Abstract
To study the effect of porcine acellular dermal matrix (ADM) on the burn wound healing. Seventy healthy Wistar rats were inflicted with 2 cm second degree burn and divided into 2 groups; one group was treated with porcine ADM and the other with Povidone Iodine Cream. Biopsies were taken on day 1, 3, 5, 7, 10, 14, 21 for histopathological and biochemical analysis to test PCNA, K19, Integrin-β1, PDGF, EGF and FGF. The results revealed relatively better and faster regeneration after treatment of porcine ADM, along with greatly increased synthesis in collagen in the experimental group. PCNA, K19, Integrin-β1 had an increase and then tapered down, and were stronger in the experimental group than in the contrast group during 21 days after burns. PDGF, EGF and FGF levels increased on day 3, peaked on day 5 and then started to decrease, while significantly enhanced expression of relevant growth factors were observed in the experimental group. Porcine ADM stimulate collagen synthesis, stem cells proliferation and differentiation, and the expression of relevant growth factors and ultimately improve the burn wound healing.
Keywords: Porcine ADM, deep partial thickness burn wounds, PCNA, K19, Integrin-β1, PDGF
Introduction
As we all know, intact skin performs a wide range of protective, perceptive, and regulatory functions. Therefore, severe skin loss due to deep partial thickness burn wounds will initiate a cascade of events like infection, edema, excessive inflammatory response and so on, which further accelerate the tissue injury and necrosis [1]. These adverse factors not only further worsen skin tissue, but also delay wound repair. Naturally, some factors can provide a possibility for wound healing [2-4]. These active factors include collagen organization, keratinocyte stem cells and some bioactive molecules which participate in the whole wound-healing process with three stages: inflammation, proliferation and remodeling period [5,6]. Thus, for serious skin defect due to the deep partial thickness burn wounds, we hope to find an ideal coverage which not only prevent the first barrier from infection, but also increase the total amount of active factors [7].
Porcine ADM, as a wound regimen has offered confidence in the treatment of deep dermal burns in the past decades. Actually, porcine ADM, as a porcine-derived tissue engineered skin, is a dermal biomaterial in which all of the cellular elements have been removed. The biologic properties of porcine ADM, because of its abilities to support tissue regeneration, repopulation with fibroblasts, revascularization, new collagen deposition and eventual absorption and replacement with native tissue, it has been widely used in tissue reconstruction. In 2001, Anil Srivastava et al [8] have documented that porcine ADM is useful as a dermal substitute in full-thickness skin defects. In 2005, Ma ZF et al [9] have also found that porcine ADM has the ability of curing full-thickness skin defects in animal experiments and enhance the quality of wound repair. On the other hand, some scholars have documented that porcine ADM bears a strong similarity to human ADM, and might be a substitute for human ADM [10]. During the past 10 years, We found porcine ADM can produces beneficial effects on the burn wound healing and summarized some correlative characters and clinical experience [11,12], which were in agreement with those demonstrated previously. Although these suggested that porcine ADM accelerates wound repair and regeneration, the precise pathological mechanisms of porcine ADM in molecular and cellular levels still remain largely unknown. There is only limited information about the inherent relationship between porcine ADM and these active factors for healing, for instance, collagen, keratinocyte stem cells, growth factors and so on.
In this article, the wound-healing process was analyzed according to three different aspects: wound-appearance changes, wound tissue pathological changes (extracellular matrix such as collagen content) and the expression of healing-promoting factors (PCNA, K19, Integrin-β1, PDGF, EGF, FGF). We hypothesized porcine ADM produce active effect on the three ones to promote wound closure. The purpose of this study is to explore the pertinent effect of porcine acellular dermal matrix (ADM) in the burn wound healing.
Materials and methods
Model building
Seventy healthy Wistar rats were utilized in this study. These rats weighing between 220 and 270 g were obtained from the Animal Center of the Sun Yat-Sen University, Guangzhou. Rats were fed a standard rat chow and tap water ad libitum. In the windowless animal quarter automatic temperature (22 ± 2°C) and lighting controls was performed. Humidity ranged from 50 to 55%. All animals received human care according to the criteria outlined in the ‘‘Guide for the Care and Use of Laboratory Animals’’ prepared by the National Academy of Sciences and published by the National Institutes of Health. We followed previously reported model of thermal injury [13,14]. After anesthesia the dorsum was shaved. A second degree burn wound of a surface 2 cm in diameter was created by using an aluminum branding iron. Aluminum branding irons were heated in a water bath to 100°C for 30 min. It was then placed without pressure for 30 s on the back of the rats. All the burned animals were then resuscitated with physiological saline solution (10 ml/kg, subcutaneously). The seventy healthy Wistar albino rats were randomly divided into 2 groups; one group was treated with porcine ADM and the other with Povidone Iodine Cream. All animals were anesthetized by intraperitoneally administration of 90 mg/kg ketamine and 10 mg/kg xylazine. Biopsies were taken on day 1, 3, 5, 7, 10, 14, 21 for histopathological and biochemical analysis for PCNA, K19, Integrin-β1, PDGF, EGF, FGF.
Histopathologic staining
The skin specimens were embedded in the paraffin blocks after they had been fixed in Bouin’s solution. Sections of 5 μm were obtained, deparaffinized and stained with Masson. Masson stain was used as a qualitative measure of the extent of collagen deposition. The skin tissue was examined and evaluated in random order under blindfold conditions with standard light microscopy.
