Abstract
Synovial sarcoma (SS) tumor cells, which have the chromosomal translocation t(X;18)(p11.2;q11.2), have an inherently greater propensity for epithelial differentiation than other mesenchymal tumors, especially spindle cell sarcomas. This is caused by de-repression of the transcription of E-cadherin by SYT-SSX1 and SYT-SSX2, which dissociate Snail or Slug, respectively, from the E-cadherin promoter. However, a subset of SS with SYT-SSX1 loses E-cadherin expression despite adequate de-repression because of mutations in E-cadherin, resulting in monophasic histology. The ratio of the expression levels of SYT-SSX1 and Snail is also associated with E-cadherin expression: the lower the SYT-SSX1/Snail ratio, the lower the level of E-cadherin expression, and vice versa, thus affecting tumor histology. In addition, Wnt signal activation caused by mutation of β-catenin, APC, or Axin 1 and 2 is associated with monophasic histology. Remodeling of the extracellular matrix is also important. Only cells that survive all of these steps can finally exhibit biphasic histology. On the other hand, the SYT-SSX2 fusion has a weaker de-repression effect on the E-cadherin promoter than does SYT-SSX1, so it is difficult for SYT-SSX2-expressing tumors to achieve sufficient capacity for epithelial differentiation to form glandular structures. This review provides an interesting model for this epithelial differentiation that shows a possible mechanism for the aberrant mesenchymal to epithelial transition of SS and suggests that it might better be considered an epithelial to mesenchymal transition.
Keywords: Synovial sarcoma, chromosomal translocation t(X;18)(p11.2;q11.2), epithelial differentiation, E-cadherin, SYT-SSX1, SYT-SSX2
Introduction
Synovial sarcoma (SS) accounts for 7–10% of all soft tissue malignancies and most commonly arises in the extremities of young adults [1]. A recurrent chromosomal translocation, t(X;18)(p11.2;q11.2), fuses the SYT gene on chromosome 18 to any of three closely related genes on the X chromosome, SSX1, SSX2, or, rarely, SSX4, resulting in the formation of SYT-SSX fusion proteins [2]. SYT-SSX fusion genes can be detected in more than 95% of cases of SS, and the detection of such fusions has been established clinically as a molecular diagnostic test for this tumor; therefore, this translocation is considered the driving oncogenic event in the development of SS. SYT-SSX fusion proteins have been shown to require chromatin-remodeling factors, such as Brg/Brm [3], to achieve their transformative potential. Quite recently, SYT-SSX fusion has been shown to interact with the SWI/SNF (BAF) complex, the best-characterized of the chromatin-remodeling complexes, by dissociating BAF47 from the complex, resulting in Sox2 activation [4].
SS is a unique mesenchymal tumor that exhibits epithelial differentiation by both morphological and immunohistochemical criteria. It is divided on the basis of morphology into two major histological subtypes: the biphasic type and the monophasic fibrous type. An intriguing observation in SS is that the specific gene fusion (i.e., SYT-SSX1 vs. SYT-SSX2) correlates strongly with the tumor phenotype (monophasic vs. biphasic histology as defined by the presence of glandular epithelial differentiation with lumen formation), and almost all biphasic SS has been shown to harbor the SYT-SSX1 fusion gene [5,6]. SS with the SYT-SSX1 fusion gene is therefore considered to be capable of epithelial differentiation as defined by histological evidence of gland formation and immunohistochemical detection of epithelial-related proteins, although the mechanism for this differentiation has not been well documented. A recent study demonstrated that SYT-SSX silencing broadened the differentiation potential of SS cells to include cell types such as osteocytes, chondrocytes, and adipocytes, providing evidence that SS is a stem cell malignancy [7]. This finding is in line with the aforementioned evidence that SYT-SSX is responsible for the histologic features specific to SS. One interesting model for this epithelial differentiation that provides a possible mechanism for this aberrant mesenchymal to epithelial transition by SS (and suggests that it might better be considered an epithelial to mesenchymal transitions) [8] posits that all SS are potentially able to undergo some degree of epithelial differentiation as evidenced by the expression of epithelial differentiation-associated genes such as E-cadherin but that the majority of such tumors lose this capability as a result of other factors, including remodeling of the extracellular matrix [9,10]. In this review, the factors that contribute to this phenomenon are explained in turn.
