Abstract
Objective In this study, we screened microRNA (miRNA) target genes of prostate cancer by integrating miRNA and mRNA expression profiles after target prediction and performed function enrichment analysis for selected candidate genes. Methods: The miRNA expression profile (GSE36802) and mRNA expression profile (GSE36801) were downloaded from the Gene Expression Omnibus database. We processed data and identified the differentially expressed miRNAs and mRNAs with R packages. Verified targets of miRNAs were identified through miRecods and miRTarBase. Then, software of Search Tool for the Retrieval of Interacting Genes was used to construct the interaction network of target genes. Finally, we performed function enrichment analysis for genes in the interaction network with the Functional Classification Tool. Results: A total of 22 upregulated and 8 downregulated miRNAs were detected in this study, of which, hsa-mir-31 was the most overexpressed miRNA in prostate cancer. Both ITGA5 and RDX, two target genes of hsa-mir-31, were found to be differentially expressed from mRNA profiles by overexpressing hsa-mir-31. The cell adhesion molecule was found to be the most significant pathway enriched by ITGA5 and RDX. Conclusion: Overexpression of hsa-mir-31 can be a significant marker to distinguish cancer tissues from benign tissues. The targets such as ITGA5 and RDX regulated by hsa-mir-31 are candidate genes of prostate cancer, which provide new treatment strategies for its gene therapy.
Introduction
Prostate cancer is more prevalent in Western countries than other parts of the world (Landis et al., 1999). Radiation therapy or prostatectomy can be used to treat prostate cancer when the cancer tissue is primarily detected. When diagnosed with advanced cancer, the patients are always treated with androgen deprivation therapy, which can easily lead to the androgen-independent phenotype. Prostate cancer is a chronic disease that often takes decades from the onset to clinical manifestation. It is mainly associated with factors such as age, race, diet, and lifestyle (Greenlee et al., 2000).
The development, invasion, and metastasis of prostate cancer involve multiple factors, multiple stages, and multiple genes. Currently, the clinical treatment of prostate cancer mainly includes surgery with adjuvant endocrine therapy, chemotherapy, and gene therapy. The genetic basis of prostate cancer, the relationships between oncogenes, tumor suppressor genes, environment such as hormones are the core issues for prostate cancer research (Hiatt et al., 1994), as well as prostate cancer susceptibility genes and metastasis-related genes.
MicroRNA (miRNA) has the potential to be used as biomarkers and therapeutic targets for the treatment of various cancers. MiRNA/mRNA expression profiles are frequently used for identifying functionally important miRNAs and their target genes. MiRNA is an endogenous noncoding single-stranded RNA with a length about 21–25nt. MiRNAs are highly conserved in evolution and act through complete or partial complementarity with the 3′UTR region of target genes, causing the degradation of mRNA or translation inhibition of the target gene to achieve its post-transcriptional regulation (Sylvestre et al., 2007). MiRNAs play multiple roles similar to oncogenes and cancer suppressors in cell growth, differentiation, and apoptosis. Ultimately, they regulate the process of tumorigenesis, development, and metastasis (Gregory and Shiekhattar, 2005).
MiR-143 is upregulated during the differentiation of prostate cancer stem cells and promotes prostate cancer metastasis by repressing FNDC3B expression (Fan et al., 2013). PCAF is upregulated in cultured PC cells, and upregulation of PCAF is associated with the downregulation of miR-17-5p (Gong et al., 2012). The regulation of Livin expression may involve miR-198 in prostate cancer cell lines (Ye et al., 2013). Kobayashi et al. (2012) report significantly higher expression of miR-30d in three prostate cell lines (PC3, DU145, and LNCaP) compared with two normal prostate cell lines (RWPE-1 and PrSc) using miRNA microarrays and qPCR. Using reporter gene assay, they identify miR-30d as a downregulator of SOCS1 expression by directly binding to 3′-UTR of SOCS1. Furthermore, miR-30d regulates the expression of phospho-STAT3, MMP-2, and MMP-9 through the downregulation of SOCS1.
MiRNA targets and functional protein interactions are a rich source of information to elucidate the function and the prognostic value of miRNAs in cancer (Alshalalfa et al., 2012). Arias et al. (2012) identified biomarkers for prostate cancer and lymph node metastasis from microarray data and the protein interaction network using the gene prioritization method. A protein–protein interaction network of established miRNA targets confirm that these proteins are highly connected and essential to the cell, affecting tumorigenesis, cell growth/proliferation, cellular death, cell assembly, and maintenance pathways (Budd et al., 2012).
