Abstract
Objective
Inflammation is both central to proper wound healing and a key driver of chronic tissue injury via a positive-feedback loop incited by incidental cell damage. We seek to derive actionable insights into the role of inflammation in wound healing in order to improve outcomes for individual patients.
Approach
To date, dynamic computational models have been used to study the time evolution of inflammation in wound healing. Emerging clinical data on histo-pathological and macroscopic images of evolving wounds, as well as noninvasive measures of blood flow, suggested the need for tissue-realistic, agent-based, and hybrid mechanistic computational simulations of inflammation and wound healing.
Innovation
We developed a computational modeling system, Simple Platform for Agent-based Representation of Knowledge, to facilitate the construction of tissue-realistic models.
Results
A hybrid equation–agent-based model (ABM) of pressure ulcer formation in both spinal cord-injured and -uninjured patients was used to identify control points that reduce stress caused by tissue ischemia/reperfusion. An ABM of arterial restenosis revealed new dynamics of cell migration during neointimal hyperplasia that match histological features, but contradict the currently prevailing mechanistic hypothesis. ABMs of vocal fold inflammation were used to predict inflammatory trajectories in individuals, possibly allowing for personalized treatment.
Conclusions
The intertwined inflammatory and wound healing responses can be modeled computationally to make predictions in individuals, simulate therapies, and gain mechanistic insights.

Yoram Vodovotz, PhD
Introduction
Wound healing is a complex process that is initiated and driven by inflammation.1,2 The first phase of the wound healing response involves the degranulation of platelets and infiltration of inflammatory cells, followed by proliferation of fibroblasts and epithelial cells that deposit collagen and cause contraction of wounds. Inflammatory mediators such as tumor necrosis factor alpha (TNF-α),3 interferon-γ,4 interleukin (IL)-6,5 IL-10,6 transforming growth factor beta-1 (TGF-β1),7 and nitric oxide8,9 modulate the wound-healing response. Wound healing is well studied in animal systems.10,11 However, even though some experimental methodologies are emerging that may allow for the study of time courses of wound healing in humans,11 it is difficult to collect time courses of primary samples from humans suffering from chronic wound-healing diseases without disturbing the very process which is being measured.
An alternative method for studying such a complex, clinically realistic system is to use a mechanistic computational model based on literature knowledge, which could be validated experimentally or clinically, which, in turn, may have applications in diagnosing/predicting wound healing trajectories of individuals or possibly in the design of novel therapeutic modalities for wound healing. Extensive work has gone into computational studies of wound healing, spanning many stages of this process, from re-epithelialization and cell migration to collagen deposition and scar formation, to angiogenesis.12,13 In this article, we review previous work on computational modeling of wound healing, and report a series of more recent spatially explicit, tissue-realistic computational models and the development of a software modeling framework that is used to facilitate the construction of such models.
Clinical Problem Addressed
Chronic, nonhealing wounds present a significant burden of both time and money to the healthcare community. Wounds, whether developed in hospital or present on admission, pose a great threat to a patient's health. They provide an opportunity for pathogens to invade and simply divert resources that the body could be using to restore health elsewhere. Furthermore, the inflammatory response incited can lead to extreme complications, especially when it becomes dysregulated. Wounds pose a specific problem to researchers in that they cannot be sampled clinically, without necessarily perturbing their course. In addition, it is essentially impossible to modulate all possible mechanisms of inflammation and wound healing in an attempt to find novel therapies. While mathematical modeling has been useful in helping integrate these mechanisms, the increasing availability of spatial, noninvasive data (such as clinical photographs) and physico-mechanical information suggests the need for new modeling approaches to leverage these new data sources to enhance the discovery of effective therapeutic regimens.
Materials and Methods
This article describes the progression of mechanistic computational models of inflammation and wound healing, starting from equation-based models and agent-based models (ABMs) that describe qualitative features of these intertwined biological processes, and progressing to tissue-realistic, agent-based, and hybrid models. We also describe a software modeling package that is developed to facilitate the construction of these new sets of models. We note that the studies described here have either been previously published or been submitted for publication elsewhere. We further note that these studies, similar to all rigorous computational biology studies, contain a comparison to in vivo data where appropriate; a step-by-step algorithmic approach to the model construction, calibration, and validation process; and a rigorous appraisal of the model.
Equation-based models of inflammation and wound healing
Differential equations are the most standard, classical method that is utilized in modeling biological processes, including inflammation and wound healing.14–16 Equation-based models have been used to explore all phases of wound healing, from inflammation,17,18 to wound closure,19,20 to tissue remodeling.21 This modeling framework, while extremely useful from a basic, mechanistic point of view, cannot be readily used to create tissue-realistic simulations that involve stochastic biological effects.