Correlation between the type of SYT-SSX fusion and the histological subtype
Kawai et al. were the first to describe the SYT-SSX fusion gene as a determinant of the morphology and prognosis of SS [5]. Although the impact of the specific fusion type on the survival of patients with SS is controversial, several independent groups have consistently observed an association between the type of SYT-SSX fusion and histological glandular differentiation [6,11-13]. Biphasic histology occurs in 38.6% of SYT-SSX1 tumors but only 3.3% of SYT-SSX2 tumors [12]. Therefore, SS with SYT-SSX1 is considered to be more capable of epithelial differentiation.
Expression of epithelial markers in synovial sarcoma
SS may express epithelial markers such as cytokeratin and epithelial membrane antigen (EMA). Approximately 90% of all SS are cytokeratin-positive. In general, the intensity of staining is stronger in the epithelial cell component than in the spindle cell component. In monophasic fibrous SS, there may be only a few cells throughout the section positive for EMA or cytokeratin. Although this feature is almost unique to SS among the spindle cell sarcomas, several other mesenchymal tumors such as glandular malignant peripheral nerve sheath tumor (MPNST) are known to show occasional morphological epithelial differentiation. This rare variant of MPNST may be difficult to distinguish from biphasic SS because the glandular element is virtually identical, and it is principally the spindle cell component that differentiates them. Subtle degrees of epithelial differentiation may be evident in the spindle cell component of biphasic SS, whereas the epithelial element in glandular MPNST invariably arises rather abruptly from a spindle cell stroma consisting of keratin-negative cells. SS may also express intercellular adhesion molecules such as E-cadherin and catenin family members. E-cadherin and catenins are intercellular adhesion molecules located at structures called adherens junctions. The adhesion protein E-cadherin plays a central part in epithelial morphogenesis. Expression of this protein is downregulated during the acquisition of metastatic potential in the late stages of epithelial tumor progression [14,15]. During this process, epithelial tumor cells also acquire fibroblastic morphology, a phenomenon known as epithelial-mesenchymal transition (EMT) [14,15]. These intercellular adhesion proteins are expressed preferentially in the glandular component of biphasic SS and in epithelial nests composed of rather short-spindled and oval to plump cells in monophasic SS [16,17]. SS may also express tight-junction-related proteins, including ZO-1, claudin-1, and occludin SS [18]. These proteins have been shown to be expressed weakly and focally in the spindle cells of biphasic and monophasic tumors [18] as well as in the glandular components of biphasic tumors.
Approximately 30–40% of SS with the SYT-SSX1 fusion show histologically glandular epithelial differentiation [12], and strong expression of E-cadherin has been shown to correspond well to the glandular component of biphasic SS [16]. However, it is not clear how these differences arise within SS with the SYT-SSX1 fusion. One interesting observation is that mutations in the zipper structure of E-cadherin, which would be expected to disrupt its function and lead to monophasic histology, occur exclusively in a subset of SS with the SYT-SSX1 fusion [9,10].
