Abstract
The tyrosinase family comprises three members, tyrosinase (Tyr), tyrosinase-related protein 1 (Tyrp1), and dopachrome tautomerase (Dct). Null mutations and deletions at the Tyr and Tyrp1 loci are known and phenotypically affect coat color due to the absence of enzyme or intracellular mislocalization. At the Dct locus, three mutations are known that lead to pigmentation phenotype. However, these mutations are not null mutations, and we therefore set out to generate a null allele at the Dct gene locus by removing exon 1 of the mouse Dct gene. Mice deficient in Dct [Dcttm1(Cre)Bee] lack Dct mRNA and dopachrome tautomerase protein. They are viable and do not show any abnormalities in Dct-expressing sites such as skin, retinal pigment epithelium, or brain. However, the mice show a diluted coat color phenotype, which is due to reduced melanin content in hair. Primary melanocytes from Dct knockout mice are viable in culture and show a normal distribution of tyrosinase and tyrosinase-related protein 1. In comparison to the knockout, the slaty mutation (Dctslt/Dctslt) has less melanin and affects growth of primary melanocytes severely. In summary, we have generated a knockout of the Dct gene in mice with effects restricted to pigment production and coat color.
The number of genes which affect pigmentation and melanocytes in mammals is still growing and is currently 127 (2). About half of them have been cloned and identified, and they affect aspects as various as melanocyte development, melanosome transport, and melanosome construction. Pigmentation is restricted to melanocytes and to the retinal pigment epithelium (RPE). Melanocytes of skin, hair follicle, inner ear, and uveal tract of the eye (iris, ciliary body, and choroid) are derived from the neural crest. On the other hand, the outer layer of the neuroectodermal optic cup, which is derived from the developing forebrain of the embryo, gives rise to the RPE, which does not undergo any migratory phase. During development, melanoblasts originating from the neural crest migrate in the dermis on the dorsolateral pathway. Melanoblasts give rise to melanocytes in dermis but also enter the epidermis, where melanocytes are then produced to populate the hair follicles and the interfollicular epidermis.
The synthesis of the melanin pigment is ascertained by tyrosinase (EC 1.14.18.1), the key enzyme in pigment synthesis, and two related enzymes, tyrosinase-related protein 1 (TYRP1) and dopachrome tautomerase (DCT) (EC 5.3.2.3), which are strongly implicated in eumelanin synthesis (10). The switch between the eumelanin and the pheomelanin pathways is thought to depend on the presence of cysteine. Thus, in the absence of cysteine, dopaquinone, the product of tyrosinase action, is transformed to cyclodopa (leucodopachrome) and then to dopachrome (and DOPA). Dopachrome gives rise to 5,6-dihydroxyindole (DHI) or is enzymatically metabolized by DCT, yielding 5,6-dihydroxyindole-2-carboxylic acid (DHICA) (40). These dihydroxyindoles might then be oxidized to eumelanin. Since DHI and its oxidation products are toxic to cells (33, 42), it was thought that DCT is required to prevent a too-high level of toxic intermediate from being produced within the cell.
The gene coding for DCT (Dct) has been identified in many species, including nonmammalian vertebrates such as chickens, Xenopus, and puffer fish (1, 6, 24). In mice, in situ hybridization assays and transgenic lacZ reporter gene assays have provided evidence that Dct is specifically expressed in cells of the pigment cell lineage, namely melanocytes and melanoblasts, and cells of the RPE (25, 36, 43). Onset of expression is at about embryonic development day 9 (E9) in RPE and at E11 in migrating melanoblasts and thus is somewhat earlier than that of the two related genes of the family, tyrosinase and Tyrp1. Moreover, in adult mouse skin, only Dct seems to be expressed in melanocyte stem cells residing in the bulge of the hair follicle, whereas Tyrp1 and the tyrosinase gene are restricted in expression to more differentiated cells and transiently amplifying cells (3, 28). Apart from the rather expected expression within cells of the pigment cell lineage, Dct is expressed in the developing telencephalon (25, 36, 43) and it was speculated that it might serve as detoxifying enzyme within neuronal tissue.
Mutations at all three tyrosinase gene family members, Tyr (tyrosinase), Tyrp1 (TYRP1), and Dct (DCT) have been found in mice (http://www.informatics.jackson.org). At the Tyr locus, the mutations range from absence of any pigmentation in the albino allele (Tyrc) to phenotypes with only slightly reduced pigmentation, such as, for example, chinchilla (Tyrc-ch). Similarly, mutations at Tyrp1 affect coat color, with the brown (Tyrp1b) mutation at the extreme representing no TYRP1 activity left. It is important to note that some of these mutations (Tyrp1b and Tyrc) still enable protein production and the phenotype is rather due to intracellular mislocalization and retention of the proteins in the endoplasmic reticulum (19, 39). Nevertheless, null mutations of both Tyrp1 and Tyr have been created by large deletions in the course of mutagenesis screens, and the corresponding phenotypes are identical to the Tyrc or Tyrp1b mutation, respectively. In contrast, only three mutations (Dctslt, Dctslt-J, and DctSlt-Lt) are known at the Dct locus, all of which lead to pigmentation phenotypes (4). They are all point mutations and still enable expression of mutant protein. It has even been argued that Dctslt, which depicts the best-characterized phenotype, might retain some DCT activity (4, 22). It therefore remained an open question of whether the absence of DCT activity leads to a coat color phenotype or is of importance in other Dct-expressing tissues, where it might contribute essential detoxifying function. To address this issue, we genetically inactivated the Dct gene by gene targeting in embryonic stem (ES) cells.
MATERIALS AND METHODS
Generation of Dct−/− mice.