Nonetheless, the compendium of equation-based models has resulted in important insights into the wound-healing response. A model of the inflammatory phase of wound healing employed ordinary differential equations (ODEs) to represent densities of various cell populations (fibroblasts, two types of macrophages) and concentrations of inflammatory mediators and other chemicals (TGF-β1, platelet-derived group factor [PDGF], hyaluronan, and collagen).17,18 Re-epithelialization has been modeled in several ways, including keratinocytes that migrate in one dimension, according to a diffusion equation with the constraint of contact inhibition.20 In a two-dimensional continuum model, epithelial cells were represented as a compressible, nonviscous fluid, meaning individual cells were not tracked, but their density was.19 The edge of this fluid progressed according to traction forces, causing the boundary of the fluid to move. Numerical solutions to this model were found using level sets. In a model of tissue re-modeling, extra-cellular matrix (ECM) components were represented as a vector field, in which a vector at each point in space pointed along the direction of collagen deposition.21 These directions (and in later models, the density of ECM at each point) influenced the direction of fibroblast growth and, therefore, the direction of new collagen deposition. Using these techniques, computational models have been used to suggest and simulate therapies that modulate wound healing.21,18
ABMs of inflammation and wound healing
Agent-based modeling is an object-oriented, rule-based, and discrete event computational modeling technique that is well suited for integrating and synthesizing cellular and molecular-level data, and it provides a useful translational tool for examining stochastic and spatial aspects of inflammation and wound healing.22 In an ABM, agents representing individual cellular or molecular components of a system populate a “virtual world,” in which their simulated behaviors are governed by rules extrapolated from known data regarding their true biological behaviors.22 Component-level mechanistic details can be included in the rules that govern the behavior of agents. Typically, both the qualitative pattern and the predicted quantitative time evolution of the agents (variables) can be assessed using ABMs. Since the rules in an ABM define local, instantaneous interactions and as the rules governing agent behavior are probabilistic, the overall behavior of the system being simulated will often be different than what was hypothesized from the rules put into the model. The power of ABM is highlighted by the degree that these system-level effects, sometimes called “emergent phenomena,” reflect real outcomes observed in traditional biological research.23
The ability of ABMs to represent spatial relationships and tissue patterning effects makes them an appealing approach for modeling wound healing biology, and there are several examples of ABMs of wound healing. However, most of these ABMs have been focused on delineating intracellular detail as an adjunct to basic research.24–27 We propose that ABMs are also well suited to a translational role to bridge basic science knowledge and the rational development of clinically applicable strategies.14,23,28,29 We have developed a series of ABMs with this specific goal in mind. Figure 1 is a schematic diagram that shows the general ABM rules used by our group for models of inflammation and wound healing. The development of a biomedical ABM very often involves the use of an existing software toolkit/development environment that allows biologists to focus on the implementation of their biological concepts as opposed to the detailed issues related to software design. ABM toolkits that have been used by biomedical researchers include NetLogo,30 Repast,31 and MASON.32 However, these are general-purpose modeling toolkits, and it is our belief that the application of ABM to biomedical issues can be enhanced via a purpose-built development environment which is tailored to the needs of representing biological systems, with the specific capabilities of facilitating high-resolution visual output and the ability to represent agents incorporating highly complicated rules. This is of particular importance as the goal of agent-based modeling expands beyond dynamic knowledge representation to augment basic research to more translational applications.
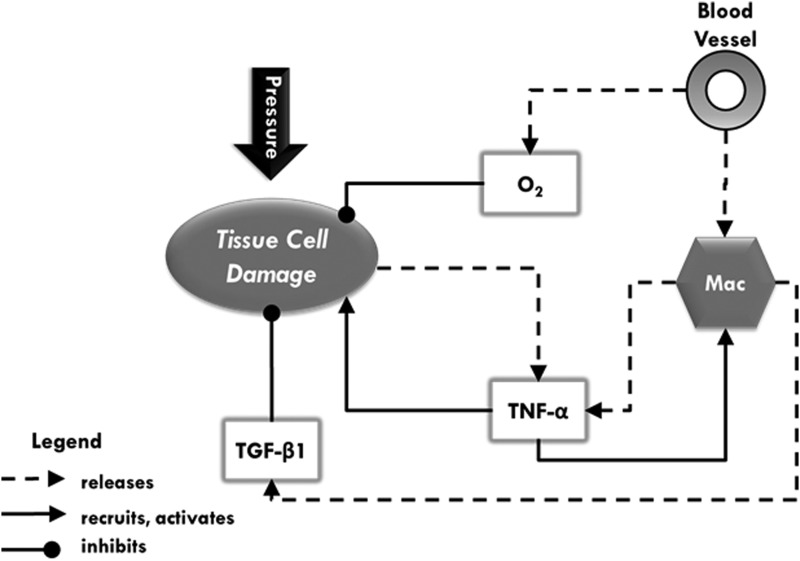
Figure 1.
A schematic of agents, data layers, and their interactions in an agent-based model of pressure ulcer formation. Dotted lines with arrowheads indicate release (e.g., activated macrophages release two types of mediators), while solid lines with arrowheads indicate chemoattraction and/or activation of cells by mediators. Solid lines with circular heads indicate inhibition (e.g., oxygen and transforming growth factor beta-1 [TGF-β1] help prevent tissue cell damage). The types of agents in this model are blood vessels, macrophages (Mac), and tissue cells. Pressure is a status that affects the components of the model. When pressure is applied, tissue cells incur damage (lose health), and blood vessels constrict. This constriction reduces the amount of material that can flow through blood vessels, which, in essence, lowers the amount of oxygen and number of macrophages entering the simulation. Molecular oxygen (O2), represented as a data layer in this model, is necessary for tissue cell health. Therefore, oxygen depletion causes further damage. Damaged tissue cells release tumor necrosis factor alpha (TNF-α), which causes activation of nearby macrophages. Activated macrophages release both TNF-α and TGF-β1, each of which are data layers that represent canonical pro- or anti-inflammatory responses, respectively. Each of these cytokines stands for a group of several pro- and anti-inflammatory mediators that participate in these respective responses. These data layers and oxygen diffuse and decay at each time step (tick). They not only act on agents in their vicinity and in a concentration-dependent manner, but also provide a chemoattractant gradient for some agents. Macrophages in the presence of TNF-α become activated. Both TGF-β1 and oxygen increase the health of nearby tissue cells.
The Simple Platform for Agent-based Representation of Knowledge
Initial ABMs of inflammation and wound healing did not attempt to mirror or explain tissue-specific data directly. As the field has matured, it became apparent that a model containing sufficient biological information to render it clinically relevant could be used as a platform on which treatment strategies could be tested in silico. Furthermore, comparisons between simulations and wounds developing in patients could point toward instantaneous, noninvasive diagnostic metrics.