SYT-SSX and transcription of E-cadherin
Blocking the SYT-SSX fusion has been shown to suppress the growth of SS cells, as occurs in other translocation sarcomas (Ewing’s, etc.) [7,19]. Therefore, a simple difference between the expression levels of the SYT-SSX fusion proteins was hypothesized to be responsible for the histological and biological differences between SYT-SSX1 and SYT-SSX2 tumors. However, one unpublished observation showed no difference in the SYT-SSX mRNA expression level as assessed by real-time PCR between tumors with the SYT-SSX1 and SYT-SSX2 fusions (Saito T and Ladanyi M; unpublished data). Functional differences between SYT-SSX1 and SYT-SSX2 are therefore expected to account for the morphological differences among SS with these different fusion genes. EMT is a phenomenon implicated in the differentiation of epithelial cells into mesenchymal cells in which E-cadherin expression is downregulated and the cells acquire a fibroblastic morphology. This aspect of EMT is reminiscent of the histology of SS, especially biphasic SS in which the E-cadherin-positive plump tumor cells form glandular structures on a background of spindle-shaped E-cadherin-negative proliferating tumor cells. As already mentioned above, E-cadherin expression can be seen in a subset of SS and can even be heterologous in the same tumor, as is cytokeratin expression. However, most SS, like other pleomorphic spindle cell sarcomas, have lost E-cadherin expression (10, and partially unpublished data). Some studies regarding the possible roles of Snail as a strong transcriptional repressor of E-cadherin demonstrated that Snail is strongly expressed in mesenchymal tissue [14,15]. Slug was subsequently shown to be able to repress E-cadherin expression in epithelial cells via the E-box elements in the proximal E-cadherin promoter [20,21]. The mRNA expression level of Snail does not differ between SS and other spindle cell sarcomas, such as pleomorphic sarcomas, leiomyosarcoma, and malignant peripheral nerve sheath tumors, suggesting that the SYT-SSX fusion protein affects not the expression levels but rather the functions of these EMT regulators (10, and partially unpublished data). SYT-SSX1 and SYT-SSX2 were recently demonstrated to interfere selectively with Snail and Slug, respectively, and release their repression of E-cadherin expression [8]. In this model, transcriptional activation of the E-cadherin gene by either SYT-SSX1 or SYT-SSX2 is caused by dissociation of Snail or Slug, respectively, from the E-cadherin promoter [8]. The SYT-SSX1 fusion protein interacts with Snail, which is a stronger repressor of E-cadherin than Slug, and dissociates Snail from the E-cadherin promoter, resulting in stronger de-repression of E-cadherin transcription (8: modified in Figure 1). This process also involves hyperacetylation of histones H3 and H4 induced by SYT-SSX1 dissociating Snail from the E-cadherin promoter [8]. The involvement of histone modification by SYT-SSX in the regulation of other genes has also been described [22].
Figure 1.
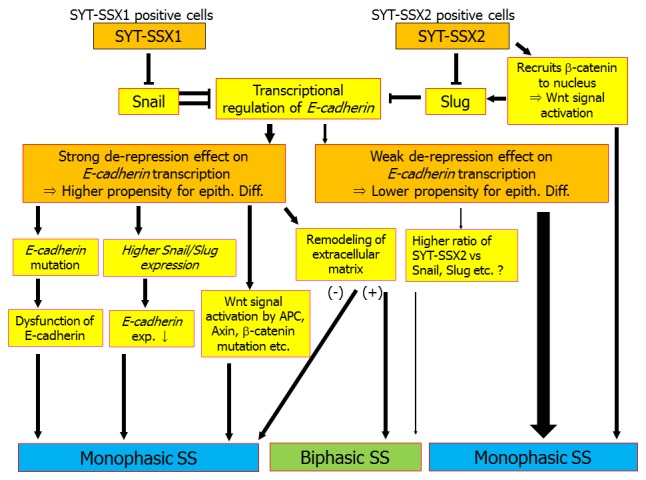
Proposed model for epithelial differentiation in synovial sarcoma. Tumor cells with the chromosomal translocation t(X;18)(p11.2;q11.2) possess an inherently higher propensity for epithelial differentiation than other mesenchymal tumors, especially spindle cell sarcomas. This is caused by dissociation of Snail or Slug from the E-cadherin promoter by SYT-SSX1 or SYT-SSX2, respectively, which relieves the repression of E-cadherin transcription. However, some SS with SYT-SSX1 lose E-cadherin expression because of mutation of E-cadherin, resulting in monophasic histology. The ratio of the expression levels of SYT-SSX1 and Snail is also associated with the expression of E-cadherin: the lower the SYT-SSX1/Snail ratio, the lower the expression of E-cadherin, thus affecting the tumor histology. In addition, Wnt signal activation caused by mutation of β-catenin, APC, or Axin1 and 2 is associated with monophasic histology. The remodeling of the extracellular matrix is also important. Only tumors that survive these steps can finally exhibit biphasic histology. On the other hand, the SYT-SSX2 fusion is a weaker de-repressor of the E-cadherin promoter than is SYT-SSX1, so it is difficult for SYT-SSX2-positive tumors to acquire enough capacity for epithelial differentiation to show glandular formation.