Immunohistochemistry analysis
The harvested skin tissues were fixed in Bouin’s, embedded in paraffin and sectioned at 5 μm thickness. Immunohistochemistry was carried out by using the DAB kit (DAKO ChemMate, DK) according to the manufacturer’s recommendations. The procedure involved the following steps: The following primary antibodies for immunohistochemical staining were used: rabbit monoclonal antibodies against PCNA, Integrin-β1, K19, FGF, PDGF, EGF (BEIJING BOISYNTHESIS BIOTECHNOLOGY CO., LTD, CHN). The sections were then incubated with each primary antibody as above in a moist chamber for 1 hour at room temperature, and washed three times in Phosphate Buffered Saline (PBS). The Goat anti-rabbit IgG biotinylated secondary antibodies (BOSTER, CHN) were applied. As negative controls, slides were incubated with PBS instead of primary antibodies.
The intensity of immunostaining for PCNA, Integrin-β1, K19, FGF, PDGF, EGF on images from two groups was quantitatively analyzed. The images obtained were analyzed for positive staining at a magnification of ×40, ×100 and ×400 using the quantitative immunohistochemical analysis software Image Pro Plus (Media Cybernetics, Baltimore, MD). Microscope was purchased from Olympus (Olympus, JPN). The total area of each analyzed tissue was approximately the same. We evaluated the average optical density (AOD) [15]. The numerical values obtained from the seven different regions in each tissue were then averaged to represent the specified marker in a given tissue.
Western blotting analysis of PCNA, Integrin-β1 and K19
The harvested skin tissues homogenates were separated in polyacrylamide-SDS gel and electroblotted onto a nitrocellulose membrane (BIO-RAD). After blocking with TBS/10% nonfat dry milk, the membrane was incubated with the following antigens: anti-PCNA (1:1000, rabbit, polyclonal) (BEIJING BOISYNTHESIS BIOTECHNOLOGY CO, LTD, CHN), anti-Integrin-β1 (1:1500, rabbit, polyclonal) (BEIJING BOISYNTHESIS BIOTECHNOLOGY CO., LTD, CHN), anti-K19 (1:1000, rabbit, polyclonal) (BEIJING BOISYNTHESIS BIOTECHNOLOGY CO., LTD, CHN), followed by incubation with a horseradish peroxidase (HRP)–conjugated secondary antibody. The signal from immunoblotting bands was captured (G:BoxiChemi camera; MSHOT, CHN) and quantified using the software GIS1000. Quantitative western blot measurements of target protein were normalized by corresponding measures of GAPDH derived from the same samples in each blot.
Statistical analysis
All statistical analyses were carried out using SPSS statistical software. All data were presented in mean (±) standard error (SEM). Dual comparisons between groups exhibiting significant values were evaluated with a t test. These differences were considered significant when probability was less than 0.05.
Results
The vivo observation of wound healing
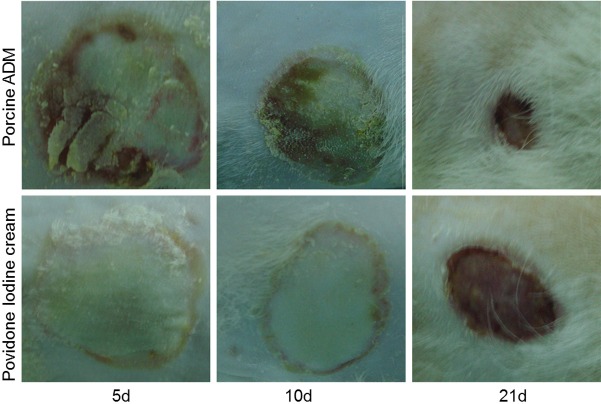
The pictures showed the representative animals treated respectively by porcine ADM and Povidone Iodine Cream. There appeared a significantly improved healing and decreased wound areas on the wounds treated by porcine ADM compared with another group (Figure 1).
Figure 1.
Serial representative changes in appearances of the wounds treated with porcine ADM and Povidone Iodine Cream on postburn day 5, 10 and 21.
Histopathological changes
The result shows representative photomicrograph of skin sections stained with masson from two groups. The collagen deposition was obviously observed on the 7th day. The wound repair process in experimental groups was better than the control group (Figure 2).
Figure 2.
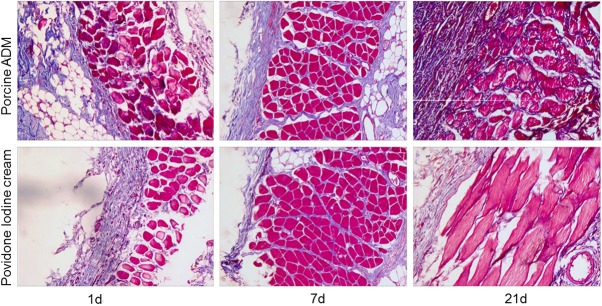
Masson staining of skin sections from two groups: Masson trichrome stain highlights collagen content (blue staining). The pictures show collagen fiber pattern is thicker in the experimental groups than in the contrast groups. All images were acquired under the same condition and displayed at the same scale. (Original magnification ×100).
Effect of porcine ADM on the localization and expression of PCNA
The expression of PCNA was first detected in both groups on the first postburn day. The expression of PCNA increased on the 3th day in burn. And it was the highest on the 5th day, and down-regulated in the following days. The expression of PCNA was detected in hair follicle cell nucleus and skin basal cell nucleus (Figure 3). PCNA positivity was stronger in the experimental group than in the control group on 1, 3, 5, 7, 10, 14, 21 days after burn (Figure 4). Results were confirmed in two independent experiments with immunohistochemistry analysis and western blot analysis.
Figure 3.