In order to facilitate this type of tissue-realistic modeling, we developed an ABM software called Simple Platform for Agent-based Representation of Knowledge (SPARK).33,34 SPARK is a stand-alone application developed in Java. It provides a user-friendly interface as well as a simple programming language for developing ABMs. To facilitate the modeling of biomedical systems, SPARK has the following features: (1) continuous space that can simulate real physical space; (2) flexible agent size and shape that can represent the relative proportions of various cell types; (3) multiple spaces that can concurrently simulate and visualize multiple scales in biomedical models; and (4) a convenient graphical user interface. In SPARK, a user-friendly language, SPARK Programming Language (SPARK-PL),with a graphical user interface is provided to modelers to build models. In addition, the software allows for agents of various sizes, sophisticated image effects, and facilitates multi-scale modeling.
The SPARK Programming Language
The SPARK-PL was developed to simplify the process of model creation by researchers who are not experienced in computer programming. Its syntax is derived primarily from the Logo programming language and the Java programming language. All models written in SPARK-PL are executed in a two-step process: They are translated into Java source code first, and then, a Java compiler is used to produce the byte code that can be executed by the SPARK simulation engine. Due to this feature, it is convenient to extend SPARK-PL with native Java constructions. As a result, SPARK-PL utilizes the full power of the Java language for ABM development. The main features of this language include object-oriented constructions, a static type system with type inference, close ties with SPARK, as well as the concise and intuitive syntax of the Logo language.35
Components of a model
The ABMs built in SPARK comprise Space, Agents, Links, Data Layers, and the Observer. Space is analogous to the physical space, and it provides a context within which the model evolves. Each Agent type typically describes one type of cells, for example, neutrophils, macrophages, and epithelial cells. Links can be used to connect two or more agents together with various strengths and distances (e.g., to allow aggregation of activated platelets). Data Layers provide a computationally efficient way of tracking smaller species, and they are often employed to represent diffusible cytokines, free radicals, oxygen, enzymes, and drugs. The Observer contains information about all spaces and agents in the model and also controls the simulation process. This allows the modeler to make queries, such as the total number of macrophages at any given time or the concentration of TNF-α near a given agent.
Each tick of the model represents one unit of real clock time (in some models 1 h, in others, 5 min, etc.). This ensures that cell life spans, molecule degradation rates, and so on are in sync so that the dynamics in each model have physical meaning. On each tick, the agents behave according to the rules specific to their type: They produce and consume; are chemo-attracted to—and activated (undergo a state change) by—mediators in data layers; and die, according to their lifespans. Some types of agents have physical properties, which allow them to, for example, change shape in order to fit into a space that is crowded with other agents. Mediators in data layers can be consumed or produced by agents or in reactions with other mediators, and undergo diffusion and evaporation (degradation) at each tick. These rules are necessarily affected over small local regions and in short time steps (one tick). Therefore, the behaviors and patterns produced by simulating several ticks of the model in succession arise as emergent phenomena resulting from the accumulated actions of a population of agents over time.
In silico trials and sensitivity analysis
After validating the behaviors observed in the models, they have been used to make predictions36–38 and as a surrogate system for testing potential wound-healing strategies and products. Sensitivity analysis, a measure of which parameters account for the greatest amount of the variance in the model's output, allowed both pressure ulcer models to identify areas of high vulnerability in each model, thus suggesting mechanisms that might be particularly susceptible to interventions. Other ABMs made predictions by simulating therapies and observing changes in model behaviors. These changes were quantified as concentrations of mediators, amount of tissue damage at and around the wound site, and so on. In several cases, changes in outcome could be measured visually. The volume of cells pushing into the neointima (the new tissue formed inside the blood vessel after injury), the size of an ulcer developing in the tissue, and the intensity of color demarcating tissue damage were visual cues that allow the modeler to understand rapidly what is occurring in the simulation.
Results
Initial equation- and agent-based computational models of inflammation and wound healing
Equation-based models of basic wound-healing mechanisms
Mathematical models of wound healing based on differential equations have been created beginning in the late 1980s, incorporating terms for mechanical stress, population dynamics, and biochemical signaling.12 Most mechanistic computational models of wound healing involve signaling among various tissue types via the actions of cytokines and growth factors.20 Such models have been used to infer which mechanisms, materials, and forces are critical to the wound-healing process. These equation-based models range from simple, equation-based models solved using level sets to represent and assess various stages of epithelial progression into a wound bed,19 to more complex models that involve inflammatory and wound-healing mechanisms.17,18,21 Such models have advanced our understanding of wound healing as it is supposed to work, suggested underlying pathological mechanisms, and tested therapies in silico.
ABMs as a tool for studies of aberrant wound healing
ABMs provide several useful capabilities in the investigation of wound healing, including being more intuitive for nonmathematicians to use, easily matching the rule-based format in which mechanistic biological knowledge is conveyed, facilitating the representation of spatial structure and tissue patterning, and allowing biological processes to evolve stochastically in both time and space.22 It is important to note that while the rules which govern model behavior are deterministic, the simulation itself is stochastic. Processes such as diffusion and Brownian motion lead to a range of different contexts that a single agent might encounter on a given time step. Different contexts will lead to a range of possible behaviors for that agent, and over a whole population of agents, the range of behaviors is observable. These features, key among them being the formation of visual patterns and stochasticity of final outcomes, facilitate the creation of virtual patients cohorts in the setting of aberrant wound healing. Next, we discuss in greater detail a series of ABMs that are developed and used to examine wound healing in different circumstances.