A genomic 18-kb NotI fragment derived from a λ clone of 129/SV DNA had been cloned into pBluescript and was provided by Peter Budd and Ian Jackson (4). This fragment contained 3.2 kb of 5′ sequence and exons 1 to 4 of the mouse Dct gene. A 4.5-kb EcoRI-XhoI fragment of the first intron was used for 3′ homology, and, following several intermediate cloning steps to remove or add restriction sites, finally cloned as an EcoRI-BamHI fragment into the EcoRI and BamHI sites of the targeting vector, pPNT (41). A 1.1-kb BglII-StuI fragment of the Dct promoter was isolated from a Dct promoter plasmid (NotI-SacI fragment) (4), and, following different intermediate cloning steps, finally linked to a 1.6-kb MluI-SalI fragment of Cre recombinase (from plasmid pMC-Cre) (17). The resulting 1.1-kb Dct-Cre expression plasmid was cloned as a NotI-XhoI fragment in the corresponding sites of pPNT. GS-1 ES cells (129S6/SvEv) were electroporated with the NotI-linearized targeting vector and selected for resistance to G418 and ganciclovir (21). Southern blot analysis was performed following PstI digestion of genomic ES cell DNA using a 32P-labeled probe (1.5-kb HindIII-BglII fragment of the Dct gene promoter). Out of 166 double-resistant clones, 4 were correctly targeted. We injected two independent clones into blastocysts from C57BL/6 mice and crossed the resulting chimeras back to C57BL/6 mice to obtain heterozygous Dct mutant mice, Dcttm1(Cre)Bee. Expression of Cre recombinase from the Dcttm1(Cre)Bee mutant mice has been described elsewhere (18) and is not discussed further in this article.
Genotyping was performed using DNA isolated from tail biopsies and analyzed by PCR using three primers (Dct sense, 5′-TTGAGAGGAGAGGAAAGGGC-3′; Dct antisense, 5′-CACGCCATCCAAGGTCATGC-3′; and Cre antisense, 5′-CATTGCTGTCACTTGGTCGT-3′), resulting in a Dct knockout-specific PCR product (∼600 bp) and a product specific for the endogenous gene (289 bp). To control for the absence of exon 1 coding sequence in knockout mice, a separate set of primers was used (Dct sense, 5′-CTGCGGAATTCTGCTCA-3′; and Dct antisense, 5′-TTCTCGGCCATTGCTCA-3′), resulting in a 224-bp fragment. For reverse transcription-PCR, total RNA was isolated from 2-day-old mouse skin. RNA was treated with DNase, and the first cDNA strand was synthesized with Superscript (Life Technologies). PCR was performed using primers situated in exons 4 and 5 of Dct (exon 4, Dct sense, 5′-ATGAGTCCTTTGCGTTGCCC-3′; and exon 5, Dct antisense, 5′-TGAAGCTGAAGGTAGAGT-3′), amplifying a 333-bp fragment. The integrity of the cDNA was controlled by amplification using β-catenin-specific primers (exon 2, β-catenin sense, 5′-CGTGGACAATGGCTACTCAA-3′; and exon 4, β-catenin antisense, 5′-CCCTCATCTAGCGTCTCAGG-3′), resulting in a 325-bp fragment.
Breeding experiments.
Except for specific analyses, mice were kept nonagouti (a) and wild type at Tyrp1 (Tyrp1+) and Tyr (Tyr+) loci. Homozygous mutant mice on an agouti background (Aw) were obtained by interbreeding the first chimeric offspring. Homozygous mutant mice with a mutation at the Tyrp1 locus (Tyrp1b) and/or the Tyr locus (Tyrc) were obtained by breeding Dcttm1(Cre)Bee homozygous mutant mice (a/a, Tyr+/Tyr+, and Tyrp1+/Tyrp1+) with BALB/c mice (A/A, Tyrp1b/Tyrp1b, and Tyrc/Tyrc). The resulting offspring were intercrossed to generate mice which are brown (Tyrp1b/Tyrp1b) and nonagouti (a) or agouti (A) but still carry the Dcttm1(Cre)Bee allele. Mice [Dcttm1(Cre)Bee/+, Tyrp1b/Tyrp1b, Tyr+/Tyr+, or Tyr+/Tyrc] were bred with each other to obtain both brown mutant mice deficient in Dct [Dcttm1(Cre)Bee/Dcttm1(Cre)Bee, Tyrp1b/Tyrp1b] and brown albino mice deficient in Dct [Dcttm1(Cre)Bee/Dcttm1(Cre)Bee, Tyrp1b/Tyrp1b, and Tyrc/Tyrc]. For comparison with slaty (Dctslt), mice with homozygous mutation at this allele (Dctslt; kindly provided by L. Lamoreux, Austin, Tex.) and kept on a C57BL/6 background were bred to Dcttm1(Cre)Bee homozygous mutant mice (a/a, Tyr+/Tyr+, and Tyrp1+/Tyrp1+). The resulting offspring were mated to each other to generate littermates of the three genotypes Dctslt/Dctslt, Dcttm1(Cre)Bee/Dcttm1(Cre)Bee, and Dctslt/Dcttm1(Cre)Bee.
Primary melanocyte culture.
To isolate primary melanocytes (14), a piece of shaved skin (adult skin, 10 to 15 cm2; 1-week-old, whole-back skin) was washed in phosphate-buffered saline (PBS), cleaned of fat, and incubated overnight at 4°C in 0.25% trypsin. Dermis was separated from epidermis, minced with scissors, and incubated for 3 to 5 h at 37°C in 10 ml of collagenase (0.1% [wt/vol]; Sigma). Following mixing (by pipetting up and down) and three rounds of centrifugation and resuspension in PBS (282 × g, 5 min), the pellet was resuspended in primary melanocyte medium (5% fetal calf serum in RPMI 1640 with Glutamax [Gibco], containing 23-ng/ml 3-isobutyl-1-methylxanthine [Sigma], 10-μg/ml bovine pituitary extract [Sigma], and 10-μg/ml insulin [Sigma]) and placed on 60- to 100-mm-diameter cell culture dishes. G418 (50 μg/ml) and 35-ng/ml tetradecanoylphorbol-13-acetate (TPA [Sigma]) were added after 24 h. Initially, we encountered problems in obtaining melanocytes from Dct knockout mice, which might be due to melanin production and accumulation of toxic intermediates such as DHI: therefore, we added catalase (100 U/ml) to the medium (42). In later experiments, catalase was no longer added to the culture medium. Proliferation was measured on 3-week-old cultures derived from 1-week-old mice. A total of 6 × 105 to 10 × 105 cells were plated on a 10-cm dish, and the total cell number per dish was determined after 8 days, following trypsinization.
