Abstract
The aim of this research was to determine the bone formation capacity in fenestration defects associated with dental implants using absorbable and non-absorbable membranes. Six dogs were used in the study. In both tibias of each animal 3 implants were installed, and around these 5 mm circular defects were created. The defects were covered with absorbable membranes (experimental group 1), non-absorbable membranes (experimental group 2), and the third defect was not covered (control group). At 3 and 8 weeks post-surgery, the animals were euthanized and the membranes with the bone tissue around the implants were processed for histological analysis. The statistical analysis was conducted with Tukey’s test, considering statistical significance when p<0.1. Adequate bone repair was observed in the membrane-covered defects. At 3 weeks, organization of the tissue, bone formation from the periphery of the defect and the absence of inflammatory infiltrate were observed in both experimental groups, but the defect covered with absorbable membrane presented statistically greater bone formation. At 8 weeks, both membrane-covered defects showed adequate bone formation without significant differences, although they did in fact present differences with the control defect in both periods (p>0.1). In the defects without membrane, continuous connective tissue invasions and bone repair deficiency were observed. There were no significant differences in the characteristics and volume of the neoformed bone in the defects around the implants covered by the different membranes, whereas the control defects produced significantly less bone. The use of biological membranes contributes to bone formation in three-wall defects.
Keywords: Bone regeneration, biological membrane, bone defect
Introduction
Bone regeneration has been used successfully in different animals and human models of oral implantology and others conditions [1-3].
In cases of bone deficiency associated with the receptor site, there is the possibility of filling the sector with bone grafts or covering it with biological membranes [3,4]. The decision to use some of these strategies is based on the characteristics of the defect, its location, vascularization of the zone, among others [5,6].
It is known that the bone graft contributes to bone neoformation in some defects using both autogenous grafts and biomaterials, where the synergy with biological membranes has proven successful [7]. The choice of membrane system, however, undergoes constant modifications on the basis of its progression and structural development.
Absorbable membranes are generally composed of collagen, which allows them to degrade; the speed of this degradation depends on its composition and the time needed for the membrane to isolate the bone defect [8]. Non-absorbable membranes are formed by other compounds like cellulose that afford them rigidity and stability and must be removed in a second surgery, which implies higher morbidity [9].
The aim of this research was to identify bone formation and quality in fenestration defects associated with the installation of dental implants.
Material and methods
Sample
Six healthy adult dogs, each weighing approximately 6 kg, from the experimental research unit of the State University of Campinas, Brazil, were used in this study. The animal procedures were approved by the research ethics committee of the State University of Campinas by 1343-9 protocol.
Surgical procedure
The animals were sedated with an intramuscular injection of ketamine hydrochloride (0.15 ml/kg) and general anesthesia with 3% sodium pentobarbital (30 mg/kg). Both tibias were operated on. Aseptic procedures and trichotomy of the surgical site were performed prior to the 5 cm horizontal incision. The soft tissues were separated and the periosteum was released and the bone exposed. Three perforation sites were established in each tibia for the insertion of implants, with 1 cm of separation between them. At each site a 9 mm deep perforation was made using the sequence of surgical drills indicated by the manufacturer (INP, Sistema de Implantes Nacionais e de Próteses Comércio Ltda.), with a 16:1 contra-angle handpiece mounted on a surgical motor at 1000 rpm and profuse physiological saline for irrigation.
Around each insertion site, circular artificial bone defects were created using a 5 mm trephine bur. In depth, the defects reached the medial tibia, thus resembling the fenestration defects present in the installation of implants in edentulous maxillae. A profuse irrigation and cleaning were performed to eliminate detritus from the preparation (Figure 1).
Figure 1.

Implants positioned in the animal’s tibia with defects 5 mm in diameter formed by trephines burs.
In each site, a titanium implant was inserted: 9 mm long and 3.5 mm wide, external-hex connection, with a rough surface due to the chemomechanical treatment with sandblasting performed by the manufacturer. The primary stability of the implants was obtained manually over 35 N with a ratchet specially designed for this phase.
Two of the three defects in each tibia were covered with membranes. An absorbable membrane was placed on the first defect (experimental group 1), and on the second defect a non-absorbable membrane (experimental group 2). The third defect was not covered (control group). Selection of the defects covered by membranes was random. For experimental group 1, a collagen membrane with a non-friable matrix derived from bovine deep flexor tendon was used; it has a flat, condensed and texturized surface with 0.004 μm pores. For experimental group 2, a cellulose membrane made from artificial skin was used; it is formed from a biosynthetic matrix of two biologically inert, semipermeable, semitransparent layers, with variable perforation diameters and tensile strengths. The inner layer is made up of a network of crystalline cellulose microfibrils that gives the membrane stiffness; the outer layer is formed of alkali cellulose of varying porosity (Figure 2).
Figure 2.