In addition, a recent paper demonstrated that SYT-SSX signal (produced by cRNA in situ hybridization) was more intensely localized in the epithelial components than in the spindle cell areas of biphasic SS [23]. In addition, nuclear expression of Snail is significantly lower in the glandular component [24]. These findings suggest the possibility that selective transcriptional up-regulation of E-cadherin in the glandular components of SS establishes and maintains the epithelial differentiation and morphology (Figure 2). One might reasonably ask whether SYT-SSX also de-represses other epithelial differentiation-related genes, such as claudin-1 and occludin, that have been shown to be expressed in SS [18] and contain E-box sequences similar to those of E-cadherin in their promoters [25]. This is not the case, however, suggesting that the regulation of epithelial differentiation-related genes is more complex than expected.
Figure 2.
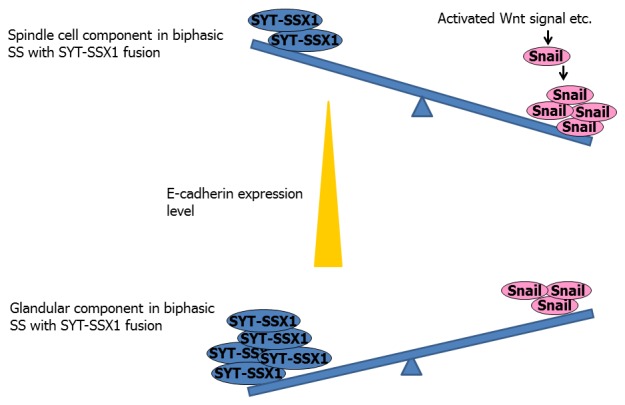
Difference of E-cadherin expression in biphasic synovial sarcoma with the SYT-SSX1 fusion. The SYT-SSX1/Snail ratio is thought to be higher in the glandular component of biphasic SS with the SYT-SSX1 fusion, causing greater de-repression of the E-cadherin promoter and leading to stronger expression of the protein. On the other hand, the SYT-SSX1/Snail ratio is thought to be lower in the spindle cell component of biphasic SS with the SYT-SSX1 fusion, resulting in weaker de-repression of the E-cadherin promoter and leading to weak or nonexistent expression of this protein. Furthermore, in the spindle cell component, the expression of Snail is more strongly up-regulated by activated Wnt signaling and thus hampers the expression of E-cadherin.
Extracellular matrix and Wnt signaling in the epithelial differentiation of SS
Matrix metalloproteinases (MMPs) are zinc proteinases responsible for the degradation of extracellular matrix macromolecules in such pathophysiological conditions as tissue remodeling and tumor invasion [26]. Expression of MMPs has been shown to be associated with tumor invasion and the patient’s prognosis [27,28]. MMP-2 expression in SS has been well described [29]: it tends to occur in biphasic SS and monophasic SS with plump cell foci but is usually absent in purely monophasic fibrous SS. In biphasic tumors, MMP-2 is more strongly expressed in the glandular than in the non-glandular component [29].