It is of great biological importance to detect the protein interaction network and to perceive the intervention of protein interactions on disease (Altieri, 2008). Search Tool for the Retrieval of Interacting Genes (STRING) is a database of known and predicted protein interactions. The interactions include direct (physical) and indirect (functional) associations. They are derived from four sources: genomic context, high-throughput experiments, (conserved) coexpression, and previous knowledge. STRING quantitatively integrates interaction data from these sources for a large number of organisms, and transfers information between these organisms where applicable. The database currently covers 2,590,259 proteins from 630 organisms (Szklarczyk et al., 2011).
In this study, we identified differentially expressed miRNAs and further integrated their verified targets of miRNAs from miRecods and miRTarBase. We also identified differentially expressed mRNAs and annotated them into protein interaction networks based on STRING database. Further, we performed function enrichment analysis for these genes in the interaction network.
Materials and Methods
Gene expression profiles
We downloaded miRNA and mRNA expression profiles from The Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/). GEO served as a public repository for high-throughput molecular abundance experimental data, allowing free distribution and shared access to comprehensive datasets (Edgar et al., 2002). The accession number of miRNA expression profile is GSE36802, containing 21 paired samples from prostate cancer tissue and benign prostate tissue. The miRNA profiles are detected on platform GPL8786-[miRNA-1_0] Affymetrix miRNA Array. The accession number of mRNA expression profile is GSE36801, containing two miRNA-31 overexpressed samples and two control samples. The platform is GPL10558-Illumina HumanHT-12 V4.0 expression beadchip (Ye et al., 2013). The miRNA expression profiles are paired prostate cancer tissue and benign prostate tissue, while the mRNA expression profiles are detected under the condition of specific miRNA overexpression. We obtained the microarray annotation data as well as the raw expression profiles.
Data preprocessing and differential analysis of miRNA
The original expression profile in CEL format was transformed into a matrix using R package Affy (Troyanskaya et al., 2001; Fujita et al., 2006). The median method was used for normalizing expression matrix. Then, the Limma package was utilized to identify differential miRNAs between 21 prostate cancer samples and 21 paired benign prostate samples (Wettenhall and Smyth, 2004). Then, package multitest with the BH method (Benjamini and Hochberg, 1995) was used for multiple test correction. If false discovery rate (FDR) <0.05 and |logFC|>1, the miRNA was considered as differentially expressed between the paired samples. The most up- and down regulated miRNAs were selected for further analysis.
Identification of target genes for differential miRNAs
Each miRNA has a plurality of target gene according to miRNA target prediction algorithms. To identify target genes with high convince, we regarded the predicted and verified miRNA targets by two algorithms as target genes. These two algorithms are miRecords and miRTarBase. MiRecords database stores the miRNA target prediction method for animals. So far, it has recorded verified target genes for 548 miRNAs involving nine species (Xiao et al., 2009). MiRTarBase is a comprehensive collection for the experimentally verified miRNA targets. Its latest update in November 2012 states that it collects 2632 relationships between 773 miRNAs and their target genes, involving 14 species (Hsu et al., 2011). In this study, all the target genes, verified by both methods, are highly identified as target genes of differential miRNAs.
Constructing interaction network of miRNA targets
One gene always acts in synergy with other partners; therefore, the interactive protein should also be studied when we explore the function of one gene and its protein (Li et al., 2004). Therefore, the online software STRING (Szklarczyk et al., 2011) was used for searching all the interactions between the differentially expressed genes (http://string-db.org). The interaction network was also constructed. The interaction is weighted by the verification of experimental data, text mining, and other ways.
Identifying differential mRNAs regulated by differential miRNA
The method of screening differential miRNAs was used to further identify genes closely related to miRNAs. In this manner, we obtained the mRNAs that were specifically differentially expressed when the miRNA was differentially expressed. If FDR<0.05 and |logFC|>1, the mRNA was considered as differentially expressed between the paired samples. Comparative analysis was performed on the differential genes and genes in the interaction network.