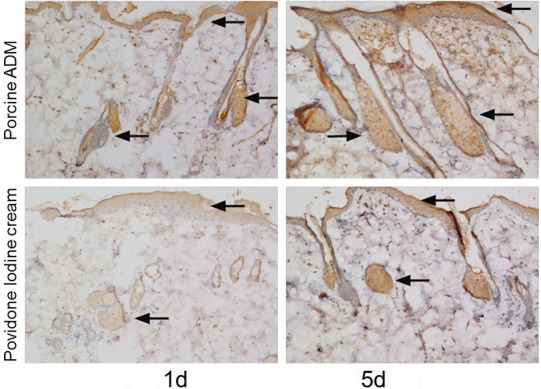
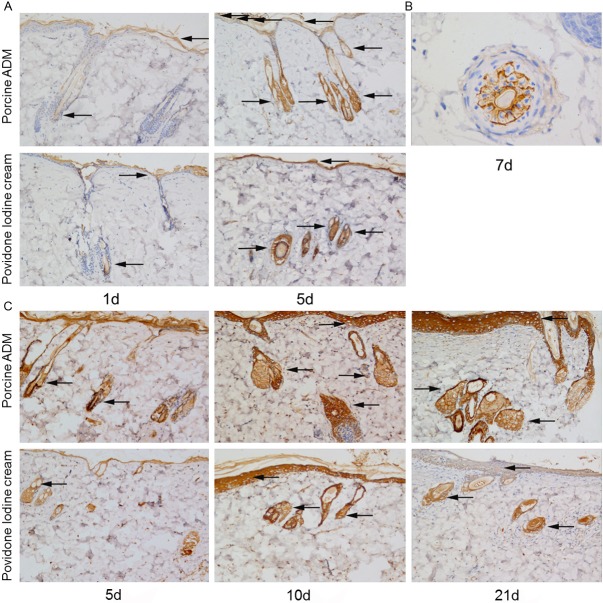
Immunohistochemistry analysis of PCNA in skin sections from two groups. Representative immunohistochemical staining images on postburn day 1 and 5. The expression of PCNA was detected in hair follicle cell nucleus and skin basal cell nucleus (brown staining, arrow). PCNA positivity was stronger in the wound tissue treated by porcine ADM than by Povidone Iodine Cream. (Original magnification ×100).
Figure 4.
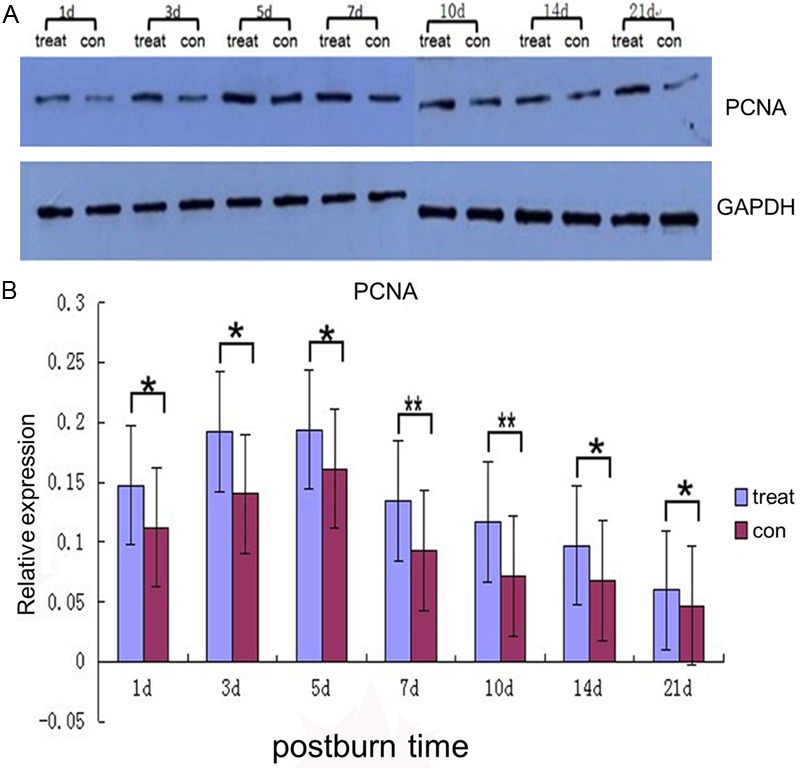
Western blot analysis for PCNA. A: Representative chemiluminescence images of blotted membranes containing protein extracts of two groups. B: Quantitative analysis of PCNA levels. Graphs depict mean PCNA density differences ± SEM of the experimental group compared to the contrast group. Star color indicates statistical significance of difference between two groups (*P < 0.05, **P < 0.01).
Effect of porcine ADM on the localization and expression of Integrin-β1 and k19
For observing keratinocyte stem cells, K19 and integrin-β1 were chosen to test by immunohistochemistry and western blot. The expression of integrin-β1 and K19 were detected on the first day (Figure 5A, 5C). The expression achieved a peak on the 5th day (Figure 5A, 5C), then declined in the following days. The expression of integrin-β1 was detected in hair follicle cells, gland cells and skin basal cells (Figure 5A, 5B). The expression of K19 was detected in hair follicle cells, gland cells and skin basal cells (Figure 5C). Meanwhile they were increased in the experimental group than in the control group during 21 days after burns (Figure 6).
Figure 5.