On the other hand, several cDNA microarray and tissue microarray studies have implicated the Wnt signaling pathway in a critical role in the formation of SS [30-34]. Nuclear β-catenin staining was reported in 30% to 60% of SS, primarily in monophasic tumors or in the spindle cell component of biphasic tumors, whereas the epithelial component of biphasic tumors shows membranous staining [16,35]. Activating mutations in this pathway have been sporadically reported in SS; these include mutations in adenomatous polyposis coli (APC) (8%) and β-catenin (8%), and all cases with such mutations have been shown to be monophasic SS [16,36]. Furthermore, among SS with mutations in E-cadherin that were considered to have abrogated E-cadherin expression, some tumors still exhibited an epithelioid morphology without any apparent formation of glandular structures [9]. The author noticed that all such cases of SS retained at least immunohistochemical evidence of membranous expression of one of three catenins [9,16], suggesting that catenins also play an important role in maintaining the morphology of SS tumor cells. This invites speculation that activation of the Wnt signaling pathway might be involved in the morphologic changes undergone by SS cells. Nuclear β-catenin was already known to influence growth (c-myc, cyclin D1, PPARδ), survival (MDR1, survivin), dedifferentiation (CDX-1, Id-2, ENK1), proteolysis (MMP-7, uPA-R, uPA), migration (laminin-5γ2), angiogenesis (VEGF), dissemination (CD44), and cellular detachment as a result of downregulation of E-cadherin expression [37-42]. MMP-2 is also a target of activated Wnt signaling [27,28]. However, histological discordance in the expression of nuclear β-catenin (mainly seen in spindle cell components) and MMP-2 (tends to be seen in glandular components or epithelioid foci) in SS suggests that MMP-2 is not a target of activated β-catenin/Wnt signaling in SS. However, MMP-2 surely plays an important role in the stromal remodeling that allows SS tumor cells to acquire a plump morphology or form glandular structures. The genes targeted by activated β-catenin/Wnt signaling in SS seem to differ somewhat from those reported elsewhere, including cyclin D1 [35]. Furthermore, SYT-SSX2 has been reported to recruit β-catenin to the nucleus, where the proteins form a transcriptionally active complex [43]. The β-catenin/Wnt signaling pathway is constitutively active in SYT-SSX2-positive SS regardless of the presence of the canonical Wnt signal [43]. Although the SYT-SSX1 fusion protein has not been reported to affect this phenomenon, these findings may explain why SS with the SYT-SSX2 fusion rarely show histological evidence of glandular epithelial differentiation and also explain the association between activated Wnt signaling and morphology in SS.
In conclusion, an interesting updated model for the epithelial differentiation mechanisms of SS has been presented. The aberrant mesenchymal to epithelial transition (MET) behavior of this unique mesenchymal tumor might better be thought of as EMT rather than MET [8], i.e., all SS progenitor cells with t(X;18)(p11.2;q11.2) are theoretically capable of some epithelial differentiation as evidenced by their expression of epithelial differentiation-associated genes such as E-cadherin, but the majority lose this character in response to other functional and physiological influences, including remodeling of the extracellular matrix [9,10].
Acknowledgements
This work was supported in part by a Grant-in-Aid for General Scientific Research from the Ministry of Education, Science, Sports and Culture (#23590434 to Tsuyoshi Saito), Tokyo, Japan.
Disclosure of conflict of interest
None.