Defects covered by non-absorbable membrane (cellulose) left, absorbable membrane (collagen) center and without cover (control) right.
In both cases, the membranes were hydrated with physiological saline to make them moldable, and they were installed on the defect around the implant taking into consideration at least 5 mm of contact between membrane and bone to stabilize its position.
All the dogs were kept under veterinary care. No complications were observed and all the dogs survived the surgical procedures.
Euthanasia and sample processing
Euthanasia periods at 3 and 8 weeks were estimated after the surgery, with 3 animals at each point. Between these periods, the animals that had not yet been euthanized were kept in the same place. For the euthanasia, the animals were first sedated under the same pharmacological protocol indicated previously, and then were given a lethal dose of 19.1% potassium chloride. To expose the implants and defects, the same steps were followed as in the first surgery. The bone blocks were removed from the tibia using a 1 cm safety margin lateral to the installed implants. The samples were fixed in 4% formaldehyde for 48 h at room temperature, washed with water and decalcified in 20% sodium citrate solution and 50% formic acid in equal parts for seven months. Each bone block was sectioned lengthwise to the implant, including the area of the artificial defect, and the bone was separated from the implant. The bone samples were dehydrated in decreasing solutions of ethyl alcohol and set in paraffin for histological analysis.
Histological and histometric analysis
Serial histological slides 6 μm thick were prepared and stained following the protocols for hematoxylin-eosin (HE) and Mallory’s trichrome (MT) staining techniques, and observed through an optical microscope. The descriptive analysis defined the presence of blood vessels, bone cells, trabeculae, medullary spaces and connective tissues, with magnifications of 10X and 100X. A 200 X 200 μm2 frame was placed on the images (0.8 mm3 with total volume count of 32 mm3) for the histometric analysis; the measurement selection was done according to described techniques [10]. A minimum count of 600 points per group was defined in each euthanasia period. 3 slides were counted for each specimen. The data were analyzed with an ANOVA for group study and Tukey’s test with a 1% level of significance.
Results
Histological analysis at three weeks
Experimental group 1, absorbable collagen membrane
Osteogenic activity was observed in the proximity of the periosteum as well as early bone formation. In the entire defect, immature trabeculated bone was observed, with lacunae, blood vessels and lax connective tissue in its interior, maintaining a clear difference between this and the adjacent bone that presented a compact pattern with Haversian canals and concentric lamellae. The bone neoformation seemed to go from the endosteal region to the inside of the defect; no areas of inflammatory resorption or inflammatory cells were observed (Figure 3).
Figure 3.

Defect covered by absorbable membrane 3 weeks: osteogenic activity in the periosteal region (RP) with differences between the neoformed bone tissue (ON) and the previously existing tissue (OP). MT 1OOX.
Experimental group 2, non-absorbable cellulose membrane
Osteoblastic activity was observed with bone neoformation filling the defect, immature bone and some blood vessels; lax connective tissue inside lacunae and multiple osteoblasts on the periphery. There was a clear limit between the defect and the adjacent bone, with no presence of osteoclasts or inflammatory infiltrate (Figure 4).
Figure 4.

Defect covered by non-absorbable membrane 3 weeks: clear differences between the neoformed bone tissue (ON) and the previously existing tissue (OP). HE 1OOX.
Control group, without membrane
The presence of immature bone tissue was observed inside the defect with ample medullary spaces and trabeculae, osteoblasts on the periphery, connective tissue and blood vessels. There was a smaller amount of bone tissue in formation compared to the membrane-covered defects; the margins between the defect and the adjacent bone were well differentiated, without finding areas of resorption although some inflammatory cells were observed (Figure 5).
Figure 5.
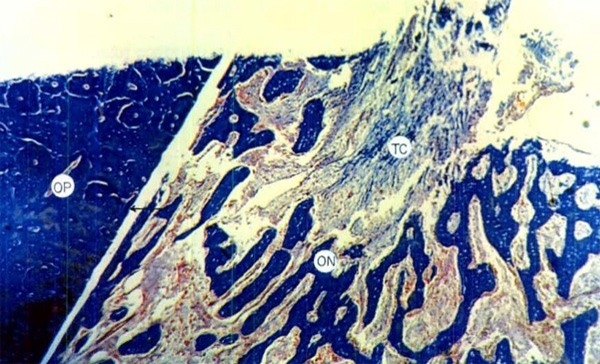
Defect without cover at 3 weeks: clear differences between the neoformed bone tissue (ON) and the previously existing tissue (OP) with presence of connective tissue (TC) inside the defect. HE 1OOX.
Histological analysis at eight weeks
Experimental group 1, absorbable collagen membrane
The defects were observed to be completely filled with neoformed bone tissue with greater maturation, a denser bone pattern and reduced medullary spaces; the presence of osteoblasts and blood vessels was also observed. In some places there was no difference between the area of bone neoformation and pre-existing bone. No areas of necrosis or presence of inflammatory cells were found (Figure 6).
Figure 6.

Defect covered by non-absorbable membrane 8 weeks: apposition of neoformed bone tissue (ON) is observed on the periphery of the defect with few differences between the defect and the previously existing bone (OP). HE 1OOX.
Experimental group 2, non-absorbable cellulose membrane
The bone defects were observed to be filled with newly formed bone, presenting reduced medullary spaces and compact trabecular. In some images the cellulose membrane was seen to be in contact with connective tissue; bone apposition of neoformed tissue on the margins of the defect with no evidence of areas of necrosis. Osteoclast activity and inflammatory infiltrate were absent (Figure 7).
Figure 7.