Diabetic foot ulcers
An initial ABM of inflammation was developed using NetLogo30 in order to clarify the mechanisms and help refine therapeutic approaches to chronic, nonhealing diabetic foot ulcers (DFU) where inflammation and wound healing are deranged and inextricably linked.38 An ideal therapy for DFU should both suppress excessive inflammation and enhance healing. An ABM was created that reproduced qualitatively much of the literature data on skin wound healing, including changes in relevant cell populations (macrophages, neutrophils, and fibroblasts) and their key effector cytokines (TNF-α, IL-1β, IL-10, and TGF-β1).38 In this simulation, a normal healing response results in tissue damage that first increases (due to wound-induced inflammation) and then decreases as the collagen levels increase. Studies by others suggest that diabetes and DFU are characterized by elevated TNF-α and reduced TGF-β1.17 Accordingly, the model simulated the genesis of DFU in two ways, either by increasing the rate of TNF-α production fourfold or by decreasing the rate of TGF-β1 production (both assumptions based on previous literature). These perturbations of the basal wound healing mechanism resulted in increased inflammation (elevated neutrophils, TNF-α, and tissue damage) and delayed healing (reduced TGF-β1 and collagen). The ABM reproduced the therapeutic effect of PDGF/platelet release treatment as well as DFU debridement. Next, the expected effect of administering a neutralizing anti-TNF-α antibody, an agent that would increase the activation of endogenous latent TGF-β1, or latent TGF-β1 (which has a longer half life than active TGF-β1), was simulated. The results of these in silico clinical trials revealed that these therapies would have similar effects regardless of the initial assumption of the derangement that underlies DFU (elevated TNF vs. reduced TGF-β1).38
Personalized models of acute vocal fold injury/phonotrauma
The vocal folds are exposed to nearly continuous biomechanical stress during phonation. Increased intrafold contact stresses associated with certain vocalization patterns can result in damage to the vocal fold mucosa.39 A NetLogo-based ABM of the inflammatory reaction to acute phonotrauma was generated and calibrated to individual subjects' mediator levels (from laryngeal secretions) before and after acute phonotrauma.36 Subjects then underwent one of several treatment strategies: rest, resonant voice exercises, or spontaneous speech. The ABM was able to recapitulate observed dynamics and to accurately predict mediator levels at time points beyond those used for calibration. Furthermore, the model was able to accurately predict mediator levels on subjects whose measurements were not used for calibration. Previous work suggested that at least in some cases, rest may not be the optimal treatment. The inflammatory trajectories revealed in this model allowed segregation of those who would benefit from rest and those who would heal optimally following resonant voice exercises. In this way, the model was used in a patient-specific manner to aid in clinical decision making.
An update to this model included more detailed inflammatory mechanisms by increasing the number of mediators in the model.37 The advantage of this increased complexity was to increase accuracy of predictions up to 24-h post-trauma. This more complex model performed at least as well as the earlier version, matching to a larger panel of mediators and membrane proteins. Interestingly, this extended model suggested that individual-specific model calibration might be necessary in order to predict outcomes of inflammation and wound healing in humans. Finally, this ABM could be calibrated with data from a rat paradigm of surgical vocal fold injury—an important surrogate for clinical surgical injury to the vocal folds, for example, in the case of cancer of the throat—and was capable of reproducing those experimental features of injury and inflammation as well.40
Incorporating tissue realism and clinically measureable physical forces to aid in clinical decision-making
The initial equation-based and ABMs discussed earlier have been used to study the time evolution of inflammation in wound healing. However, these models depend on calibration to quantities such as concentrations of mediators, which are not only variable throughout the areas of a wound, but also difficult to obtain without corrupting a wound. More readily available sources of clinical data include noninvasive, quantitative measures of tissue properties such as blood flow, as well as images of the macroscopic appearance of evolving wounds. Next, we discuss examples of these approaches to modeling wound healing, using the SPARK modeling platform to facilitate tissue-realistic modeling.
Post-spinal cord injury pressure ulcers: a hybrid model incorporating tissue blood flow
Pressure ulcers are costly and life-threatening complications for people with spinal cord injury (SCI). People with SCI also exhibit differential blood flow properties in non-ulcerated skin. A hybrid equation- and ABM of ischemia-induced hyperemia and pressure ulcer formation was developed to provide insights into the pathogenesis and effective treatment of post-SCI pressure ulcers.41 The model combined ODEs related to blood flow along with an ABM of skin injury, inflammation, and ulcer formation. The relationship between pressure and the course of ulcer formation, as well as several other important characteristic patterns of pressure ulcer formation, was demonstrated in this model. The equation-based portion of this model was calibrated to data related to blood flow following experimental pressure responses in noninjured human subjects or to data from people with SCI. This hybrid model predicted a higher propensity to form ulcers in response to pressure in people with SCI versus noninjured control subjects, and, thus, may serve as a novel diagnostic platform for post-SCI ulcer formation.41 Notably, the abstracted vascular resistance parameter can be obtained in individual patients by doing subject-specific fitting, raising the possibility of individualized prediction of pressure ulcer risk.41
Post-SCI pressure ulcers: a visually realistic ABM
Serial, noninvasive wound imaging combined with ABMs could, in theory, allow for the investigation of both space- and time-dependent dynamics via visually realistic, mechanistic simulations. Accordingly, we created an ABM in which repeated cycles of ischemia/reperfusion over a bony prominence cause tissue damage and induce inflammation, which lead to cycles of additional damage caused by pro-inflammatory positive-feedback mechanisms (unpublished observations). The ABM was calibrated against serial images of post-SCI pressure ulcers obtained from patients following Institutional Review Board approval and informed consent. Behaviors encoded in the ABM at the cellular level led to tissue-level phenotypes described in the literature. The model recapitulated visual patterns of ulcer formation in SCI patients on which it was not calibrated. Sensitivity Analysis of model parameters revealed that increasing either oxygen availability or the rate at which pressure is applied and released (simulating a patient being turned) led to predictions of improved outcomes.