Immunohistochemical staining of integrin-β1 and K19 in skin sections from two groups. A: Representative images of integrin-β1 on postburn day 1 and 5. B: Representative images of integrin-β1 on the 7th day in burn. C: Representative images of K19 on postburn day 1, 5 and 21. The expression of integrin-β1 and K19 was detected in hair follicle cells, gland cells and skin basal cells (brown staining, arrow). Integrin-β1 and K19 positivity in the epithelial cells was stronger in porcine ADM-treated wounds than in Povidone Iodine Cream-treated wounds. (Original magnification ×100 except (B), which is ×400).
Figure 6.
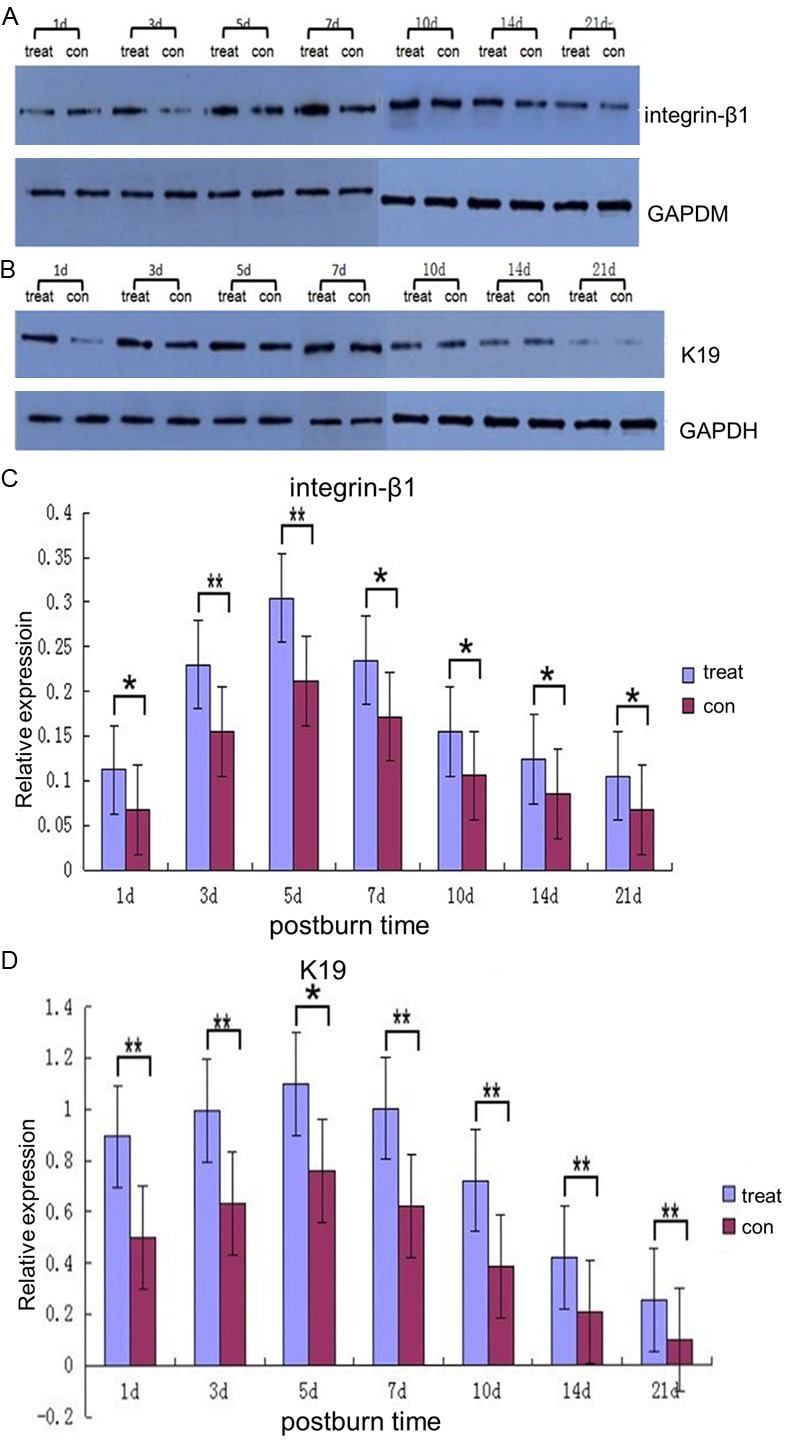
Western blot analysis for integrin-β1 and K19. A, B: Representative chemiluminescence images of blotted membranes containing protein extracts of two groups. C, D: Quantitative analysis of integrin-β1 and K19 levels. Graphs depict mean K19 and integrin-β1 density differences ± SEM of the experimental group compared to the contrast group. Star color indicates statistical significance of difference between two groups (*P < 0.05, **P < 0.01).
Effect of porcine ADM on the expression of PDGF, EGF and FGF
For observing the relationship between porcine ADM and growth factors during wound repair, three key growth factors, PDGF, EGF and FGF were studied. We detected the expression of them by immunohistochemistry and semi-quantitative analysis by measuring the average optical density (AOD). The representative pictures were offered (Figures 7, 8 and 9). The result indicated the target factors were secreted within 21 days post-burn, the peak occurred on the fifth day. In addition, significantly enhanced expression of relative target proteins were observed in the experimental group compared with in the control group for 21 days (Figure 10).
Figure 7.