All antibody stainings were performed at room temperature in 24-well plates. Cells were grown on coverslips coated with poly-d-lysine (0.1 mg/ml) (Sigma) and fixed for 15 min in 2% paraformaldehyde. After being washed with PBS, the cells were permeabilized with Triton X-100 (0.1% [wt/vol]) and 1% bovine serum albumin (BSA) for 10 min at room temperature. Nonspecific binding sites were blocked for 30 min with 5% BSA and 5% fetal calf serum in PBS. The cells were then incubated for 90 min at 37°C with the primary antibody (anti-PEP7, anti-PEP8, and anti-PEP1) and for 30 min with the secondary antibody (CY3-coupled donkey anti-rabbit immunoglobulin G). Nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole) for 10 to 20 min at room temperature. Coverslips were mounted on slides with 1,4-diazobicyclo(2,2,2)-octane (DABCO). Confocal microscopy of cells grown on coverslips was performed according to standard procedures (12).
Total melanin.
Melanin was extracted from dorsal hair (1 to 4 mg) of 4- to 8-week-old mice by treatment with alkali (1 ml of 1 M NaOH, 4 h at 85°C). Relative net melanin content (per gram of hair) was determined by spectrophotometric determination of A475. Each hair sample was measured in triplicate. Data are presented as means ± standard error. Statistical significance was assessed by the t test.
Western blot.
Total protein extracts were isolated from adult mouse skin. Western blots were performed as described previously (11, 12). Briefly, 100 to 150 μg of protein was boiled for 5 min in reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer and electrophoresed on sodium dodecyl sulfate-8% polyacrylamide gels. The proteins were transferred to a nitrocellulose membrane in a mixture of 20% methanol, 40 mM glycine, and 50 mM Tris base. After transfer, membranes were saturated overnight with PBS containing 4% milk, 1% BSA, and 0.25% Tween 20. Membranes were incubated with the primary antibody (anti-PEP1 or anti-PEP8) overnight at 4°C, washed, and incubated with the secondary antibody (horseradish peroxidase, mouse anti-rabbit) for 30 min. Finally, the membrane was washed again, and immunocomplexes were visualized by a chemiluminescent reaction (ECL; Amersham Life Science, Otelfingen, Switzerland).
Histology and immunohistochemistry.
For histological analysis, organs of adult mice were dissected, fixed overnight in 4% paraformaldehyde, and embedded in paraffin. Seven-micrometer sections were stained with hematoxylin and eosin. For immunofluorescence, cryostat sections of E14.5 embryos were fixed for 3 min in ice-cold acetone, blocked in PBS-1% BSA, and incubated for 1 h at room temperature with primary antibodies. DCT and tyrosinase were detected with anti-PEP8 and anti-PEP7 antibodies (kindly provided by V. Hearing). The sections were incubated for 30 min at room temperature with the secondary antibody (donkey anti-rabbit CY3), washed three times in PBS, and mounted in Citifluor (type 40-88; Aldrich-Chemie, Steinheim, Germany).
RESULTS
Gene targeting at exon 1 of the mouse Dct gene locus.
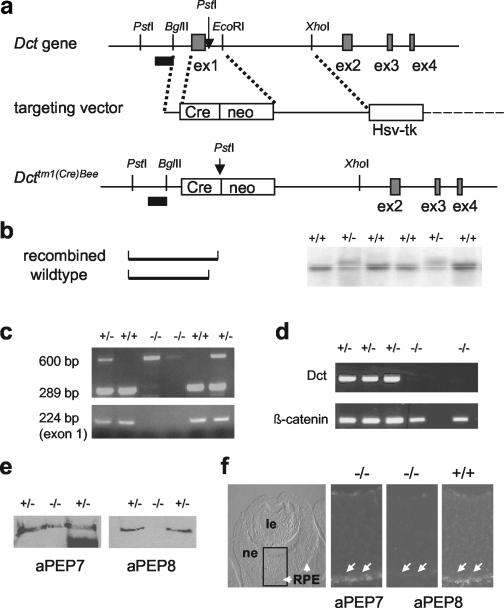
A genomic fragment containing Dct promoter sequences and the first four exons of Dct was used to construct a replacement-type targeting vector. The knockout was performed at exon 1, which contains the ATG and codes for the signal peptide (see Fig. 1 for details). Mice with heterozygous mutation of the targeted allele [Dcttm1(Cre)Bee] were obtained following mating of chimeras to C57BL/6 females. Matings of male and female heterozygous mutant mice derived from two independent ES cell clones resulted in the birth of homozygous mutant mice, which were born in a ratio consistent with the Mendelian pattern of inheritance. The knockout mice were deficient for exon 1, as evidenced by failure of exon 1-specific PCR amplification, and lacked Dct mRNA. Furthermore, absence of DCT protein was corroborated by using anti-PEP8 antibody in Western blots of skin of newborn mice and on cryosections of E14.5 embryonic eyes. We thus conclude that we have successfully generated mice lacking DCT.
FIG. 1.
Gene targeting at the Dct gene locus. (a) Scheme of the gene targeting strategy. Only exons 1 to 4 are indicated, and restriction sites are illustrated with relevance to cloning and Southern blot detection. The external 5′ probe is indicated as a black bar. neo, neomycin resistance gene cassette (Pgk-neo-pA). (b) Use of PstI digestion and an external probe (a) allowed us to distinguish between the recombined and wild-type alleles. Note that the exact localization of the 5′ PstI site is not known and is only inferred from the size of the hybridizing fragments. (c) PCR analysis confirms removal of exon 1. (Top panel) Standard PCR analysis with three primers allows us to distinguish between the targeted and wild-type allele. (Bottom panel) Exon 1-specific primers lead to amplification in DNA from heterozygous (+/−) and wild-type (+/+) mice but not from homozygous Dcttm1(Cre)Bee mutant (−/−) mice. (d) Reverse transcription-PCR analysis of RNA isolated from newborn skin demonstrates absence of Dct transcripts in Dcttm1(Cre)Bee mutant mice. The reaction was controlled by detection of β-catenin transcripts. (e) Western blot of skin extracts shows the presence of tyrosinase (anti-PEP7) but absence of DCT (anti-PEP8) from homozygous Dcttm1(Cre)Bee mutant mice. (f) DCT is absent from embryonic RPE. Cryostat sections of homozygous Dcttm1(Cre)Bee mutant E14.5 embryos do reveal expression of tyrosinase (anti-PEP7), but no expression of DCT (anti-PEP8; arrows), in comparison to the wild-type (+/+) control. In the left panel, RPE is indicated by arrows. ne, neuroretina; le, lens.