The ABM was then used to investigate potential treatments in silico. While the use of corticosteroids is broadly considered a risk factor for chronic, nonhealing wounds,42 it is also known to be an effective suppressor of acute inflammation. In an in silico clinical trial of steroid treatment, steroids applied early enough and at a high enough dose were effective in stemming the local inflammatory response, leading to predictions of improved outcomes in the early stages of pressure ulcer formation and development. This ABM incorporated damage-associated molecular pattern (DAMP) molecules as key drivers of postinjury inflammation. The most well-studied DAMP is high-mobility group protein-B1 (HMGB1), and has, therefore, emerged as a therapeutic target for inflammatory diseases.43–45 Accordingly, we carried out an in silico trial of a putative, neutralizing anti-HMGB1 antibody therapy. In our simulations, this strategy did not prove to be successful in stemming the inflammatory response to repeated ischemia/reperfusion injury. We hypothesize that compensatory mechanisms encoded in the ABM were able to compensate for the lack of traditional DAMPs, allowing for activation of the inflammatory response via cell damage, without an explicit diffusible signal (unpublished observations).
While this ABM exhibits stochastic changes that lead to variability among simulation outputs, these variations arise from a fixed set of parameters, with no adjustments for patient-specific input. The model outputs were validated as a whole set against clinical images from a cohort of SCI patients who developed pressure ulcers. Thus, in its current form, the model is able to recapitulate visual features that are common to many of the pressure ulcers from the cohort. Ultimately, however, the goal would be to define patient-specific inputs in order to facilitate personalized diagnosis and therapy.
Post-angioplasty and in-stent restenosis
Restenosis is a complication of percutaneous coronary intervention.46,47 An ABM was developed for modeling of the biological and physical influences induced by per cutaneous transluminal intervention, and subsequent inflammation and healing will lead to improved mechanistic insights and therapy (unpublished observations). The ABM simulated the dynamic formation of a neointima after balloon overstretch injury in the absence of stents or drugs. The ABM contains four major compartments in a simulated arterial cross-section: lumen, endothelial cells (ECs), tunica media consisting of smooth muscle cells (SMCs), and the tunica adventitia. ECs and SMCs are represented as connected, elastic, and physical agents. The model also included platelets and macrophages, along with cytokines such as TNF-α and TGF-β1. The life spans of the different cells types in the ABM were set to the correct relative proportions based on literature data. The ABM was further calibrated against the published consensus time course of restenosis in experimental porcine balloon overstretch injury. The patterns produced by the ABM were similar to published findings in swine, and suggested that a complex, individual-specific combination of degree of injury, platelet aggregation, elaboration of cytokines, and SMC migration leads to neointima formation. Though the prevailing notion is that the magnitude of neointima is roughly proportional to the size/extent of the original injury to the tunica media, our simulations suggest that the ultimate cross-sectional appearance is due to physical forces imparted by migrating SMCs, interacting nonlinearly with inflammatory/healing mechanisms (unpublished observations).
Discussion
In silico mechanistic models provide unique opportunities for studying inflammation and wound healing. Recent ABMs not only allow a mechanistic insight, but also provide an inexpensive and accessible platform for hypothesis testing, simulating clinical trials, designing patient-specific therapies, and developing diagnostic tools. By providing a means of synthesizing disparate aspects of biomedical knowledge, mechanistic computational modeling provides a plethora of opportunities to continue exploring, make new insights, and ultimately help patients.2,13–15,29,48,49 At the level of the population, an ABM of DFU was used to give insights into how current therapies work (or do not) in given patients, and also to suggest novel therapies.38 At the other end of the spectrum, an ABM of acute phonotrauma was not only able to recapitulate inflammatory mediator dynamics observed in the laryngeal secretions of human subjects, but also able to accurately predict those levels and time points beyond model calibration range.36 That model was also used to simulate different treatment strategies and pointed to a less intuitive strategy as more effective than the standard prescription of rest.36 These insights were garnered by incorporating only a small set of mediators and cell types into the model.
Incorporating physically and physiologically relevant information into ABMs, such as blood flow in tissues and forces between cells, allowed for new mechanistic predictions. In the case of the former, a simple approximation of tissue blood flow differences between noninjured subjects and SCI patients, when juxtaposed on a stochastic model of inflammation and tissue injury, led to the prediction of higher propensity to ulcerate in SCI patients versus controls. Even visual information can be deceiving, as illustrated by agent-based simulations of restenosis. Static cross-sections of arterial restenosis suggested that neointima entered through a break in the external elastic lamina of a coronary artery, and, therefore, the degree of hyperplasia was thought to be proportional to the size of the rupture.50,51 However, time courses yielded by dynamic simulations revealed an alternative possibility. Infiltrating cells appeared to be actually pushing on the edges of the ruptured external elastic lamina, forcing it wider. This insight might impact the analysis of histological samples of balloon-injured arteries, and may also affect the design of novel therapies that are aimed at mitigating these physical forces.
Incorporating tissue realism into ABMs of pressure ulcer formation has raised the possibility of noninvasive diagnostics and possibly therapy based on serial macroscopic images of nonhealing wounds. Due to the individualized and context-dependent nature of the inflammatory response, a tool that could help clinicians decide the best course of treatment based only on visual information could be revolutionary. Such an in silico diagnostic could improve patient care. This model has also been used to simulate a variety of treatment strategies, paving the way for fast, inexpensive, and early-stage trials of newly designed treatments.
There are, of course, limitations to this approach. Simulations of biological processes are necessarily approximations. In order to balance computational cost with a model's utility, models should be parsimonious, including the smallest number of elements that will still yield a model which is useful for gaining mechanistic insights, clinical applications, or both. Often, modeling choices can be driven by the availability of data rather than which elements might be most critical to or informative in a process. These choices are subjective, and one can always find a justification to include additional elements in a given model, as long as those elements increase the validity or utility of the model. An implication of this simplified version of reality is that a model can only be useful to a certain level of resolution. It is important to keep in mind the scope a model can reach when probing it for insights.