Gene Ontology and pathway enrichment analysis of gene sets in the interaction network
Traditional analysis always focuses on single genes, which ignores the functional interactions between genes. The gene set enrichment analysis considers functionally similar or function-related genes as a whole. In this strategy, we can identify the biological functions or biological properties by calculating the overall significance of gene expression changes in this gene set (Nam and Kim, 2008).
In this study, the P-value represents the possibility of a gene possessing a Gene Ontology term. The smaller the P-value, the less possible that the gene module is random. The genes in the module perform specific and significant biological functions in synergy (Allison et al., 2006).
Functional Classification Tool (Huang da et al., 2009) utilized the iced clustering algorithm heuristic partitioning procedure to screen the complex functions and pathways for genes of interest. We performed Gene Ontology and pathway enrichment analysis for the genes in the interaction network (FDR<0.05).
Results
Identified differential miRNAs
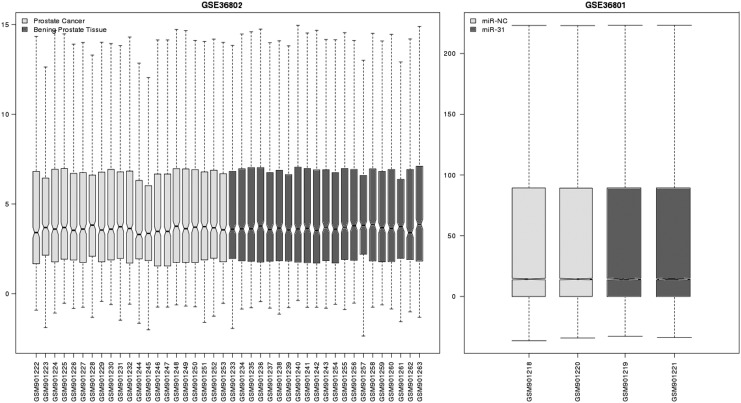
The original expression profiles are well preprocessed and normalized, as shown in Figure 1. After normalization, we performed differential analysis between prostate cancer samples and benign prostate samples using limma. FDR multiple test correction was used for identifying differential genes. Finally, 30 miRNAs were regarded as significantly differentially expressed under the threshold of FDR<0.05 and |logFC|>1, of which, 22 were upregulated such as hsa-mir-31, hsa-mir-145, hsa-mir-455, and hsa-mir-505, while 8 were downregulated including hsa-mir-33a, hsa-mir-25, hsa-mir-130b, and hsa-mir-769. Hsa-mir-31 was the most overexpressed miRNA in prostate cancer. Therefore, it was selected for further study (Table 1).
FIG. 1.
Boxplot of normalized expression profiles. MiRNA expression profiles are shown in the left figure. Light grey and dark grey boxes represent the cancer and benign samples. Gene expression profiles by overexpressing miRNA-31 are shown in the right figure. The light grey and dark grey boxes represent the control and miR-31 overexpressed samples. The black line in the box represents the median of gene expression. The black line can indicate the level of data standardization. The black lines of samples are almost at the same level, indicating that the data are well normalized.
Table 1.
List of Differentially Expressed miRNAs
| miRNA_ID_LIST | Adjusted P-value | logFC |
|---|---|---|
| hsa-mir-31 | 0.000269 | 2.4511 |
| hsa-mir-145 | 1.55E-06 | 2.30637 |
| hsa-mir-455 | 4.35E-06 | 2.12955 |
| hsa-mir-221 | 8.92E-05 | 1.77595 |
| hsa-mir-222 | 3.23E-08 | 1.69902 |
| hsa-mir-143 | 8.92E-05 | 1.6839 |
| hsa-mir-221 | 3.23E-08 | 1.64293 |
| hsa-mir-133b | 0.000146 | 1.55934 |
| hsa-mir-376c | 8.55E-05 | 1.53589 |
| hsa-mir-187 | 0.0227 | 1.47433 |
| hsa-mir-139 | 0.000439 | 1.43215 |
| hsa-mir-455 | 3.57E-06 | 1.37154 |
| hsa-mir-224 | 0.00286 | 1.29457 |
| hsa-mir-204 | 0.00314 | 1.22415 |
| hsa-mir-505 | 8.23E-05 | 1.21126 |
| hsa-mir-149 | 0.00216 | 1.20354 |
| hsa-mir-222 | 0.0401 | 1.08908 |
| hsa-mir-34a | 0.0174 | 1.08451 |
| hsa-mir-152 | 3.36E-05 | 1.04373 |
| hsa-mir-30e | 0.00376 | 1.01954 |
| hsa-mir-377 | 0.0401 | 1.01744 |
| hsa-mir-181c | 0.000268 | 1.00151 |
| hsa-mir-33a | 0.0227 | −1.00405 |
| hsa-mir-25 | 8.55E-05 | −1.01443 |
| hsa-mir-18b | 0.0401 | −1.04336 |
| hsa-mir-130b | 0.000734 | −1.07332 |
| hsa-mir-769 | 0.0129 | −1.11036 |
| hsa-mir-182 | 0.000254 | −1.16549 |
| hsa-mir-148a | 0.000304 | −1.20802 |
| hsa-mir-96 | 0.00709 | −1.31404 |
logFC, log fold change.
