for such therapy.
Hitre et al. (2005) in a prospective study of patients with CRC treated with 5-FU adjuvant therapy found that subjects with germline high TS polymorphisms had longer DFS and OS. The authors concluded that the combination of 5′-TSER and 3′-TSER TS polymorphisms, measured from PBMC of CRC patients receiving adjuvant 5-FU-based chemotherapy, is an independent prognostic marker (Hitre et al., 2005). In addition, the authors suggest that the germline TS genotypes leading to high TS expression (Danenberg, 2004; Mandola et al., 2004) can predict significantly better DFS and OS.
It is very important to point out that the published data refer to various populations with different ethnic background that may impact on the study outcome. As an example, the Hungarian study by Hitre et al. (2005) included a majority of patients (82%) with high TS polymorphisms, whereas in our study only 22.1% had high TS polymorphisms. The more ethnic differences in the metabolism of the folate cofactor of 5-FU may also affect the final study results, as well as findings suggesting a relationship between the folate level and TS polymorphisms (Chen et al., 2003).
It should be stated that some other genetic factors may influence the 5-FU response, like microsatellite stability (MSI) and TP53 mutations. Some studies suggest a relationship between p53 status, MSI, and TS expression. Kristensen et al. (2010) revealed that TS expression is significantly higher in MSI tumors compared with microsatellite stable tumors, and Popat et al. (2006) found that CRC cells with high TS levels are more likely to overexpress p53. However, clinical studies are not sufficient to reliably predict a response to 5-FU on the basis of MSI, TP53, and TS status.
On the basis of our results, it can be concluded that CRC tumors located proximal to the splenic flexure were more frequently associated with high TS polymorphisms than distal tumors. Patients with high TS polymorphisms had a significantly greater risk of early recurrence during the first 20 months after surgery. The high TS polymorphism patients should be subjected to a strict follow-up protocol for early detection of cancer recurrence.
Acknowledgment
This work was supported by the Pomeranian Medical University Research Program grant no. WLBiML-401-01/3/12.
Author Disclosure Statement
No competing financial interests exist.
References
- Allegra CJ. Paik S. Colangelo LH, et al. Prognostic value of thymidylate synthase, Ki-67, and p53 in patients with Dukes' B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol. 2003;21:241–250. doi: 10.1200/JCO.2003.05.044. [DOI] [PubMed] [Google Scholar]
- Broll R. Busch P. Duchrow M, et al. Influence of thymidylate synthase and p53 protein expression on clinical outcome in patients with colorectal cancer. Int J Colorectal Dis. 2005;20:94–102. doi: 10.1007/s00384-004-0621-5. [DOI] [PubMed] [Google Scholar]
- Carreras CW. Santi DV. The catalytic mechanism and structure of thymidylate synthase. Annu Rev Biochem. 1995;64:721–762. doi: 10.1146/annurev.bi.64.070195.003445. [DOI] [PubMed] [Google Scholar]
- Cascinu S. Graziano F. Valentini M, et al. Vascular endothelial growth factor expression, S-phase fraction and thymidylate synthase quantitation in node-positive colon cancer: relationships with tumor recurrence and resistance to adjuvant chemotherapy. Ann Oncol. 2001;12:239–244. doi: 10.1023/a:1008339408300. [DOI] [PubMed] [Google Scholar]
- Chen J. Hunter DJ. Stampfer MJ, et al. Polymorphism in the thymidylate synthase promoter enhancer region modifies the risk and survival of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:958–962. [PubMed] [Google Scholar]
- Danenberg PV. Thymidylate synthetase—a target enzyme in cancer chemotherapy. Biochim Biophys Acta. 1977;473:73–92. doi: 10.1016/0304-419x(77)90001-4. [DOI] [PubMed] [Google Scholar]
- Danenberg PV. Pharmacogenomics of thymidylate synthase in cancer treatment. Front Biosci. 2004;9:2484–2494. doi: 10.2741/1410. [DOI] [PubMed] [Google Scholar]