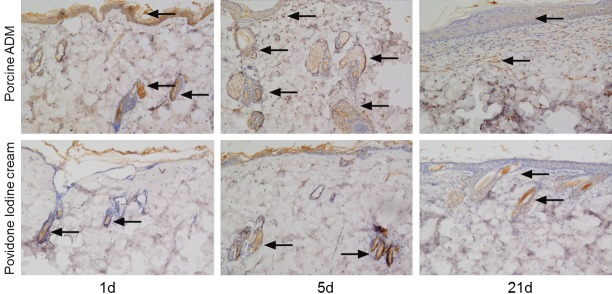
Immunohistochemical staining of PDGF in skin sections from two groups. Representative images of PDGF on 1, 5 and 21 days after burn. The expression of PDGF was stronger in wounds treated by porcine ADM treated wounds than by Povidone Iodine Cream treated. (Brown staining, arrow) (Original magnification ×100).
Figure 8.
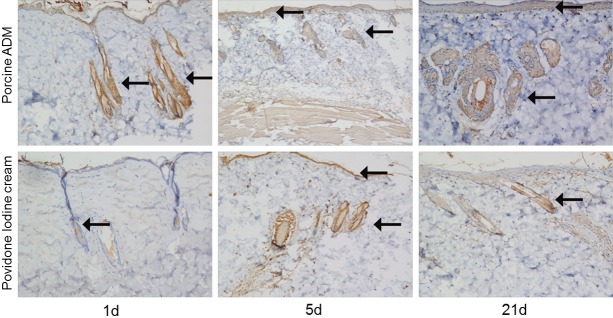
Immunohistochemical staining of EGF in skin sections from two groups. Representative images of EGF on 1, 5 and 21 days after burn. The expression of EGF was stronger in the experimental group than in the control one. (Brown staining, arrow) (Original magnification ×100).
Figure 9.
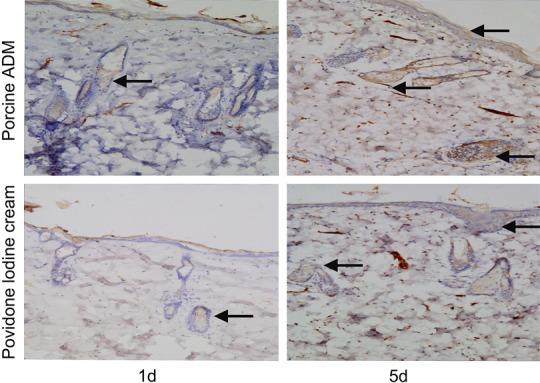
Immunohistochemical staining of FGF in skin sections from two groups. Representative images of FGF on postburn day 1 and 5. The expression of FGF was stronger in porcine ADM-treated wounds than in Povidone Iodine Cream-treated wounds. (Original magnification ×100).
Figure 10.
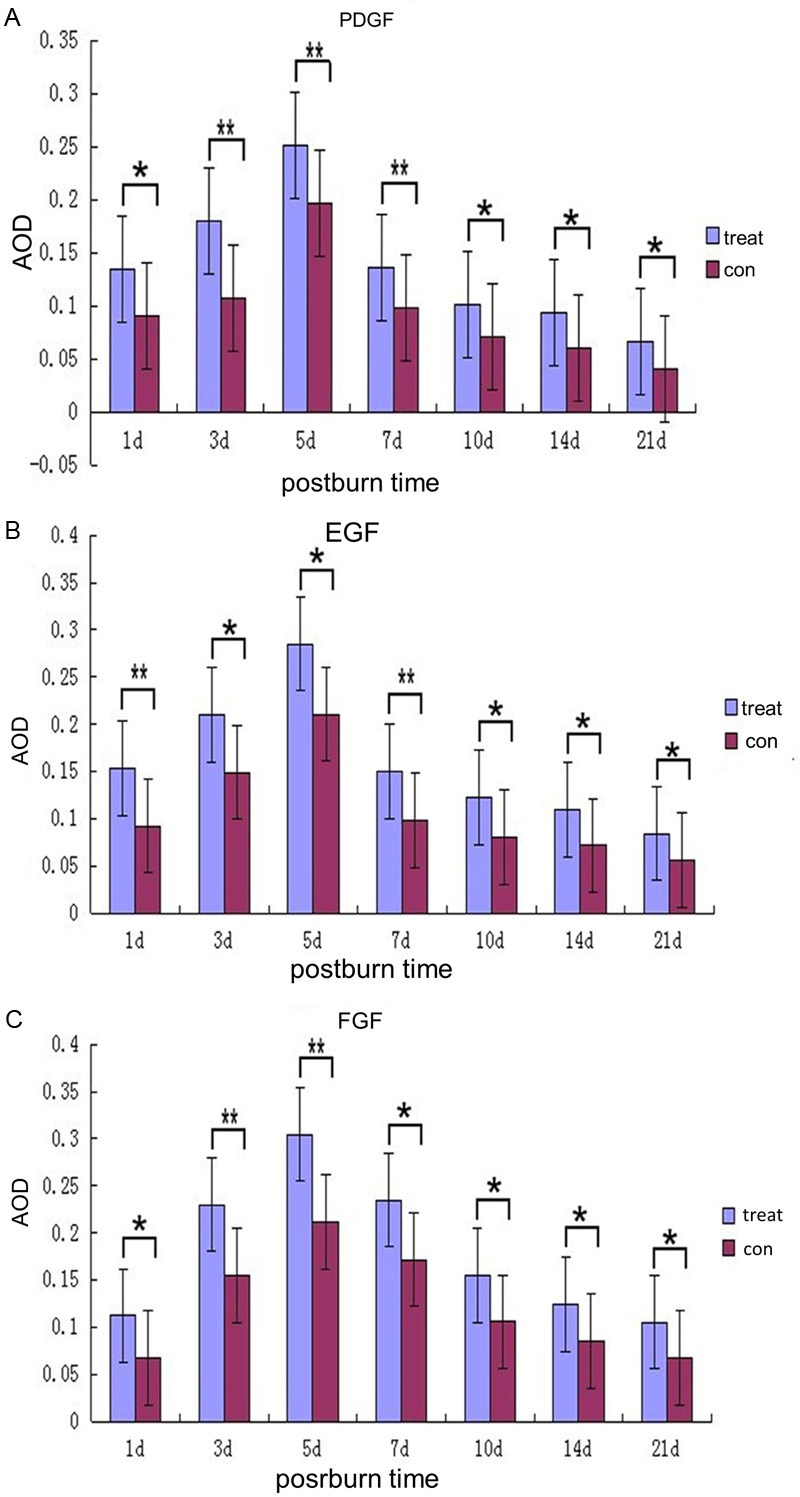
Semi-quantitative analysis of PDGF, EGF and FGF levels by measuring the average optical density (AOD). Graphs depict mean PDGF, EGF and FGF density differences ± SEM of the experimental group compared to the control group. Star color indicates statistical significance of difference between two groups (*P < 0.05, **P < 0.01).