In 1998, Murray et al.52 wrote,
Even if the mechanisms were well understood—and certainly they are not at this stage—mathematics would be required to explore the consequences of manipulating the various parameters associated with any particular wound management scenario. The number of options that are fast becoming available to wound managers will become overwhelming unless we can find a way to simulate particular treatment protocols before applying them in practice.
These predictions are beginning to be realized. Previous computational models have given a general sense of how certain treatment strategies could affect wound-healing dynamics. We have now reached the stage where we are simulating wounds as they form and as they heal. We are able to simulate treatment strategies and observe their effects on the tissue in the simulation just as we do in the patient. Thus, we are now beginning to see the potential for personalized medicine of mechanistic computational modeling.49 In particular, the agent-based approach could allow delineation among subjects who have different wound-healing trajectories. In an extension of previous statistically based modeling,53 we may soon use the output of mechanistic simulations as a guide to understand a given patient's wound-healing trajectory and, hopefully, treat that patient based on model predictions. Moreover, we may probe such mechanistic models in order to determine which model parameters/behaviors are at the root of patient-specific differences in the inflammatory and wound-healing responses, and use these insights for new therapeutic targets or diagnostic biomarkers.
The next frontier for mechanistic computational modeling is really to form a bridge between visual simulation outputs and clinical images. In an ABM, wound formation and evolution is marked by morphological outputs of the model. These morphological features—along with numerical data also generated during simulations—provide a rich space of output features to which hypotheses can be linked. Using image analysis to extract features and machine learning to compare clinical images to simulations, it will be possible to extract pertinent details that cause some simulations/subjects to resolve their wounds, while others progress to deranged inflammation and inappropriate healing.
Key Findings.
Equation- and agent-based are two frameworks for mechanistic computational simulations that have been employed in the study of inflammation and wound healing.
ABMs simulate complex systems by synthesizing many independent pieces of mechanistic information into a framework that can evolve in space and time.
ABMs can be calibrated to individual subjects, allowing for personalized predictions and diagnostics.
Adding tissue realism and physical forces to the agents in these ABMs added a new level of insight, and opened up many new possibilities for diagnostics.
Innovation
Mechanistic computational models have been initially employed to increase understanding of the mechano-biology and tissue remodeling events involved in wound healing and, more recently, to suggest novel therapies and predict the responses of individuals. Such computational models are poised to serve as the basis for personalized, preventative interventions.
Abbreviations and Acronyms
- ABM
agent-based model
- DAMP
damage-associated molecular pattern molecule
- DFU
diabetic foot ulcers
- EC
endothelial cell
- HMGB1
high-mobility group protein B1
- IL
interleukin
- PCI
per cutaneous transluminal intervention
- PDGF
platelet-derived group factor
- SCI
spinal cord injury
- SMC
smooth muscle cell
- SPARK
Simple Platform for Agent-based Representation of Knowledge
- SPARK-PL
SPARK Programming Language
- TNF-α
tumor necrosis factor alpha
- TGF-β1
transforming growth factor beta-1
Acknowledgments and Funding Sources
This work was supported in part by the National Institutes of Health grants P50GM53789, R33HL-089082, R01DC008290, and UO1 DK072146; National Institute on Disability and Rehabilitation Research grant H133E070024; a Shared University Research Award from IBM, Inc.; and grants from the Commonwealth of Pennsylvania, the Pittsburgh Lifesciences Greenhouse, and the Pittsburgh Tissue Engineering Initiative/Department of Defense.
Author Disclosure and Ghostwriting
Y.V. is a co-founder of and a stakeholder in Immunetrics, Inc. G.A. is a consultant to Immunetrics, Inc. Y.V., G.A., and Q.M. are co-inventors on U.S. Patent No. 48,165,819 (“Modeling Wound Healing”). The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Cordelia Ziraldo, PhD, recently graduated from the Carnegie Mellon-University of Pittsburgh Joint PhD Program in Computational Biology. Her dissertation research, under the direction of Dr. Yoram Vodovotz, focused on mechanistic modeling of inflammation and wound healing. Qi Mi, PhD, is an Assistant Professor of Sports Medicine and Nutrition at the University of Pittsburgh School of Health and Rehabilitation Science. He directs the modeling core of the Center for Inflammation and Regenerative Modeling (CIRM; www.mirm.pitt.edu/cirm) at the McGowan Institute for Regenerative Medicine. Gary An, MD, is an Associate Professor of Surgery and Co-Director of the Surgical Intensive Care Unit at the University of Chicago and a Senior Fellow at the Computation Institute at the University of Chicago. He is the founder and director of the Fellowship in Translational Systems Biology at the University of Chicago. Yoram Vodovotz, PhD, is a Professor of Surgery, Immunology, Computational and Systems Biology, Bioengineering, Clinical and Translational Science, and Communication Science and Disorders at the University of Pittsburgh School of Medicine. He is the Director of the Center for Inflammation and Regenerative Modeling (CIRM; www.mirm.pitt.edu/cirm) at the McGowan Institute for Regenerative Medicine. Dr. Vodovotz is a co-founder and current President of the Society for Complexity in Acute Illness (www.scai-med.org), and a co-founder of Immunetrics, Inc. (www.immunetrics.com), a Pittsburgh-based company that is commercializing this mathematical modeling work in the context of the pharmaceutical industry.