Target genes of differential miRNAs
The validated relationship between miRNA and its target genes were downloaded from database miRecords and miRTarBase. As shown in Table 2, the miRNA targets of hsa-mir-31, which are confirmed by miRecords or miRTarBase, were defined as its target genes.
Table 2.
Differentially Expressed Genes Among miRNA Targets
| miRecord | miRTarBase |
|---|---|
| —— | ARPC5 |
| —— | CASR |
| —— | CXCL12 |
| —— | DACT3 |
| —— | DKK1 |
| —— | DMD |
| —— | ETS1 |
| FOXP3 | FOXP3 |
| Fzd3 | FZD3 |
| —— | HOXC13 |
| —— | ICAM1 |
| ITGA5 | ITGA5 |
| —— | JAZF1 |
| —— | KLF13 |
| LATS2 | LATS2 |
| MMP16 | MMP16 |
| MPRIP | MPRIP |
| —— | NFAT5 |
| —— | NUMB |
| PPP2R2A | PPP2R2A |
| RDX | RDX |
| —— | RET |
| RHOA | RHOA |
| —— | SELE |
| —— | TIAM1 |
| —— | YY1 |
| FIH | —— |
The interaction network for target genes of miRNA
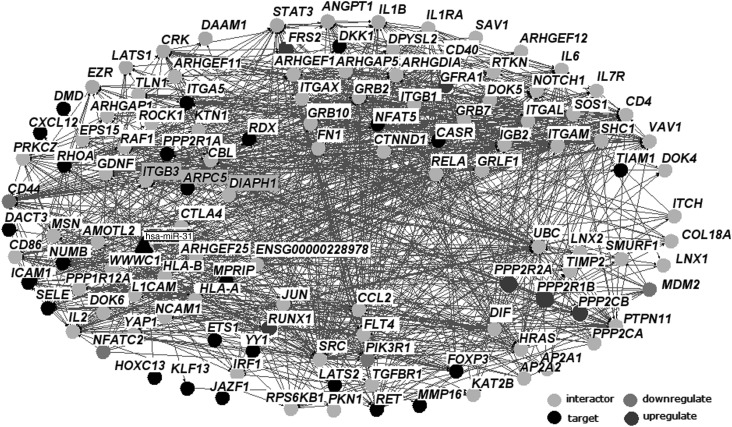
The online software STRING is utilized to predict interactions between target genes of hsa-mir-31. As a result, we identified a total of 935 significantly differentially expressed genes (DEGs) that are influenced by the overexpression of hsa-mir-31. The interaction network of miRNAs mapped by DEGs included 6 upregulated genes, 4 downregulated genes, and 25 target genes (Fig. 2). Combined scores that weigh the degree of confidence for each interaction are illustrated in Supplementary Table S1 (Supplementary materials are available online at www.liebertpub.com.gtmb).
FIG. 2.
The interaction network of miRNA target genes. Triangle node represents hsa-miR-31, black nodes represent miRNA target genes from the miRecords and miRTarBase, the middle grey and dark grey nodes represent down- and upregulated genes, the remaining light grey nodes are the proteins in the interactive partners of miRNA target genes.