A coat color phenotype of Dct-deficient mice.
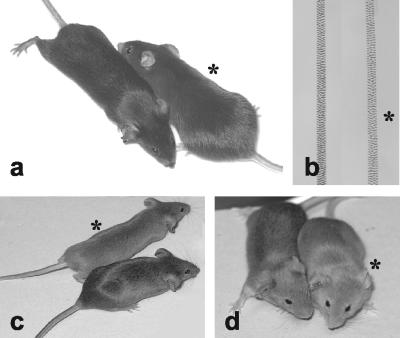
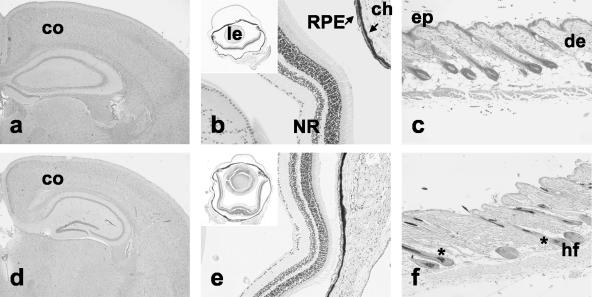
No difference was seen in heterozygous mutant mice and wild-type mice. However, homozygous mutant mice were clearly recognized by a diluted and lighter coat color. This was observed on both agouti and nonagouti backgrounds (Fig. 2) and was also seen in single hair samples (Fig. 2b). Organs which express DCT were then analyzed by histology to determine whether they were altered due to Dct deficiency (Fig. 3). No significant differences were observed between brains of wild-type mice and homozygous mutant mice (Fig. 3a and d). Eyes were histologically normal, with normal pigmentation of iris, ciliary body, and RPE (Fig. 3b and e). Equally, choroidal melanocytes were normally, homogenously, and heavily pigmented in homozygous mutant mice. Skin was histologically normal and did not show any obvious difference in pigmentation (Fig. 3c and f). In addition, other organs which do not express Dct, such as testis, lung, pancreas, liver, spleen, kidney, muscle, stomach, and intestine, were equally sectioned and revealed to be normal. Thus, we conclude that the phenotypic consequences of Dct deficiency in vivo are restricted to melanocytes with effects on coat color.
FIG. 2.
Absence of DCT affects coat color. (a) Heterozygous or homozygous Dcttm1(Cre)Bee mutant nonagouti black mice. (b) Single hairs from mice depicted in panel a. Decreased pigmentation is seen in the homozygous Dcttm1(Cre)Bee mutant hair (asterisk). (c and d) The coat color is equally diluted on an agouti (Aw) or agouti brown (A, Tyrp1b) genetic background. In panels a to d, mice (and hair) from homozygous Dcttm1(Cre)Bee mutants are indicated by asterisks.
FIG. 3.
Morphology of Dct-expressing tissues is not altered in Dct-deficient mice. Forebrain (a and d), eye (b and e), and skin (c and f) samples from adult C57BL/6 (a to c) and Dcttm1(Cre)Bee mutant mice (d to f) were analyzed on paraffin sections. co, cortex; NR, neuroretina; ep, epidermis; de, dermis; hf, hair follicle; ch, choroid; le, lens.
The Dct-deficient mice were wild type at the tyrosinase and the Tyrp1 locus. Hence, potential effects due to lacking enzyme activity or function or integrity of the melanosomal complex might have been compensated for to a certain degree by tyrosinase or TYRP1. We thus crossed the Dcttm1(Cre)Bee allele to mice carrying alleles encoding nonfunctional tyrosinase (albino, Tyrc) and Tyrp1 (brown, Tyrp1b). We first generated mice with homozygous mutation at both the Tyrp1 and Dct loci. These mice were again lighter in coat color (Fig. 2d), and breedings did not reveal any deviation from the expected Mendelian ratios. Some of these mice were additionally heterozygous for the albino allele (Tyrc) and were used to obtain albino brown mutant mice deficient in DCT. Mice with homozygous mutation of all three tyrosinase family genes were obtained, and again, analysis of paraffin sections of all major organs, including brain, eye, and skin, did not reveal any difference from wild-type mice and heterozygous [Dcttm1(Cre)Bee/+] littermates (not shown). Even though we cannot exclude that activity of DCT, for example, in brain, might be compensated for by other proteins, we suggest that absence of all tyrosinase family proteins only affects pigment cells and melanin synthesis.
Culture of Dct-deficient primary melanocytes.
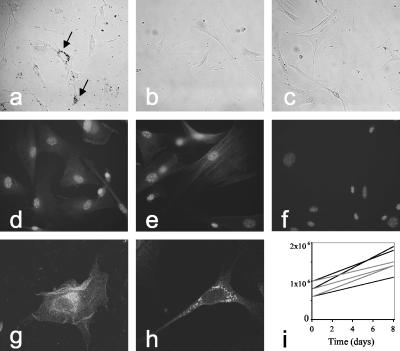
Even though we have not seen any evidence for changes in melanocytes in the living mouse, toxic effects might be tampered with or compensated for by surrounding keratinocytes or fibroblasts. We hence isolated primary melanocytes from skin of Dct knockout mice. In all of these experiments, melanocyte numbers were not obviously different when beginning with the isolation procedure, at a point at which fibroblasts and keratinocytes are still present. Dct-deficient primary melanocytes could be isolated and seemed not to be affected in viability or proliferation (Fig. 4a and i). When counting cell numbers in such primary cultures, viability of Dct-deficient melanocytes was not reduced compared to that in wild-type melanocytes (not shown). Equally, primary melanocytes were grown from albino mice lacking tyrosinase and TYRP1 function (Tyrc/Tyrc, Tyrp1b/Tyrp1b) and with either homozygous or heterozygous mutation of the Dct knockout allele. Again, deficiency of Dct did not affect primary melanocyte cultures (Fig. 4b and c). Cultures of pigmented Dct knockout melanocytes were then used for immunofluorescence analysis, which confirmed the absence of DCT expression (Fig. 4f) and demonstrated the normal expression and localization of tyrosinase and TYRP1 (Fig. 4d and e), depicting the punctate pattern, with perinuclear density representing melanosomes and trans-Golgi network (TGN) localization (Fig. 4g and h). The results show that Dct-deficient melanocytes grow in culture and suggest that absence of DCT does not affect localization of tyrosinase and TYRP1.