References
- 1.Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. 4th edn. Philadelphia: Mosby; 2001. [Google Scholar]
- 2.Clark J, Rocques PJ, Crew AJ, Gill S, Shipley J, Chan AM, Gusterson BA, Cooper CS. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994;7:502–508. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- 3.Nagai M, Tanaka S, Tsuda M, Endo S, Kato H, Sonobe H, Minami A, Hiraga H, Nishihara H, Sawa H, Nagashima K. Analysis of transforming activity of human synovial sarcoma-associated chimeric protein SYT-SSX1 bound to chromatin remodeling factor hBRM/hSNF2 alpha. Proc Natl Acad Sci U S A. 2001;98:3843–3848. doi: 10.1073/pnas.061036798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadoch C, Crabtree GR. Reversible disruption of mSWI/SNF (BAF) complexes by the SS18-SSX oncogenic fusion in synovial sarcoma. Cell. 2013;153:71–85. doi: 10.1016/j.cell.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai A, Woodruff J, Healey JH, Brennan MF, Antonescu CR, Ladanyi M. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med. 1998;338:153–160. doi: 10.1056/NEJM199801153380303. [DOI] [PubMed] [Google Scholar]
- 6.Antonescu CR, Kawai A, Leung DH, Lonardo F, Woodruff JM, Healey JH, Ladanyi M. Strong association of SYT-SSX fusion type and morphologic epithelial differentiation in synovial sarcoma. Diagn Mol Pathol. 2000;9:1–8. doi: 10.1097/00019606-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Naka N, Takenaka S, Araki N, Miwa T, Hashimoto N, Yoshioka K, Joyama S, Hamada K, Tsukamoto Y, Tomita Y, Ueda T, Yoshikawa H, Itoh K. Synovial sarcoma is a stem cell malignancy. Stem Cells. 2010;28:1119–1131. doi: 10.1002/stem.452. [DOI] [PubMed] [Google Scholar]
- 8.Saito T, Nagai M, Ladanyi M. SYT-SSX1 and SYT-SSX2 interfere with repression of E-cadherin by Snail and Slug. A potential mechanism for aberrant mesenchymal to epithelial transition in human synovial sarcoma. Cancer Res. 2006;66:6919–6927. doi: 10.1158/0008-5472.CAN-05-3697. [DOI] [PubMed] [Google Scholar]
- 9.Saito T, Oda Y, Sugimachi K, Kawaguchi K, Tamiya S, Tanaka K, Matsuda S, Sakamoto A, Iwamoto Y, Tsuneyoshi M. E-cadherin gene mutations frequently occur in synovial sarcoma as a determinant of histological features. Am J Pathol. 2001;159:2117–2124. doi: 10.1016/s0002-9440(10)63063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito T, Oda Y, Kawaguchi K, Sugimachi K, Yamamoto H, Tateishi N, Tanaka K, Matsuda S, Iwamoto Y, Ladanyi M, Tsuneyoshi M. E-cadherin mutation and Snail overexpression as alternative mechanisms of E-cadherin inactivation in synovial sarcoma. Oncogene. 2004;23:8629–8638. doi: 10.1038/sj.onc.1207960. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Sun B, Wang J, Cai W, Zhao X, Zhang S, Hao X. Prognostic implication of SYT-SSX fusion type and clinicopathological parameters for tumor-related death, recurrence, and metastasis in synovial sarcoma. Cancer Sci. 2009;100:1018–1025. doi: 10.1111/j.1349-7006.2009.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladanyi M, Antonescu CR, Leung DH, Woodruff JM, Kawai A, Healey JH, Brennan MF, Bridge JA, Neff JR, Barr FG, Goldsmith JD, Brooks JS, Goldblum JR, Ali SZ, Shipley J, Cooper CS, Fisher C, Skytting B, Larsson O. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res. 2002;62:135–40. [PubMed] [Google Scholar]
- 13.Guillou L, Benhattar J, Bonichon F, Gallagher G, Terrier P, Stauffer E, Somerhausen Nde S, Michels JJ, Jundt G, Vince DR, Taylor S, Genevay M, Collin F, Trassard M, Coindre JM. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J. Clin. Oncol. 2004;22:4040–4050. doi: 10.1200/JCO.2004.11.093. [DOI] [PubMed] [Google Scholar]