References
- 1.Broughton G. Janis JE. Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 2.Vodovotz Y. Constantine G. Faeder J. Mi Q. Rubin J. Sarkar J. Squires R. Okonkwo DO. Gerlach J. Zamora R. Luckhart S. Ermentrout B. An G. Translational systems approaches to the biology of inflammation and healing. Immunopharmacol Immunotoxicol. 2010;32:181. doi: 10.3109/08923970903369867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapala K. The effect of tumor necrosis factor-alpha on wound healing. An experimental study. Ann Chir Gynaecol Suppl. 1996;211:1. [PubMed] [Google Scholar]
- 4.Adelmann-Grill BC. Hein R. Wach F. Krieg T. Inhibition of fibroblast chemotaxis by recombinant human interferon gamma and interferon alpha. J Cell Physiol. 1987;130:270. doi: 10.1002/jcp.1041300213. [DOI] [PubMed] [Google Scholar]
- 5.Gallucci RM. Simeonova PP. Matheson JM. Kommineni C. Guriel JL. Sugawara T. Luster MI. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14:2525. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- 6.Ohshima T. Sato Y. Time-dependent expression of interleukin-10 (IL-10) mRNA during the early phase of skin wound healing as a possible indicator of wound vitality. Int J Legal Med. 1998;111:251. doi: 10.1007/s004140050163. [DOI] [PubMed] [Google Scholar]
- 7.Roberts AB. Sporn MB. Transforming growth factor-b. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1996. [Google Scholar]
- 8.Efron DT. Most D. Barbul A. Role of nitric oxide in wound healing. Curr Opin Clin Nutr Metab Care. 2000;3:197. doi: 10.1097/00075197-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Schwentker A. Vodovotz Y. Weller R. Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002;7:1. doi: 10.1016/s1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 10.Dorsett-Martin WA. Rat models of skin wound healing: a review. Wound Repair Regen. 2004;12:591. doi: 10.1111/j.1067-1927.2004.12601.x. [DOI] [PubMed] [Google Scholar]
- 11.Lindblad WJ. Considerations for selecting the correct animal model for dermal wound-healing studies. J Biomater Sci Polym Ed. 2008;19:1087. doi: 10.1163/156856208784909390. [DOI] [PubMed] [Google Scholar]
- 12.Sherratt JA. Dallon JC. Theoretical models of wound healing: past successes and future challenges. C R Biol. 2002;325:557. doi: 10.1016/s1631-0691(02)01464-6. [DOI] [PubMed] [Google Scholar]
- 13.Vodovotz Y. Translational systems biology of inflammation and healing. Wound Repair Regen. 2010;18:3. doi: 10.1111/j.1524-475X.2009.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An G. Faeder J. Vodovotz Y. Translational systems biology: introduction of an engineering approach to the pathophysiology of the burn patient. J Burn Care Res. 2008;29:277. doi: 10.1097/BCR.0b013e31816677c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vodovotz Y. An G. Systems biology, inflammation. In: Yan Q, editor. Systems Biology in Drug Discovery and Development: Methods and Protocols. Totowa, NJ: Springer Science & Business Media; 2009. [Google Scholar]
- 16.Vodovotz Y. Clermont G. Chow C. An G. Mathematical models of the acute inflammatory response. Curr Opin Crit Care. 2004;10:383. doi: 10.1097/01.ccx.0000139360.30327.69. [DOI] [PubMed] [Google Scholar]
- 17.Waugh HV. Sherratt JA. Macrophage dynamics in diabetic wound dealing. Bull Math Biol. 2006;68:197. doi: 10.1007/s11538-005-9022-3. [DOI] [PubMed] [Google Scholar]
- 18.Waugh HV. Sherratt JA. Modeling the effects of treating diabetic wounds with engineered skin substitutes. Wound Repair Regen. 2007;15:556. doi: 10.1111/j.1524-475X.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- 19.Arciero JC. Mi Q. Branca MF. Hackam DJ. Swigon D. Continuum model of collective cell migration in wound healing and colony expansion. Biophys J. 2011;100:535. doi: 10.1016/j.bpj.2010.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wearing HJ. Sherratt JA. Keratinocyte growth factor signalling: a mathematical model of dermal-epidermal interaction in epidermal wound healing. Math Biosci. 2000;165:41. doi: 10.1016/s0025-5564(00)00008-0. [DOI] [PubMed] [Google Scholar]
- 21.McDougall S. Dallon J. Sherratt J. Maini P. Fibroblast migration and collagen deposition during dermal wound healing: mathematical modelling and clinical implications. Philos Transact A Math Phys Eng Sci. 2006;364:1385. doi: 10.1098/rsta.2006.1773. [DOI] [PubMed] [Google Scholar]
- 22.An G. Mi Q. Dutta-Moscato J. Solovyev A. Vodovotz Y. Agent-based models in translational systems biology. Wiley Interdiscip Rev Syst Biol Med. 2009;1:159. doi: 10.1002/wsbm.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foteinou PT. Calvano SE. Lowry SF. Androulakis IP. Translational potential of systems-based models of inflammation. Clin Transl Sci. 2009;2:85. doi: 10.1111/j.1752-8062.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christley S. Lee B. Dai X. Nie Q. Integrative multicellular biological modeling: a case study of 3D epidermal development using GPU algorithms. BMC Syst Biol. 2010;4:107. doi: 10.1186/1752-0509-4-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern JR. Christley S. Zaborina O. Alverdy JC. An G. Integration of TGF-beta- and EGFR-based signaling pathways using an agent-based model of epithelial restitution. Wound Repair Regen. 2012;20:862. doi: 10.1111/j.1524-475X.2012.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker DC. Georgopoulos NT. Southgate J. From pathway to population—a multiscale model of juxtacrine EGFR-MAPK signalling. BMC Syst Biol. 2008;2:102. doi: 10.1186/1752-0509-2-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker DC. Hill G. Wood SM. Smallwood RH. Southgate J. Agent-based computational modeling of wounded epithelial cell monolayers. IEEE Trans Nanobioscience. 2004;3:153. doi: 10.1109/tnb.2004.833680. [DOI] [PubMed] [Google Scholar]