FIG. 4.
Culture of primary melanocytes. (a) Homozygous Dcttm1(Cre)Bee mutant melanocytes isolated from pigmented mice. Arrows indicate melanin-containing melanosomes. (b) Primary melanocytes from unpigmented (Tyrc and Tyrp1b) mice lacking DCT (Dcttm1(Cre)Bee, homozygous). (c) Primary melanocytes from unpigmented (Tyrc and Tyrp1b) Dcttm1(Cre)Bee heterozygous mutant mice. (d to f) Primary melanocytes from homozygous Dcttm1(Cre)Bee mutant mice (kept in catalase-containing medium) express TYRP1 (anti-PEP1; d) or tyrosinase (anti-PEP7; e) but no DCT (anti-PEP8; f). Note that nuclei are revealed by DAPI. (g and h) Confocal microscopy of homozygous Dcttm1(Cre)Bee mutant primary melanocytes reveals localization of TYRP1 (g) and tyrosinase (h) in a punctate pattern with perinuclear density, as in wild-type cells (not shown). (i) Primary cultures of melanocytes from 1-week-old Dcttm1(Cre)Bee mutant mice show doubling times of about 8 days, both with catalase (three cultures, dotted gray lines) and without catalase (three cultures, solid black lines).
The knockout phenotype is different from slaty.
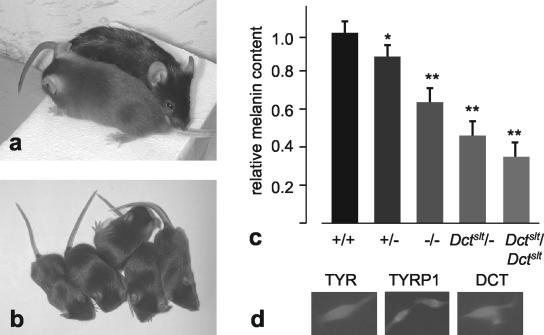
The Dctslt mutation was described as a mutation with very low activity of DCT (22). Mice homozygous for this mutation have a coat color phenotype (Fig. 5a) very similar to that of the knockout mice (Fig. 2a). To directly compare the slaty mutation with the knockout, we obtained Dctslt homozygous mutant mice which had been maintained on a C57BL/6 genetic background. These mice were mated to homozygous Dct-knockout mice [Dcttm1(Cre)Bee]. The resulting offspring were indistinguishable in coat color from the two parental strains, thus confirming that the two mutations are allelic and affect eumelanin synthesis similarly (Fig. 5b). By breeding such compound heterozygotes between each other or with homozygous knockout mice, littermates were generated that carry all three genotypes [Dctslt/Dctslt, Dctslt/Dcttm1(Cre)Bee, and Dcttm1(Cre)Bee/Dcttm1(Cre)Bee]. Since these three genotypes were not distinguishable by coat color, we measured relative melanin content (Fig. 5c) and compared the values to those of wild-type C57BL/6 mice (1.017 ± 0.079, n = 6) and heterozygous mutant mice [Dcttm1(Cre)Bee/+, 0.895 ± 0.050, n = 12]. As expected from the coat color phenotype, hair of knockout mice and of slaty mutant mice had significantly lower melanin content than that of C57BL/6 mice. However, the relative melanin contents among the three genotypes [Dcttm1(Cre)Bee/Dcttm1(Cre)Bee, 0.664 ± 0.074, n = 42; Dctslt/Dcttm1(Cre)Bee, 0.480 ± 0.068, n = 34; and Dctslt/Dctslt, 0.393 ± 0.058, n = 10] differed significantly (P < 0.001), with the slaty values being inferior to the knockout values. We therefore conclude that the coat color phenotype of Dct knockout [Dcttm1(Cre)Bee/Dcttm1(Cre)Bee] mice is similar to the slaty coat color phenotype but the hair melanin content is more severely affected in slaty mice (Dctslt/Dctslt).
FIG. 5.
The slaty mutation (Dctslt). (a) A homozygous mutant Dctslt mouse in comparison to a C57BL/6 mouse shows a similar dilution of coat color to that of a homozygous Dcttm1(Cre)Bee mutant mouse (Fig. 2a). (b) Littermates of Dcttm1(Cre)Bee/Dcttm1(Cre)Bee and Dcttm1(Cre)Bee/Dctslt genotypes are indistinguishable by coat color. (c) Relative melanin contents (see Materials and Methods) of hair are different among Dcttm1(Cre)Bee homozygous (−/−; n = 42), Dctslt homozgyous (n = 10), and compound mice. The relative melanin values for wild-type (C57BL/6, +/+; n = 6) and heterozygous knockout (+/−; n = 12) mice are equally depicted (*, P < 0.01; **, P < 0.001; n = 34). (d) DCT, tyrosinase, and TYRP1 are expressed in Dctslt primary melanocytes. Note that nuclei are revealed by DAPI.
Dctslt and DctSlt-Lt melanocytes are very difficult to grow in culture (9). We equally tried to establish cultures from homozygous mutant Dctslt mice and, using standard medium, never achieved any survival of primary melanocytes. We then added catalase to the medium, to counteract the effects of the absence of DCT. Surprisingly, inclusion of catalase only partially overcame the problem, and cells grew only very slowly. Dctslt mutant melanocytes express tyrosinase and TYRP1 but equally express DCT as recognized by the anti-PEP8 antibody (Fig. 5d). We thus suggest that the mutant DCT from the Dctslt allele is expressed in primary melanocytes and that its effect on melanin production and primary melanocyte growth cannot be explained solely by a reduction in enzyme activity.