- 14.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transition by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 15.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 16.Saito T, Oda Y, Sakamoto A, Tamiya S, Kinukawa N, Hayashi K, Iwamoto Y, Tsuneyoshi M. Prognostic value of the preserved expression of the E-cadherin and catenin families of adhesion molecules and of β-catenin mutation in synovial sarcoma. J Pathol. 2000;192:342–350. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH705>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa T, Yokoyama R, Matsuno Y, Shimoda T, Hirohashi S. Prognostic significance of histologic grade and nuclear expression of beta-catenin in synovial sarcoma. Human Pathol. 2001;32:257–263. doi: 10.1053/hupa.2001.22764. [DOI] [PubMed] [Google Scholar]
- 18.Billings SD, Walsh SV, Fisher C, Nusrat A, Weiss SW, Folpe AL. Aberrant expression of tight junction-related proteins ZO-1, claudin-1 and occluding in synovial sarcoma: an immunohistochemical study with ultrastructural correlation. Mod Pathol. 2004;17:141–149. doi: 10.1038/modpathol.3800042. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Skytting B, Nilsson G, Gasbarri A, Haslam K, Bartolazzi A, Brodin B, Mandahl N, Larsson O. SYT-SSX is critical for cyclin D1 expression in synovial sarcoma cells: a gain of function of the t(X;18)(p11.2;q11.2) translocation. Cancer Res. 2002;62:3861–3867. [PubMed] [Google Scholar]
- 20.Bolós V, Hector Peinado H, Pérez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- 21.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- 22.Lubieniecka JM, de Bruijn DR, Su L, van Dijk AH, Subramanian S, van de Rijn M, Poulin N, van Kessel AG, Nielsen TO. Histone deacetylase inhibitors reverse SS18-SSX-mediated polycomb silencing of the tumor suppressor early growth response 1 in synovial sarcoma. Cancer Res. 2008;68:4303–4310. doi: 10.1158/0008-5472.CAN-08-0092. [DOI] [PubMed] [Google Scholar]
- 23.Kanemitsu S, Hisaoka M, Shimajiri S, Matsuyama A, Hashimoto H. Molecular detection of SS18-SSX fusion gene transcripts by cRNA in situ hybridization in synovial sarcoma using formalin-fixed, paraffin-embedded tumor tissue specimens. Diagn Mol Pathol. 2007;16:9–17. doi: 10.1097/PDM.0b013e318031f02f. [DOI] [PubMed] [Google Scholar]
- 24.Subramaniam MM, Navarro S, Llombart-Bosch A. Immunohistochemical study of correlation between histologic subtype and expression of epithelial-mesenchymal transition-related proteins in synovial sarcomas. Arch Pathol Lab Med. 2011;135:1001–1009. doi: 10.5858/2010-0071-OAR1. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Estrada OM, Cullerés A, Soriano FX, Peinado H, Bolós V, Martínez FO, Reina M, Cano A, Fabre M, Vilaró S. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2006;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsons SL, Watson SA, Brown PD, Collins HM, Steele RJ. Matrix metalloproteinases. Br J Surg. 1997;84:160–166. [PubMed] [Google Scholar]
- 27.Yang Y, Jiao L, Hou J, Xu C, Wang L, Yu Y, Li Y, Yang C, Wang X, Sun Y. Dishevelled-2 silencing reduces androgen-dependent prostate tumor cell proliferation and migration and expression of Wnt-3a and matrix metalloproteinases. Mol Biol Rep. 2013;40:4241–50. doi: 10.1007/s11033-013-2506-6. [DOI] [PubMed] [Google Scholar]