Discussion
Wound healing is a complicated biological process. The deep partial thickness burn wound repair involves steps that include inflammation around the site of injury, angiogenesis and the development of granulation tissue, repair of the connective tissue and epithelium, and ultimately remodeling that leads to a healed wound. Among these steps, connective tissue plays a pivotal role. Collagen, produced by fibroblasts, is a principal component of connective tissues, which provides a structural framework, strength and milieu for the regenerating tissue of the wound [16]. We detected that a greatly increased collagen deposit in the experimental group compared with the control one. It indicated that the treatment of the burn wound with porcine ADM results in a significantly increased collagen synthesis, which provides a possibility for shortening the duration of wound healing and enhancing the quality of wound regeneration.
Re-epithelialization is necessary in the repair of deep partial thickness burn wounds, which is a process of restoring the epidermis. During the process, keratinocytes proliferate and migrate from the wound edge to resurface epidermal defects. Thus, proliferating keratinocytes are a source that ensures an adequate supply of cells to migrate and cover the wound [17]. For evaluating the healing quality by the proliferative aspect, we determined the expression levels of proliferating cell nuclear antigen (PCNA) by immunohistochemistry and western blot [18,19]. Immunohistochemistry analysis revealed the localization of PCNA was observed within the hair follicle structures indicating the origin of epithelial cells and skin basal cell nucleus, and a faster rate of re-epidermis growth, a greater thickness of the cuticular layer in porcine ADM treated wounds at 5 days after burns. Meanwhile, western blot results showed that the expression levels of PCNA in the group treated with porcine ADM were much higher than that in the control group. These results indicated re-epithelialization was significantly increased in the experimental group in comparison with the contrast group, which suggested that porcine ADM could be effective in the improvement of the wound-healing quality by promoting re-epithelialization.
Keratinocyte stem cells (KSC) residing in skin appendages are regard as proliferation units, can self-renew and are responsible for generating the various lineages present in the mature tissue [20-26]. Ito et al have found keratinocyte stem cells constitute almost solely to final re-epithelialization in deep dermal wounds [27]. From the embryologic viewpoint, several literatures have also documented KSCs assist in development, regeneration and repair of skin and skin appendages [28,29]. Current studies have identified three populations of KSCs in epidermis. These include the interfollicular (IF) epidermal stem cells in the epidermal basal layer, the hair follicle (HF) stem cells of the bulge and the sebaceous gland (SG) stem cells located immediately above the hair bulge as well. For observing KSCs and the relationship of porcine ADM and these stem cells during the burn wound healing, integrin-β1 and keratin (K)19 were employed to identify KSCs by immunohistochemical staining and western blotting. K19 and Integrin-β1 are informative in defining the stem cell phenotype and have been used as potential markers [30-32]. The result indicated the expression of integrin-β1 and K19 were detected in hair follicle cells, gland cells and skin basal cells. Western blotting analysis designated that Integrin-β1 and K19 immunoreactivities were first detected within 24 h, peaked on day 5 and then started to decrease until complete healing. Meanwhile, they are stronger in the experimental group than in the contrast group during 21 days after burn. The above results indicated thermal trauma access to closure depending on KSCs, which are in agreement with those demonstrated previously [27-29], and KSCs abundantly proliferate around porcine ADM-treated wound compared with the control. Thus, we concluded porcine ADM was effective in promoting KSCs to participate in burn wound repair.