DISCUSSION
The tyrosinase family consists of three members, tyrosinase, TYRP1, and DCT (formerly tyrosinase-related protein 2), with conservation throughout the vertebrate lineage (6, 7, 24). Whereas many mutations, including null mutations, are known at the mouse tyrosinase locus (formerly the c or albino locus) and at the Tyrp1 locus (former the b or brown locus) (http://www.informatics.jackson.org), only three mutations have been described at the third locus, Dct (former the slaty locus) (4). Moreover, many mutations in humans have been found in tyrosinase, causing oculocutaneous albinism type 1 (OCA1) (29), and a few have been found in TYRP1 (OCA3) (35). No such mutation has been found in the human DCT gene, thus making it possible that DCT is an indispensable enzyme. We have now successfully inactivated the Dct gene in mice.
DCT has been shown to be an enzyme of the eumelanin biosynthetic pathway (22, 40). In presence of the enzyme, dopachrome is metabolized to DHICA. The part of dopachrome which is not metabolized will be converted spontaneously to DHI. Most of these intermediate substances, such as DHI, DHICA, and, equally, DOPA, are cytotoxic in cell culture (32, 33, 42), which is mainly due to oxidation products, such as H2O2. For example, antiproliferative effects of DHI could be abrogated in the presence of catalase (42). More DHI should be produced in primary melanocytes lacking DCT, thus causing cytotoxic effects. In consequence, when we began with isolation of primary melanocytes from pigmented Dct-deficient mice, we sometimes encountered problems in proliferation and growth (not shown) like those described for exogenously added DHI (42). Since similar problems have been mentioned for melanocytes of Dctslt mice (9), we argued that this effect is due to the accumulation of toxic by-products, including H2O2, and added catalase to the melanocyte culture medium, which helped to improve viability. This effect was clearly linked to pigment production and to the absence of DCT, since albino melanocytes lacking DCT were not affected and always grew as controls. In later experiments, we started isolation with more cells and avoided inclusion of cellular debris and hairs. Under such conditions, Dct knockout melanocytes grew and proliferated similarly with or without catalase in the medium (Fig. 4). In vivo, in Dct knockout mice, we have not seen any obvious decrease in melanocyte numbers. When isolating primary melanocytes from mice, no difference in number was observed. It is feasible that, in skin, interaction with keratinocytes and fibroblasts might somehow prevent accumulation of cytotoxic products.
Besides pigment cells, Dct expression is found within the developing telencephalon (31, 36), and it had been suggested that DCT might exert detoxifying functions, similar to the potency to avoid reactive oxygen species generated by autooxidation of melanin precursors (4). Brain sections of adult Dct knockout mice revealed no difference from wild-type mice and thus make it unlikely that absence of DCT has any effect in the developing telencephalon. A toxic effect would have been apparent in our experiment, even though we cannot completely exclude having missed minor effects or that brains of knockout mice are more sensitive to toxic insult. It might nevertheless be feasible that the expression of Dct in telencephalon is without functional relevance.
Interestingly, the 5′ regulatory region of only 6 kb of the mouse tyrosinase gene promoter leads to expression of the reporter gene lacZ in the developing telencephalon (38), without obvious expression of mRNA or detectable enzyme activity (15, 20). Use of an enlarged 5′ regulatory region, encompassing the tyrosinase enhancer/locus control region, apparently prevents this expression pattern (5, 13, 34). It might thus be possible that tyrosinase expression in forebrain has to be inhibited, and, hence, additional control elements have evolved. On the other side, expression of Dct in forebrain might be of no functional relevance and does not require to be suppressed.
The Dct locus was formerly called the slaty locus, according to the sole mutation known at this locus (16). In the meantime, two other mutations have been identified, DctSlt-lt (slaty light), with a coat color effect already evident in heterozygotes, and Dctslt-2J, which is similar to the original slaty (Dctslt) mutation (4). This mutation, which changes an arginine to a glutamine in the first copper binding domain, was reported to yield about 10 to 30% of wild-type DCT activity in eye extracts (22). In contrast, when a Dct cDNA carrying the slaty mutation was expressed in cells in culture, no enzymatic activity was detected. According to Western blots, protein was produced from both the wild-type and the slaty mutant (Dctslt) cDNA, even though the antibody reacted less with the protein encoded by the mutant cDNA (23). From our data, we suggest that the slaty mutation leads to a more severe reduction in melanin than the Dct knockout. All three members of the tyrosinase family are type 1 membrane-bound melanosomal glycoproteins with similar structural features, and all three are believed to interact with one another in melanosomes (30). TYRP1 and DCT might act together to modulate tyrosinase activity (26). Thus, a nonfunctional but processed DCT protein might prevent tyrosinase from being fully active and/or reaching the melanosome. Along this line, it is now clear that mutations in tyrosinase and TYRP1 affect the association of each with calnexin and Bip, thus leading to retention of mutant products in the endoplasmic reticulum (39). Even though the effect seems to be weaker, association of DCT with calnexin was slightly increased in a tyrosinase or TYRP1 mutant situation. From these experiments, it was concluded that tyrosinase and TYRP1, as well as, to some extent, DCT, associate. A mutation in any of these genes might thus affect maturation and degradation of the others (39). DCT was reported to have a maturation pathway distinct from tyrosinase and TYRP1 and is not only confined to melanosomes but distributed perinuclearly in the TGN (27). Hence, DCT might have additional regulatory function within the melanocyte (27). Consequently, the point mutation in the copper binding domain of the slaty (Dctslt) mutation might not only affect DCT activity within the melanosome but also might affect interaction with yet unknown proteins within the TGN.