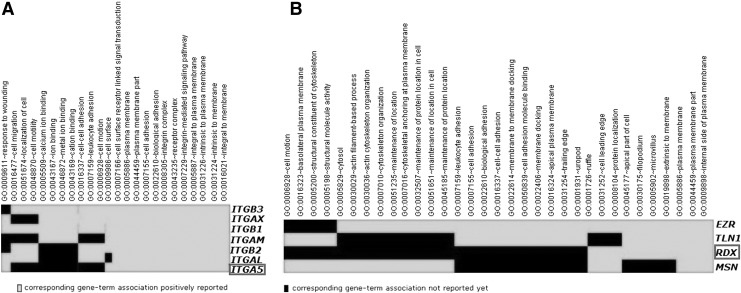
Function enrichment analysis of genes in the interaction network
Function enrichment analysis was performed for the target genes of differential miRNAs, using the Functional Classification Tool. We found two enriched functional clusters, containing 25 and 39 Gene Ontology terms, respectively (Fig. 3, Table S2). Both ITGA5 and RDX are validated as target genes of hsa-mir-31 using luciferase reporter assay, qRT-PCR, and Western blot. We also obtained six enriched pathways of DEGs in the network. The most significant pathway was cell adhesion molecules (CAMs), which is a key process in cancer metastasis. The first step of cancer invasion is to change CAMs, which endow the tumor metastasis ability, with subsequent adhesion of circulating tumor cells, vascular endothelial cells, and stroma (Fujita et al., 2008) (Table 3).
FIG. 3.
Two significant functional clusters and genes enriched in clusters. Genes in the boxes represent experimentally verified target genes of hsa-miR-31. The rest are genes in the interaction network and they are enriched in the same functional cluster as ITGA5 or RDX.
Table 3.
The Pathways Enriched by the Genes in the Interaction Network
| Term | Count | FDR |
|---|---|---|
| hsa04514:Cell adhesion molecules (CAMs) | 14 | 1.20E-04 |
| hsa04144:Endocytosis | 15 | 9.39E-04 |
| hsa04012:ErbB signaling pathway | 11 | 9.51E-04 |
| hsa04662:B cell receptor signaling pathway | 10 | 0.00241898 |
| hsa04062:Chemokine signaling pathway | 14 | 0.006649006 |
| hsa04350:TGF-beta signaling pathway | 10 | 0.008463694 |
FDR, false discovery rate.
Discussion
Prostate cancer is the most commonly lethal cancer in men. Unlike other major types of cancer, no single gene has been identified as being mutated in the majority of prostate tumors. This implies that the expression profiling of genes, including the noncoding miRNAs, may substantially vary across individual cases of this cancer (Zhang et al., 2012).
MiRNA degrades or inhibits the translation of its target genes by fully or partially hybridizing binding with the 3′-UTR of target genes (Fujita et al., 2008). They mainly regulate gene expression at the post-transcriptional level. MiRNAs may potentially impact a series of important processes of life, such as development, cell proliferation, apoptosis, and cell differentiation. A number of studies have reported that miRNAs exhibit up- or downregulation in cancer samples (Galardi et al., 2007). MiRNAs are always differentially expressed in prostate cancer. There is widespread deregulation of miRNA expression in human prostate cancer (Ozen et al., 2008). Ectopic miR-34a expression results in cell cycle arrest and growth inhibition and attenuates hemoresitance to the anticancer drug camptothecin by inducing apoptosis, suggesting a potential role of miR-34a for the treatment of p53-defective prostate cancer (Fujita et al., 2008). MiR-221 and miR-222 expression affects the proliferation potential of the human prostate carcinoma cell (Galardi et al., 2007). MicroRNA145 targets BNIP3 and suppresses prostate cancer progression (Chen et al., 2012). These miRNAs are all differentially expressed in prostate cancer. Therefore, differential miRNAs can be considered as potential biomarkers for prostate cancer.
In this study, we combined miRNA and mRNA expression profiles, integrated miRNA target information from miRecods and miRTarBase, and considered the protein interactions to identify candidate miRNAs and their target genes. The screened miRNA-mRNA pairs may be candidates for further verification.
It is found that hsa-mir-31 is the most overexpressed miRNA in prostate cancer. Integrin α5 (ITGA5) and radixin (RDX) are target genes of hsa-mir-31 according to miRTarBase. ITGA5 and RDX are two migration-related genes and play an important role in mediating cell adhesion and migration in cancer (Andorfer et al., 2011). Shih et al. (2009) have identified that the expression of ITGA5 can increase the formation of mother vessels by stimulating the VEGF-A pathway. In addition, Li et al. (2010) have suggested that miR-31 also blocks breast cancer metastasis through the suppression of cell migration and is functionally linked to ITGA5 and RDX. Moreover, Andrea et al. have stated that miR-31 as antimetastatic miRNA prevents all steps of metastasis through downregulating the expression of ITGA5 and RDX (Creighton et al., 2010). Our results may create a new insight into the role of ITGA5 and RDX in prostate cancer. ITGA5 and RDX differentially expressed after the overexpression of hsa-mir-31, according to the mRNA expression profile, suggesting that ITGA5 and RDX can become candidate genes of prostate cancer and allow a new treatment strategy for its gene therapy. Moreover, the overexpression of hsa-mir-31 can distinguish tissues from prostate cancer and benign surroundings.