Some bioactive molecules, such as growth factors, carry a big weight in the wound remodeling process [33]. Among them, PDGF, EGF, FGF are well known as a significant and active factor for wound healing process. Rollman et al [34] have documented that PDGF exerts autocrine, mitogenic effect on keratinocytes. This indicates that overexpression of PDGF in keratinocytes could support epidermal proliferation and stabilization of the dermoepidermal junction during wound closure. In addition, it stimulates vessel maturation by recruitment and differentiation of pericytes to the immature endothelial channels [35]. Besides the above, the epidermal growth factor (EGF) is also known as a key promoter associated with keratinocyte proliferation and differentiation at wound site to accelerate wound healing, even involved in skin appendages regeneration [36-40]. Meanwhile, several studies have also indicated that EGF might carry a big weight in inducing the development and regeneration of sweat glands, which is still an unsolved issue after deep burns [41,42]. Although the ideal condition, the deep burns heal well with satisfactory functional restoration, continues to be a major one in the treatment of burns, the mentioned above suggests that the overexpression of EGF can be effective in settlement. On the other hand, FGF has been documented to be clinical benefit due to burn wounds markedly rapid closure [43,44]. Actually, the basic fibroblast growth factor (FGF) is a member of the fibroblast growth factors family, which consists of at least 23 homologous peptides and is present in the basal membrane and the subendothelial extracellular matrix of blood vessels. Several literatures present it stimulates the proliferation of fibroblast and capillary endothelial cells and keratinocyte division in vitro and collagen synthesis and granulation tissue formation and epidermal regeneration in vivo, and further hypothesis on the therapeutic potential of FGF in healing wounds, curing bone damage, grafting vasculature and regenerating lens [43,45,46]. Moreover, a clinical trial that encompassed 600 patients from 32 hospitals across China investigated the efficacy of topical FGF application in enhancing the healing of partial-thickness second-degree burns [47]. In our study, we employed immunohistochemical staining to explore the relationship between porcine ADM with the three key growth factors. The result displayed that secretion of target factors were observed within 21 postburn-days, the peak occurred on the day 5, which are agreement with the result of PCNA, K19 and Integrin-β1 by immunohistochemistry and western blot. In addition, significantly enhanced expression of relative target proteins were observed in the experimental group compared with in the contrast group for 21 days. According to it, we have the reason to believe that porcine ADM not only provides a protective barrier but also clinically irritate the overexpression of growth factors, especially PDGF, EGF, FGF, to assist in burn wound repair.
In summary, our results put forward new documents about superiority of the treatment of deep partial thickness burn wounds with porcine ADM. Porcine ADM involve in and accelerate the process of burn wound repair by stimulating collagen synthesis, stem cells proliferation and differentiation, and the expression of relevant growth factors.
Acknowledgements
This paper was supported by the National Natural Science Foundation of China (Grant No. 81071557 and No. 81372062), Program of National Clinical Specialties, the Medical Research Project of Science and Technology Bureau of Foshan City (Grant No. 201008084) and the Project from the Department of health of Guangdong of Guangdong Province (Grant No. A2012638) and should be attributed to department of burns in the First Affiliated Hospital of Sun Yat-Sen University.
References
- 1.Kao CC, Garner WL. Acute burns. Plast Reconstr Surg. 2000;101:2482–2493. [PubMed] [Google Scholar]
- 2.Chung AS, Kao WJ. Fibroblasts regulate monocyte response to ECM-derived matrix: the effects on monocyte adhesion and the production of inflammatory, matrix remodeling, and growth factor proteins. J Biomed Mater Res A. 2009;89:841–853. doi: 10.1002/jbm.a.32431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong F, Cheng X, Wang S, Zhao Y, Gao Y, Cai H. Heparin-immobilized polymers as non-inflammatory and non-thrombogenic coating materials for arsenic trioxide eluting stents. Acta Biomater. 2010;6:534–546. doi: 10.1016/j.actbio.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Liang Y, Liu W, Han B, Yang C, Ma Q, Zhao W, Rong M, Li H. Fabrication and characters of a corneal endothelial cells scaffold based on chitosan. J Mater Sci Mater Med. 2011;22:175–183. doi: 10.1007/s10856-010-4190-6. [DOI] [PubMed] [Google Scholar]
- 5.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 6.Menke MN, Menke NB, Boardman CH, Diegelmann RF. Biologic therapeutics and molecular profiling to optimize wound healing. Gynecol Oncol. 2008;111:S87–91. doi: 10.1016/j.ygyno.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava A, De Sagun EZ, Jennings LJ, Sethi S, Phuangsab A, Hanumadass M, Reyes HM, Walter RJ. Use of porcine acellular derm matrix as a dermal substitute in rats. Ann Surg. 2001;233:400–408. doi: 10.1097/00000658-200103000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma ZF, Chai JK, Yang HM, Liu Q, Xu MH, Yin HN. Acellular porcine dermal matrix produced with different methods and an experimental study on its transplantation to skin wound. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2005;17:92–94. [PubMed] [Google Scholar]
- 10.Ge L, Zheng S, Wei H. Comparison of histological structure and biocompatibility between human acellular dermal matrix (ADM) and porcine ADM. Burns. 2009;35:46–50. doi: 10.1016/j.burns.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Feng X, Shen R, Tan J, Chen X, Pan Y, Ruan S, Zhang F, Lin Z, Zeng Y, Wang X, Lin Y, Wu Q. The study of inhibiting systematic inflammatory response syndrome by applying xenogenic (porcine) acellular dermal matrix on second-degree burns. Burns. 2007;33:477–479. doi: 10.1016/j.burns.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Feng X, Tan J, Pan Y, Wu Q, Ruan S, Shen R, Chen X, Du Y. Control of hypertrophic scar from inception by using xenogenic (porcine) acellular dermal matrix (ADM) to cover deep second degree burn. Burns. 2006;32:293–298. doi: 10.1016/j.burns.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Gurel A, Armutcu F, Hosnuter M, Unalacak M, Kargi E, Altinyazar C. Caffeic acid phenethyl ester improves oxidative organ damage in rat model of thermal trauma. Physiol Res. 2004;53:675–682. [PubMed] [Google Scholar]