DCT is expressed in most melanoma cells and is used as melanoma differentiation antigen (37). It was even reported that the presence of DCT in melanoma is correlated with resistance to certain chemotherapeutic agents (8). Thus, it is possible that the absence of DCT [Dcttm1(Cre)Bee] or altered DCT protein conformation and/or localization (Dctslt or DctSlt-Lt) will affect melanoma formation or melanoma sensitivity. This can now be tested by using Dct mutant and knockout mice following breeding with transgenic melanoma models.
Acknowledgments
Thanks are due to Christelle Richard, Barbara Canepa, and Julien Ackermann for help with blastocyst injections, genotyping, RNA analyses, and melanocyte cultures; Ian Jackson and Peter Budd for supplying genomic clones of mouse Dct; the MIM facility for help with histology and immunofluorescence; Lynn Lamoreux for providing Dctslt mice; and to Edith Hummler for precious advice on ES cell work and comments on the manuscript.
The present work was supported by grant 3100-066796.01 (F.B.) from the Swiss National Science Foundation and by the National Center of Competence in Research Molecular Oncology, a research instrument of the Swiss National Science Foundation.
REFERENCES
- 1.April, C., I. Jackson, and S. Kidson. 1998. Molecular cloning and sequence analysis of a chicken cDNA encoding tyrosinase-related protein-2/DOPAchrome tautomerase. Gene 219:45-53. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, D., and M. L. Lamoreux. 2003. The color loci of mice—a genetic century. Pig. Cell Res. 16:333-344. [DOI] [PubMed] [Google Scholar]
- 3.Botchkareva, N. V., M. L. Khlgatian, B. J. Longley, V. A. Botchkarev, and B. A. Gilchrest. 2001. SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J. 15:645-658. [DOI] [PubMed] [Google Scholar]
- 4.Budd, P., and I. Jackson. 1995. Structure of the mouse tyrosinase-related protein-2/dopachrome tautomerase (Tyrp2/Dct) gene and sequence of two novel slaty alleles. Genomics 29:35-43. [DOI] [PubMed] [Google Scholar]
- 5.Camacho-Hübner, A., and F. Beermann. 2001. Increased transgene expresssion by the mouse tyrosinase enhancer is restricted to neural crest-derived pigment cells. Genesis 29:180-187. [DOI] [PubMed] [Google Scholar]
- 6.Camacho-Hübner, A., C. Richard, and F. Beermann. 2002. Genomic structure and evolutionary conservation of the tyrosinase family from Fugu. Gene 285:59-68. [DOI] [PubMed] [Google Scholar]
- 7.Camacho-Hübner, A., A. Rossier, and F. Beermann. 2000. The Fugu rubripes tyrosinase gene promoter targets transgene expression to pigment cells in the mouse. Genesis 28:99-105. [DOI] [PubMed] [Google Scholar]
- 8.Chu, W., B. Pak, M. Bani, M. Kapoor, S. Lu, A. Tamir, R. Kerbel, and Y. Ben-David. 2000. Tyrosinase-related protein 2 as a mediator of melanoma specific resistance to cis-diamminedichloroplatinum(II): therapeutic implications. Oncogene 19:395-402. [DOI] [PubMed] [Google Scholar]
- 9.Costin, G., J. Valencia, W. Vieira, M. Lamoreux, and V. Hearing. 2003. Characterization of two new mouse melanocyte cell lines carrying the slaty and slaty light mutations. Pig. Cell Res. 16:419. [Google Scholar]
- 10.del Marmol, V., and F. Beermann. 1996. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 381:165-168. [DOI] [PubMed] [Google Scholar]
- 11.Foletti, A., J. Ackermann, A. Schmidt, E. Hummler, and F. Beermann. 2002. Absence of fibroblast growth factor 2 does not prevent tumor formation originating from the RPE. Oncogene 21:1841-1847. [DOI] [PubMed] [Google Scholar]
- 12.Foletti, A., F. Vuadens, and F. Beermann. 2003. Nuclear localization of mouse fibroblast growth factor 2 requires N-terminal and C-terminal sequences. Cell. Mol. Life Sci. 60:2254-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganss, R., L. Montoliu, A. Monaghan, and G. Schütz. 1994. A cell-specific enhancer far upstream of the mouse tyrosinase gene confers high level and copy number-related expression in transgenic mice. EMBO J. 13:3083-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gause, P., M. Lluria-Prevatt, W. Keith, A. Balmain, S. Linardopolous, J. Warneke, and M. Powell. 1997. Chromosomal and genetic alterations of 7,12-dimethylbenz(a)anthracene-induced melanoma from TP-ras transgenic mice. Mol. Carcinog. 20:78-87. [DOI] [PubMed] [Google Scholar]
- 15.Giménez, E., A. Lavado, P. Giraldo, and L. Montoliu. 2003. Tyrosinase gene expression is not detected in mouse brain outside the retinal pigment epithelium. Eur. J. Neurosci. 18:2673-2676. [DOI] [PubMed] [Google Scholar]
- 16.Green, M. 1973. Chromosome 14. Mouse Newsl. 47:36. [Google Scholar]
- 17.Gu, H., J. D. Marth, P. C. Orban, H. Mossmann, and K. Rajewsky. 1994. Deletion of a DNA polymerase β gene segment in T cells using cell type-specific gene targeting. Science 265:103-106. [DOI] [PubMed] [Google Scholar]
- 18.Guyonneau, L., A. Rossier, C. Richard, E. Hummler, and F. Beermann. 2002. Expression of Cre recombinase in pigment cells. Pig. Cell Res. 15:305-309. [DOI] [PubMed] [Google Scholar]
- 19.Halaban, R., S. Svedine, E. Cheng, Y. Smicun, R. Aron, and D. Hebert. 2000. Endoplasmic reticulum retention is a common defect associated with tyrosinase-negative albinism. Proc. Natl. Acad. Sci. USA 97:5889-5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hearing, V. 2000. The melanosome: the perfect model for cellular responses to the environment. Pig. Cell Res. 13(Suppl. 8):23-34. [DOI] [PubMed] [Google Scholar]