- 28.Kamino M, Kishida M, Kibe T, Ikoma K, Iijima M, Hirano H, Tokudome M, Chen L, Koriyama C, Yamada K, Arita K, Kishida S. Wnt-5a signaling is correlated with infiltrative activity in human glioma by inducing cellular migration and MMP-2. Cancer Sci. 2011;102:540–548. doi: 10.1111/j.1349-7006.2010.01815.x. [DOI] [PubMed] [Google Scholar]
- 29.Saito T, Oda Y, Sakamoto A, Tamiya S, Iwamoto Y, Tsuneyoshi M. Matrix metalloproteinase-2 expression correlates with morphological and immunohistochemical epithelial characteristics in synovial sarcoma. Histopathology. 2002;40:279–285. doi: 10.1046/j.1365-2559.2002.01345.x. [DOI] [PubMed] [Google Scholar]
- 30.Nagayama S, Katagiri T, Tsunoda T, Hosaka T, Nakashima Y, Araki N, Kusuzaki K, Nakayama T, Tsuboyama T, Nakamura T, Imamura M, Nakamura Y, Toguchida J. Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res. 2002;62:5859–5866. [PubMed] [Google Scholar]
- 31.Segal NH, Pavlidis P, Antonescu CR, Maki RG, Noble WS, DeSantis D, Woodruff JM, Lewis JJ, Brennan MF, Houghton AN, Cordon-Cardo C. Classification and subtype prediction of adult soft tissue sarcoma by functional genomics. Am J Pathol. 2003;163:691–700. doi: 10.1016/S0002-9440(10)63696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baird K, Davis S, Antonescu CR, Harper UL, Walker RL, Chen Y, Glatfelter AA, Duray PH, Meltzer PS. Gene expression profiling of human sarcomas: insight into sarcoma biology. Cancer Res. 2005;65:9226–9235. doi: 10.1158/0008-5472.CAN-05-1699. [DOI] [PubMed] [Google Scholar]
- 33.Ng TL, Gown AM, Barry TS, Cheang MC, Chan AK, Turbin DA, Hsu FD, West RB, Nielsen TO. Nuclear beta-catenin in mesenchymal tumors. Mod Pathol. 2005;18:68–74. doi: 10.1038/modpathol.3800272. [DOI] [PubMed] [Google Scholar]
- 34.Saito T, Nagai M, Tsuda M, Ladanyi M. Differentially expressed genes defined by expression profiling of sarcomas with chimeric transcription factors provide an enriched source of candidate transcriptional targets. Abstracts of LXX Cold Spring Harbor Symposium. 2005:225. [Google Scholar]
- 35.Saito T, Oda Y, Yamamoto H, Kawaguchi K, Tanaka K, Matsuda S, Iwamoto Y, Tsuneyoshi M. Nuclear β-catenin correlates with cyclin D1 expression in spindle and pleomorphic sarcomas but not in synovial sarcoma. Hum Pathol. 2006;37:689–697. doi: 10.1016/j.humpath.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Saito T, Oda Y, Sakamoto A, Kawaguchi K, Tanaka K, Matsuda S, Tamiya S, Iwamoto Y, Tsuneyoshi M. APC mutations in synovial sarcoma. J Pathol. 2002;196:445–449. doi: 10.1002/path.1066. [DOI] [PubMed] [Google Scholar]
- 37.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. β-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumor. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 39.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-myc as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 40.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 41.Hiendlmeyer E, Regus S, Wassermann S, Hlubek F, Haynl A, Dimmler A, Koch C, Knoll C, van Beest M, Reuning U, Brabletz T, Kirchner T, Jung A. β-catenin up-regulates the expression of the urokinase plasminogen activator in human colorectal tumors. Cancer Res. 2004;64:1209–1214. doi: 10.1158/0008-5472.can-3627-2. [DOI] [PubMed] [Google Scholar]
- 42.Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of β-catenin signaling, slug, and MAPK. J Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pretto D, Barco R, Rivera J, Neel N, Gustavson MD, Eid JE. The synovial sarcoma translocation protein SYT-SSX2 recruits β-catenin to the nucleus and associates with it in an active complex. Oncogene. 2006;25:3661–3669. doi: 10.1038/sj.onc.1209413. [DOI] [PubMed] [Google Scholar]