CAMs, which are enriched by the interaction network of the target genes of hsa-mir-3, are closely related to tumor invasion and metastasis by binding with ligands from the extracellular matrix or cells and triggering a variety of signaling pathways. Coordinated changes are observed in expression of CAMs in prostate cancer (Murant et al., 1997). Prostate cancer cells exhibit a diverse expression of cell-CAMs and their signaling intermediates. The expression of these adhesion molecules has a close association with the invasive phenotype of these cells. Indeed, characteristics of the tumor cells have been altered by the overexpression of adhesion molecules (Davies et al., 2000). Therefore, tumor CAMs act as a biomarker to diagnose the invasion and metastasis of tumor cells.
On one hand, the identification and determination of miRNAs, which are closely related to the tumor occurrence and development, contribute to the study of their regulatory networks, elucidate the molecular mechanisms of prostate cancer, and provide new insights into early diagnosis and treatment. On the other hand, studies on candidate genes, which are related with the incidence of prostate cancer, not only benefit the early diagnosis, but also provide a reliable basis for its prognosis based on gene therapy.
The structure and size of miRNAs make them free from the attacks of ribonuclease. Although progress has been made to elaborate the roles of miRNAs in cancer research, their specific action mechanism in prostate cancer remains to be further studied. The occurrence of prostate cancer involves multiple genes and multiple factors, which finally leads to extremely complex biological phenotypes through multiple stages.
The proposed method proposes a novel method to identify candidate miRNAs that can be biomarkers of prostate cancer. Moreover, target genes regulated by miRNA and differentially expressed after miRNA dysregulation can be designed as new treatment strategies for antiprostate cancer therapy in the future.
Supplementary Material
Author Disclosure Statement
All authors declare that no competing financial interests exist.
References
- Allison DB. Cui X. Page GP, et al. Microarray data analysis: from disarray to consolidation and consensus. Nat Rev Genet. 2006;7:55–65. doi: 10.1038/nrg1749. [DOI] [PubMed] [Google Scholar]
- Alshalalfa M. Bader GD. Goldenberg A, et al. Detecting microRNAs of high influence on protein functional interaction networks: a prostate cancer case study. BMC Syst Biol. 2012;6:112. doi: 10.1186/1752-0509-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- Andorfer CA. Necela BM. Thompson EA, et al. MicroRNA signatures: clinical biomarkers for the diagnosis and treatment of breast cancer. Trends Mol Med. 2011;17:313–319. doi: 10.1016/j.molmed.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Arias CR. Yeh HY. Soo VW. Biomarker identification for prostate cancer and lymph node metastasis from microarray data and protein interaction network using gene prioritization method. Sci World J. 2012;2012:842727. doi: 10.1100/2012/842727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y. Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc Ser B. 1995;57:12. [Google Scholar]
- Budd WT. Weaver DE. Anderson J, et al. microRNA dysregulation in prostate cancer: network analysis reveals preferential regulation of highly connected nodes. Chem Biodivers. 2012;9:857–867. doi: 10.1002/cbdv.201100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Liu Q. Huang Z, et al. Tripchlorolide induces cell death in lung cancer cells by autophagy. Int J Oncol. 2012;40:1066–1070. doi: 10.3892/ijo.2011.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton CJ. Fountain MD. Yu Z, et al. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res. 2010;70:1906–1915. doi: 10.1158/0008-5472.CAN-09-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. Jiang WG. Mason MD. Cell-cell adhesion molecules and signaling intermediates and their role in the invasive potential of prostate cancer cells. J Urol. 2000;163:985–992. [PubMed] [Google Scholar]