- Edler D. Glimelius B. Hallström M, et al. Thymidylate synthase expression in colorectal cancer: a prognostic and predictive marker of benefit from adjuvant fluorouracil-based chemotherapy. J Clin Oncol. 2002;20:1721–1728. doi: 10.1200/JCO.2002.07.039. [DOI] [PubMed] [Google Scholar]
- Elsaleh H. Joseph D. Grieu F, et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–1750. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- Etienne MC. Chazal M. Laurent-Puig P, et al. Prognostic value of tumoral thymidylate synthase and p53 in metastatic colorectal cancer patients receiving fluorouracil-based chemotherapy: phenotypic and genotypic analyses. J Clin Oncol. 2002;20:2832–2843. doi: 10.1200/JCO.2002.09.091. [DOI] [PubMed] [Google Scholar]
- Fernández-Contreras ME. Sánchez-Hernández JJ. González E, et al. Combination of polymorphisms within 5′ and 3′ untranslated regions of thymidylate synthase gene modulates survival in 5 fluorouracil-treated colorectal cancer patients. Int J Oncol. 2009;34:219–229. [PubMed] [Google Scholar]
- Formentini A. Henne-Bruns D. Kornmann M. Thymidylate synthase expression and prognosis of patients with gastrointestinal cancers receiving adjuvant chemotherapy: a review. Langenbecks Arch Surg. 2004;389:405–413. doi: 10.1007/s00423-004-0510-y. [DOI] [PubMed] [Google Scholar]
- Hitre E. Budai B. Adleff V, et al. Influence of thymidylate synthase gene polymorphisms on the survival of colorectal cancer patients receiving adjuvant 5-fluorouracil. Pharmacogenet Genomics. 2005;15:723–730. doi: 10.1097/01.fpc.0000175598.42141.59. [DOI] [PubMed] [Google Scholar]
- Horie N. Aiba H. Oguro K, et al. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5′-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct. 1995;20:191–197. doi: 10.1247/csf.20.191. [DOI] [PubMed] [Google Scholar]
- Iacopetta B. Grieu F. Joseph D. Elsaleh H. A polymorphism in the enhancer region of the thymidylate synthase promoter influences the survival of colorectal cancer patients treated with 5-fluorouracil. Br J Cancer. 2001;85:827–830. doi: 10.1054/bjoc.2001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa W. Uetake H. Shirota Y, et al. Combination of dihydropyrimidine dehydrogenase and thymidylate synthase gene expressions in primary tumors as predictive parameters for the efficacy of fluoropyrimidine-based chemotherapy for metastatic colorectal cancer. Clin Cancer Res. 2003;9:786–791. [PubMed] [Google Scholar]
- Ju J. Pedersen-Lane J. Maley F. Chu E. Regulation of p53 expression by thymidylate synthase. Proc Natl Acad Sci USA. 1999;96:3769–3774. doi: 10.1073/pnas.96.7.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K. Watanabe G. Identification and functional analysis of single nucleotide polymorphism in the tandem repeat sequence of thymidylate synthase gene. Cancer Res. 2003;63:6004–6007. [PubMed] [Google Scholar]
- Kornmann M. Link KH. Galuba I, et al. Association of time to recurrence with thymidylate synthase and dihydropyrimidine dehydrogenase mRNA expression in stage II and III colorectal cancer. J Gastrointest Surg. 2002;6:331–337. doi: 10.1016/s1091-255x(02)00018-5. [DOI] [PubMed] [Google Scholar]
- Kornmann M. Schwabe W. Sander S, et al. Thymidylate synthase and dihydropyrimidine dehydrogenase mRNA expression levels: predictors for survival in colorectal cancer patients receiving adjuvant 5-fluorouracil. Clin Cancer Res. 2003;9:4116–4124. [PubMed] [Google Scholar]
- Kralovánszky J. Köves I. Orosz Z, et al. Prognostic significance of the thymidylate biosynthetic enzymes in human colorectal tumors. Oncology. 2002;62:167–174. doi: 10.1159/000048263. [DOI] [PubMed] [Google Scholar]
- Kristensen MH. Weidinger M. Bzorek M, et al. Correlation between thymidylate synthase gene variants, RNA and protein levels in primary colorectal adenocarcinomas. J Int Med Res. 2010;38:484–497. doi: 10.1177/147323001003800212. [DOI] [PubMed] [Google Scholar]