- 28.An GC. Translational systems biology using an agent-based approach for dynamic knowledge representation: an evolutionary paradigm for biomedical research. Wound Rep Reg. 2010;18:8. doi: 10.1111/j.1524-475X.2009.00568.x. [DOI] [PubMed] [Google Scholar]
- 29.Vodovotz Y. Csete M. Bartels J. Chang S. An G. Translational systems biology of inflammation. PLoS Comput Biol. 2008;4:1. doi: 10.1371/journal.pcbi.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilensky U. NetLogo. Evanston, IL: Center for Connected Learning and Computer-Based Modeling; 1999. [Google Scholar]
- 31.North MJ. Collier NT. Vos JR. Experiences creating three implementations of the repast agent modeling toolkit. ACM Trans Model Comput Simul. 2006;16:1. [Google Scholar]
- 32.Luke S. Balan GC. Panait L. Cioffi-Revilla C. Paus S. MASON: a Java multi-agent simulation library; Proceedings of the Agent 2003 Conference; 2003. [Google Scholar]
- 33.Solovyev A. Mikheev M. Zhou L. Ziraldo C. An G. Mi Q. SPARK: a framework for multi-scale agent-based biomedical modeling. Int J Agent Technol Syst. 2010;2:18. doi: 10.4018/jats.2010070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solovyev A. Mi Q. Simple Platform for Agent-Based Representation of Knowledge (SPARK) [Online] 2012. www.pitt.edu/∼cirm/spark www.pitt.edu/∼cirm/spark
- 35.2012 MIT Media Lab. http://el.media.mit.edu/logo-foundation/resources/index.html http://el.media.mit.edu/logo-foundation/resources/index.html
- 36.Li NYK. Verdolini K. Clermont G. Mi Q. Hebda PA. Vodovotz Y. A patient-specific in silico model of inflammation and healing tested in acute vocal fold injury. PLoS ONE. 2008;3:e2789. doi: 10.1371/journal.pone.0002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li NYK. Vodovotz Y. Kim KH. Mi Q. Hebda PA. Verdolini Abbott K. Biosimulation of acute phonotrauma: an extended model. Laryngoscope. 2011;121:2418. doi: 10.1002/lary.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mi Q. Rivière B. Clermont G. Steed DL. Vodovotz Y. Agent-based model of inflammation and wound healing: insights into diabetic foot ulcer pathology and the role of transforming growth factor-β1. Wound Rep Reg. 2007;15:617. doi: 10.1111/j.1524-475X.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 39.Li NY. Verdolini AK. Rosen C. An G. Hebda PA. Vodovotz Y. Translational systems biology and voice pathophysiology. Laryngoscope. 2010;120:511. doi: 10.1002/lary.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li NYK. Vodovotz Y. Hebda PA. Verdolini K. Biosimulation of inflammation and healing in surgically injured vocal folds. Ann Otol Rhinol Laryngol. 2010;119:412. doi: 10.1177/000348941011900609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solovyev A. Mi Q. Tzen YT. Brienza D. Vodovotz Y. Hybrid equation/agent-based model of ischemia-induced hyperemia and pressure ulcer formation predicts greater propensity to ulcerate in Subjects with spinal cord injury. PLoS Comput Biol. 2013;9:e1003070. doi: 10.1371/journal.pcbi.1003070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller P. Wille J. van Ramshorst B. van der Werken C. Pressure ulcers in intensive care patients: a review of risks and prevention. Intensive Care Med. 2002;28:1379. doi: 10.1007/s00134-002-1487-z. [DOI] [PubMed] [Google Scholar]
- 43.Andersson U. Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H. Tracey KJ. Targeting HMGB1 in inflammation. Biochim Biophys Acta. 2010;1799:149. doi: 10.1016/j.bbagrm.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu S. Li W. Ward MF. Sama AE. Wang H. High mobility group box 1 protein as a potential drug target for infection- and injury-elicited inflammation. Inflamm Allergy Drug Targets. 2010;9:60. doi: 10.2174/187152810791292872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jukema JW. Ahmed TA. Verschuren JJ. Quax PH. Restenosis after PCI. Part 2: prevention and therapy. Nat Rev Cardiol. 2012;9:79. doi: 10.1038/nrcardio.2011.148. [DOI] [PubMed] [Google Scholar]
- 47.Jukema JW. Verschuren JJ. Ahmed TA. Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2012;9:53. doi: 10.1038/nrcardio.2011.132. [DOI] [PubMed] [Google Scholar]
- 48.An G. Bartels J. Vodovotz Y. In silico augmentation of the drug development pipeline: examples from the study of acute inflammation. Drug Dev Res. 2011;72:1. doi: 10.1002/ddr.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mi Q. Li NYK. Ziraldo C. Ghuma A. Mikheev M. Squires R. Okonkwo DO. Verdolini Abbott K. Constantine G. An G. Vodovotz Y. Translational systems biology of inflammation: potential applications to personalized medicine. Personalized Med. 2010;7:549. doi: 10.2217/pme.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guzman LA. Mick MJ. Arnold AM. Forudi F. Whitlow PL. Role of intimal hyperplasia and arterial remodeling after balloon angioplasty: an experimental study in the atherosclerotic rabbit model. Arterioscler Thromb Vasc Biol. 1996;16:479. doi: 10.1161/01.atv.16.3.479. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz RS. Huber KC. Murphy JG. Edwards WD. Camrud AR. Vlietstra RE. Holmes DR. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992;19:267. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- 52.Murray JD. Cook J. Tyson R. Lubkin SR. Spatial pattern formation in biology: I. Dermal wound healing. II. Bacterial patterns. J Franklin Inst. 1998;335:303. [Google Scholar]
- 53.Robson MC. Hill DP. Woodske ME. Steed DL. Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg. 2000;135:773. doi: 10.1001/archsurg.135.7.773. [DOI] [PubMed] [Google Scholar]