- Edgar R. Domrachev M. Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. Chen X. Deng W, et al. Up-regulated microRNA-143 in cancer stem cells differentiation promotes prostate cancer cells metastasis by modulating FNDC3B expression. BMC Cancer. 2013;13:61. doi: 10.1186/1471-2407-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A. Sato JR. Rodrigues Lde O, et al. Evaluating different methods of microarray data normalization. BMC Bioinform. 2006;7:469. doi: 10.1186/1471-2105-7-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y. Kojima K. Hamada N, et al. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem Biophys Res Commun. 2008;377:114–119. doi: 10.1016/j.bbrc.2008.09.086. [DOI] [PubMed] [Google Scholar]
- Galardi S. Mercatelli N. Giorda E, et al. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem. 2007;282:23716–23724. doi: 10.1074/jbc.M701805200. [DOI] [PubMed] [Google Scholar]
- Gong AY. Eischeid AN. Xiao J, et al. miR-17-5p targets the p300/CBP-associated factor and modulates androgen receptor transcriptional activity in cultured prostate cancer cells. BMC Cancer. 2012;12:492. doi: 10.1186/1471-2407-12-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee RT. Murray T. Bolden S, et al. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- Gregory RI. Shiekhattar R. MicroRNA biogenesis and cancer. Cancer Res. 2005;65:3509–3512. doi: 10.1158/0008-5472.CAN-05-0298. [DOI] [PubMed] [Google Scholar]
- Hiatt RA. Armstrong MA. Klatsky AL, et al. Alcohol consumption, smoking, and other risk factors and prostate cancer in a large health plan cohort in California (United States) Cancer Causes Control. 1994;5:66–72. doi: 10.1007/BF01830728. [DOI] [PubMed] [Google Scholar]
- Hsu SD. Lin FM. Wu WY, et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 2011;39:D163–D169. doi: 10.1093/nar/gkq1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W. Sherman BT. Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N. Uemura H. Nagahama K, et al. Identification of miR-30d as a novel prognostic maker of prostate cancer. Oncotarget. 2012;3:1455–1471. doi: 10.18632/oncotarget.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SH. Murray T. Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. 1. [DOI] [PubMed] [Google Scholar]
- Li S. Armstrong CM. Bertin N, et al. A map of the interactome network of the metazoan C elegans. Science. 2004;303:540–543. doi: 10.1126/science.1091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Zhang M. Chen H, et al. Ratio of miR-196s to HOXC8 messenger RNA correlates with breast cancer cell migration and metastasis. Cancer Res. 2010;70:7894–7904. doi: 10.1158/0008-5472.CAN-10-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murant SJ. Handley J. Stower M, et al. Co-ordinated changes in expression of cell adhesion molecules in prostate cancer. Eur J Cancer. 1997;33:263–271. doi: 10.1016/s0959-8049(96)00418-2. [DOI] [PubMed] [Google Scholar]
- Nam D. Kim SY. Gene-set approach for expression pattern analysis. Brief Bioinform. 2008;9:189–197. doi: 10.1093/bib/bbn001. [DOI] [PubMed] [Google Scholar]
- Ozen M. Creighton CJ. Ozdemir M, et al. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27:1788–1793. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- Shih S-C. Zukauskas A. Li D, et al. The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis. Cancer Res. 2009;69:3272–3277. doi: 10.1158/0008-5472.CAN-08-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre Y. De Guire V. Querido E, et al. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem. 2007;282:2135–2143. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D. Franceschini A. Kuhn M, et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39:D561–D568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanskaya O. Cantor M. Sherlock G, et al. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17:520–525. doi: 10.1093/bioinformatics/17.6.520. [DOI] [PubMed] [Google Scholar]
- Wettenhall JM. Smyth GK. limmaGUI: a graphical user interface for linear modeling of microarray data. Bioinformatics. 2004;20:3705–3706. doi: 10.1093/bioinformatics/bth449. [DOI] [PubMed] [Google Scholar]
- Xiao F. Zuo Z. Cai G, et al. miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009;37:D105–D110. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L. Li S. Ye D, et al. Livin expression may be regulated by miR-198 in human prostate cancer cell lines. Eur J Cancer. 2013;49:734–740. doi: 10.1016/j.ejca.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Zhang W. Edwards A. Fan W, et al. miRNA-mRNA correlation-network modules in human prostate cancer and the differences between primary and metastatic tumor subtypes. PLoS One. 2012;7:e40130. doi: 10.1371/journal.pone.0040130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.