- Laurie JA. Moertel CG. Fleming TR, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol. 1989;7:1447–1456. doi: 10.1200/JCO.1989.7.10.1447. [DOI] [PubMed] [Google Scholar]
- Leichman CG. Lenz HJ. Leichman L, et al. Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J Clin Oncol. 1997;15:3223–3229. doi: 10.1200/JCO.1997.15.10.3223. [DOI] [PubMed] [Google Scholar]
- Lurje G. Manegold PC. Ning Y, et al. Thymidylate synthase gene variations: predictive and prognostic markers. Mol Cancer Ther. 2009;8:1000–1007. doi: 10.1158/1535-7163.MCT-08-0219. [DOI] [PubMed] [Google Scholar]
- Mandola MV. Stoehlmacher J. Muller-Weeks S, et al. A novel single nucleotide polymorphism within the 5′ tandem repeat polymorphism of the thymidylate synthase gene abolishes USF-1 binding and alters transcriptional activity. Cancer Res. 2003;63:2898–2904. [PubMed] [Google Scholar]
- Mandola MV. Stoehlmacher J. Zhang W, et al. A 6 bp polymorphism in the thymidylate synthase gene causes message instability and is associated with decreased intratumoral TS mRNA levels. Pharmacogenetics. 2004;14:319–327. doi: 10.1097/00008571-200405000-00007. [DOI] [PubMed] [Google Scholar]
- Marsh S. Collie-Duguid ES. Li T, et al. Ethnic variation in the thymidylate synthase enhancer region polymorphism among Caucasian and Asian populations. Genomics. 1999;58:310–312. doi: 10.1006/geno.1999.5833. [DOI] [PubMed] [Google Scholar]
- Marsh S. McKay JA. Cassidy J. McLeod HL. Polymorphism in the thymidylate synthase promoter enhancer region in colorectal cancer. Int J Oncol. 2001;19:383–386. doi: 10.3892/ijo.19.2.383. [DOI] [PubMed] [Google Scholar]
- Midgley RS. Yanagisawa Y. Kerr DJ. Evolution of nonsurgical therapy for colorectal cancer. Nat Clin Pract Gastroenterol Hepatol. 2009;6:108–120. doi: 10.1038/ncpgasthep1337. [DOI] [PubMed] [Google Scholar]
- Paradiso A. Simone G. Petroni S, et al. Thymidilate synthase and p53 primary tumour expression as predictive factors for advanced colorectal cancer patients. Br J Cancer. 2000;82:560–567. doi: 10.1054/bjoc.1999.0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popat S. Matakidou A. Houlston RS. Thymidylate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2004;22:529–536. doi: 10.1200/JCO.2004.05.064. [DOI] [PubMed] [Google Scholar]
- Popat S. Wort R. Houlston RS. Inter-relationship between microsatellite instability, thymidylate synthase expression, and p53 status in colorectal cancer: implications for chemoresistance. BMC Cancer. 2006;6:150. doi: 10.1186/1471-2407-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall F. Ostwald C. Schiffmann L. Barten M. Do thymidylate synthase gene promoter polymorphism and the C/G single nucleotide polymorphism predict effectiveness of adjuvant 5-fluorouracil-based chemotherapy in stage III colonic adenocarcinoma? Oncol Rep. 2007;18:203–209. [PubMed] [Google Scholar]
- Quasar Collaborative Group. Gray R. Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- Rahman L. Voeller D. Rahman M, et al. Thymidylate synthase as an oncogene: a novel role for an essential DNA synthesis enzyme. Cancer Cell. 2004;5:341–351. doi: 10.1016/s1535-6108(04)00080-7. [DOI] [PubMed] [Google Scholar]
- Sulzyc-Bielicka V. Domagala P. Majdanik E, et al. Nuclear thymidylate synthase expression in sporadic colorectal cancer depends on the site of the tumor. Virchows Arch. 2009;454:695–702. doi: 10.1007/s00428-009-0787-x. [DOI] [PubMed] [Google Scholar]
- Tsuji T. Hidaka S. Sawai T, et al. Polymorphism in the thymidylate synthase promoter enhancer region is not an efficacious marker for tumor sensitivity to 5-fluorouracil-based oral adjuvant chemotherapy in colorectal cancer. Clin Cancer Res. 2003;9:3700–3704. [PubMed] [Google Scholar]
- Wolpin BM. Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134:1296–1310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y. Nakajima G. Schmitz JC, et al. Multi-level gene expression profiles affected by thymidylate synthase and 5-fluorouracil in colon cancer. BMC Genomics. 2006;7:68. doi: 10.1186/1471-2164-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]