- 14.Zhu H, Wei X, Bian K, Murad F. Effects of nitric oxide on skin burn wound healing. J Burn Care Res. 2008;29:804–814. doi: 10.1097/BCR.0b013e3181848119. [DOI] [PubMed] [Google Scholar]
- 15.Burke RE, Cadet JL, Kent JD, Karanas AL, Jackson-Lewis V. An assessment of the validity of densitometric measures of striatal tyrosine hydroxylase-positive fibers: relationship to apomorphine-induced rotations in 6-hydroxydopamine lesioned rats. J Neurosci Methods. 1990;35:63–73. doi: 10.1016/0165-0270(90)90095-w. [DOI] [PubMed] [Google Scholar]
- 16.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Wen H, Wu G, Chen W, Yang H, Fu J. Topical application of leptin promotes burn wound healing in rats. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:703–706. [PubMed] [Google Scholar]
- 19.Kulac M, Aktas C, Tulubas F, Uygur R, Kanter M, Erboga M, Ceber M, Topcu B, Ozen OA. The effects of topical treatment with curcumin on burn wound healing in rats. J Mol Histol. 2013;44:83–90. doi: 10.1007/s10735-012-9452-9. [DOI] [PubMed] [Google Scholar]
- 20.Bickenbach JR. Isolation, characterization, and culture of epithelial stem cells. Methods Mol Biol. 2005;289:97–102. doi: 10.1385/1-59259-830-7:097. [DOI] [PubMed] [Google Scholar]
- 21.Bickenbach JR, Grinnell KL. Epidermal stem cells: interactions in developmental environments. Differentiation. 2004;72:371–380. doi: 10.1111/j.1432-0436.2004.07208003.x. [DOI] [PubMed] [Google Scholar]
- 22.Bickenbach JR, Stern MM. Plasticity of epidermal stem cells: survival in various environments. Stem Cell Rev. 2005;1:71–77. doi: 10.1385/SCR:1:1:071. [DOI] [PubMed] [Google Scholar]
- 23.Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watt FM, Jensen KB. Epidermal stem cell diversity and quiescence. EMBO Mol Med. 2009;1:260–267. doi: 10.1002/emmm.200900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb A, Kaur P. Epidermal stem cells. Front Biosci. 2006;11:1031–1041. doi: 10.2741/1861. [DOI] [PubMed] [Google Scholar]
- 26.Webb A, Li A, Kaur P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation. 2004;72:387–395. doi: 10.1111/j.1432-0436.2004.07208005.x. [DOI] [PubMed] [Google Scholar]
- 27.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs E, Byrne C. The epidermis: rising to the surface. Curr Opin Genet Dev. 1994;4:725–36. doi: 10.1016/0959-437x(94)90140-x. [DOI] [PubMed] [Google Scholar]
- 29.Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 30.Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109:1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- 31.Fu X, Sun X, Li X, Sheng Z. Dedifferentiation of epidermal cells to stem cells in vivo. Lancet. 2001;358:1067–1068. doi: 10.1016/S0140-6736(01)06202-X. [DOI] [PubMed] [Google Scholar]
- 32.Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 33.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 34.Rollman O, Jensen UB, Ostman A, Bolund L, Gustafsdottir SM, Jensen TG. Platelet derived growth factor (PDGF) responsive epidermis formed from human keratinocytes transduced with the PDGF beta receptor gene. J Invest Dermatol. 2003;120:742–749. doi: 10.1046/j.1523-1747.2003.12129.x. [DOI] [PubMed] [Google Scholar]
- 35.Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103–114. doi: 10.1007/978-3-540-78281-0_7. [DOI] [PubMed] [Google Scholar]
- 36.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 37.Martin P. Wound healing – aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 38.Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Zeigler ME, Krause S, Karmiol S, Varani J. Growth factor-induced epidermal invasion of the dermis in human skin organ culture: dermal invasion correlated with epithelial cell motility. Invasion Metastasis. 1996;16:3–10. [PubMed] [Google Scholar]
- 40.Shikiji T, Minami M, Inoue T, Hirose K, Oura H, Arase S. Keratinocytes can differentiate into eccrine sweat ducts in vitro: involvement of epidermal growth factor and fetal bovine serum. J Dermatol Sci. 2003;33:141–150. doi: 10.1016/j.jdermsci.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Fu X, Sun X, Sun T, Sheng Z. The interaction between epidermal growth factor and matrix metalloproteinases induces the development of sweat glands in human fetal skin. J Surg Res. 2002;106:258–263. doi: 10.1006/jsre.2002.6469. [DOI] [PubMed] [Google Scholar]
- 42.Sheng Z, Fu X, Cai S, Lei Y, Sun T, Bai X, Chen M. Regeneration of functional sweat gland-like structures by transplanted differentiated bone marrow mesenchymal stem cells. Wound Repair Regen. 2009;17:427–435. doi: 10.1111/j.1524-475X.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa T, Terai S, Urata Y, Marumoto Y, Aoyama K, Sakaida I, Murata T, Nishina H, Shinoda K, Uchimura S, Hamamoto Y, Okita K. Fibroblast growth factor 2 facilitates the differentiation of transplanted bone marrow cells into hepatocytes. Cell Tissue Res. 2006;323:221–231. doi: 10.1007/s00441-005-0077-0. [DOI] [PubMed] [Google Scholar]
- 44.Moya ML, Garfinkel MR, Liu X, Lucas S, Opara EC, Greisler HP, Brey EM. Fibroblast growth factor-1 (FGF-1) loaded microbeads enhance local capillary neovascularization. J Surg Res. 2010;160:208–212. doi: 10.1016/j.jss.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Lin S, Xiao Y, Huang Y, Tan Y, Cai L, Li X. Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci. 2008;82:190–204. doi: 10.1016/j.lfs.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Fu X, Shen Z, Chen Y, Xie J, Guo Z, Zhang M, Sheng Z. Randomized placebo-controlled trial of use of topical recombinant bovine basic fibroblast growth factor for second-degree burns. Lancet. 1998;352:1661–1664. doi: 10.1016/S0140-6736(98)01260-4. [DOI] [PubMed] [Google Scholar]