- 21.Hummler, E., A. M. Mérillat, I. Rubera, B. C. Rossier, and F. Beermann. 2002. Conditional gene targeting of the Scnn1a (αENaC) gene locus. Genesis 32:169-172. [DOI] [PubMed] [Google Scholar]
- 22.Jackson, I., D. Chambers, K. Tsukamoto, N. Copeland, D. Gilbert, N. Jenkins, and V. Hearing. 1992. A second tyrosinase-related protein, TRP-2, maps to and is located at the mouse slaty locus. EMBO J. 11:527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroumpouzos, G., K. Urabe, T. Kobayashi, C. Sakai, and V. Hearing. 1994. Functional analysis of the slaty gene product (TRP2) as dopachrome tautomerase and the effect of a point mutation on its catalytic function. Biochem. Biophys. Res. Commun. 202:1060-1068. [DOI] [PubMed] [Google Scholar]
- 24.Kumasaka, M., S. Sato, I. Yajima, and H. Yamamoto. 2003. Isolation and developmental expression of tyrosinase family genes in Xenopus laevis. Pig. Cell Res. 16:455-462. [DOI] [PubMed] [Google Scholar]
- 25.MacKenzie, M., S. Jordan, P. Budd, and I. Jackson. 1997. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev. Biol. 192:99-107. [DOI] [PubMed] [Google Scholar]
- 26.Manga, P., K. Sato, L. Ye, F. Beermann, M. L. Lamoreux, and S. Orlow. 2000. Mutational analysis of the modulation of tyrosinase by tyrosinase-related proteins 1 and 2 in vitro. Pig. Cell Res. 13:364-374. [DOI] [PubMed] [Google Scholar]
- 27.Negroiu, G., R. Dwek, and S. Petrescu. 2003. The inhibition of early N-glycan processing targets TRP-2 to degradation in B16 melanoma cells. J. Biol. Chem. 278:27035-27042. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura, E. K., S. A. Jordan, H. Oshima, H. Yoshida, M. Osawa, M. Moriyama, I. J. Jackson, Y. Barrandon, Y. Miyachi, and S. Nishikawa. 2002. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416:854-860. [DOI] [PubMed] [Google Scholar]
- 29.Oetting, W., J. Fryer, S. Shriram, and R. King. 2003. Oculocutaneous albinism type 1: the last 100 years. Pig. Cell Res. 16:307-311. [DOI] [PubMed] [Google Scholar]
- 30.Orlow, S., B. Zhou, A. Chakraborty, M. Drucker, S. Pifko-Hirst, and J. Pawelek. 1994. High-molecular weight forms of tyrosinase and the tyrosinase-related proteins: evidence for a melanogenic complex. J. Investig. Dermatol. 103:196-201. [DOI] [PubMed] [Google Scholar]
- 31.Pavan, W. J., and S. M. Tilghman. 1994. Piebald lethal (sl) acts early to disrupt the development of neural crest-derived melanocytes. Proc. Natl. Acad. Sci. USA 91:7159-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pawelek, J., A. Korner, A. Bergstrom, and J. Bologna. 1980. New regulators of melanin biosynthesis and the autodestruction of melanoma cells. Nature 286:617-619. [DOI] [PubMed] [Google Scholar]
- 33.Pawelek, J., and A. Lerner. 1978. 5,6-Dihydroxyindole is a melanin precursor showing potent cytotoxicity. Nature 276:626-628. [DOI] [PubMed] [Google Scholar]
- 34.Porter, S. D., and C. J. Meyer. 1994. A distal tyrosinase upstream element stimulates gene expression in neural-crest-derived melanocytes of transgenic mice: position-independent and mosaic expression. Development 120:2103-2111. [DOI] [PubMed] [Google Scholar]
- 35.Sarangarajan, R., and R. Boissy. 2001. Tyrp1 and oculocutaneous albinism type 3. Pig. Cell Res. 14:437-444. [DOI] [PubMed] [Google Scholar]
- 36.Steel, K. P., D. C. Davidson, and I. J. Jackson. 1992. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development 115:1111-1119. [DOI] [PubMed] [Google Scholar]
- 37.Sun, Y., M. Song, S. Stevanovic, C. Jankowiak, A. Paschen, H. Rammensee, and D. Schadendorf. 2000. Identification of a new HLA-A(*)0201-restricted T-cell epitope from the tyrosinase-related protein 2 (TRP2) melanoma antigen. Int. J. Cancer 87:399-404. [DOI] [PubMed] [Google Scholar]
- 38.Tief, K., A. Schmidt, A. Aguzzi, and F. Beermann. 1996. Tyrosinase is a new marker for cell populations in the mouse neural tube. Dev. Dyn. 205:445-456. [DOI] [PubMed] [Google Scholar]
- 39.Toyofuku, K., I. Wada, J. Valencia, T. Kushimoto, V. Ferrans, and V. Hearing. 2001. Oculocutaneous albinism types 1 and 3 are ER retention diseases: mutation of tyrosinase or Tyrp1 can affect the processing of both mutant and wild-type proteins. FASEB J. 15:2149-2161. [DOI] [PubMed] [Google Scholar]
- 40.Tsukamoto, K., I. Jackson, K. Urabe, P. Montague, and V. Hearing. 1992. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed dopachrome tautomerase. EMBO J. 11:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tybulewicz, V., M. Tremblay, M. Lamarca, R. Willemsen, B. Stubblefield, S. Winfield, B. Zablocka, E. Sidransky, B. Martin, S. Huang, K. Mintzer, H. Westphal, R. Mulligan, and E. Ginns. 1992. Animal model of Gaucher's disease from targeted disruption of the mouse glucocerebrosidase gene. Nature 357:407-410. [DOI] [PubMed] [Google Scholar]
- 42.Urabe, K., P. Aroca, K. Tsukamoto, D. Mascagna, A. Palumbo, G. Prota, and V. Hearing. 1994. The inherent cytotoxicity of melanin precursors: a revision. Biochim. Biophys. Acta 1221:272-278. [DOI] [PubMed] [Google Scholar]
- 43.Zhao, S., and P. Overbeek. 1999. Tyrosinase-related protein 2 promoter targets transgene expression to ocular and neural crest-derived tissues. Dev. Biol. 216:154-163. [DOI] [PubMed] [Google Scholar]