Defect covered by non-absorbable membrane 8 weeks: apposition of the neoformed bone tissue (ON) is observed on the bone that was there previously (OP); in contact with the membrane areas of connective tissue and immature bone matrix are observed. HE 1OOX.
Control group, without membrane
Multiple areas of fibrous tissue were observed without presence of osteoblast activity. In most of the lamellae, isolated immature bone tissue was observed with invagination of the connective tissue to the inside of the defect. The neoformed bone tissue was observed on the periphery of the defect, with clear differences from the pre-existing bone. No inflammatory cells were observed (Figure 8).
Figure 8.

Defect covered by non-absorbable membrane 8 weeks: apposition of the neoformed bone tissue (ON) much smaller and clear differences between the defect and the previously existing bone are observed. Abundant connective tissue was observed with invasion of the defect towards the inside of the cavity. HE 1OOX.
Histometric analysis
Statistically significant differences were observed between the created defects and their bone repair. The defects covered by collagen membrane showed a statistically greater bone repair than those covered by cellulose membranes (p<0.1) and those without membrane in the three-week period (p<0.1). At 8 weeks, the defects protected by absorbable and non-absorbable membrane did not present any significant differences but were statistically greater than the defect without membrane protection (p<0.1). In general terms, the formed bone volume was greater in defects covered by collagen membrane, followed by those covered by cellulose membrane and the defect without cover (Table 1).
Table 1.
Measurements of bone volume formed at different euthanasia periods (mm3)
| Defect | 3 wks. | P (3 wks.) | 8 wks. | P (8 wks.) |
|---|---|---|---|---|
| Defect with absorbable membrane (collagen) | 22.13 | P<0.1 | 25.12 | P<0.1 |
| Defect with non-absorbable membrane (cellulose) | 18.29 | P>0.1 | 25.71 | P<0.1 |
| Control defect | 14.83 | P>0.1 | 18.45 | P>0.1 |
Application of Tukey’s test with statistical significance if P<0.1.
Discussion
Bone repair is influenced by several factors, including the size and shape of the defect, cortical or spongy bone quality, quality of the adjacent periosteum, general systemic conditions of the subject and others [5]. Different publications have reported on the difficulty of bone repair when the defects are of a critical size [11] or the vascular capacity of the peripheral bone tissue is deficient, circumstances in which the contribution of elements like the biological membranes and bone grafts or substitutes can improve the repair process.
Guided bone regeneration has been used in the management of defects in long bones [1], regeneration of dental alveoli after exodontia [2] and in the management of defects adjacent to installed dental implants [12,13].
The results of this study demonstrated that the bone repair in the artificially created defects is of better quality and greater volume when biological membranes are used, because these act as a physical barrier to separate the overlying connective tissue from the bone and prevent the soft tissue from invading the defect, while also protecting the blood clot during the first stages of healing. Although these membranes partially isolate the bone defect from the periosteum in the bone repair, the porosity of the membrane may allow some interaction between the periosteum and the defect [5], which affords the adjacent bone a fundamental role in the bone neoformation phase. Thus, the treatment of patients with different types of membranes, with or without the use of bone graft, has been successful [14], showing versatility and adequate bone formation.
The circular model of defect used in this study resembles the conditions found in fenestration defects and peri-implant resorptions that affect implants installed in both the maxilla and the mandible, offering an adequate comparison, unlike other models that generate quadrilateral defects, with straight walls and angles, those that are covered by different types of membranes but do not effectively replicate the healing process of the circular defect, which begins from the periphery, as we observed in all the cases studied.
Considerations about the bone defect without membrane protection
In defects without biological membranes, even though they were not large, the collapse of the repair was observed due to connective tissue entering the defect and to the fact that in the absence of a bone graft, the repair began from the periphery of the defect. These results were observed in previous studies [15] and more extreme results were observed [16] where was established that the fenestration of implants was covered by bone tissue in 3.5% of the cases that did not use membranes and 75% of the cases that did. Other authors [17] showed experimental surgery in defects created in rats, where the second week the expression of alkaline phosphatase, osteopontin and osteocalcin was observed, whereas in the soft tissue outside the membrane there was no expression. At the fourth week adequate bone repair was observed, concluding that the collagen-based membrane fibers might also contribute to the bone formation of the defect. Our results showed that in defects without membranes there as a disorganized area of connective tissue with islands of bone tissue on the periphery of the defect, confirming these authors’ findings.
Considerations about membrane chose
Perhaps the most important bone regeneration results are bound up with the quality of the membrane and its composition. The fibrillar composition of the collagen membrane can also act as an osteoconductive factor in bone neoformation [18]; on the other hand, rough surfaces can act with greater cell adhesion [19] since the size of the membrane pores is also related to the type of cells able to permeate the membrane [8].
The collagen-based composition allows hemostatic activity, facilitates clot stabilization and does not cause immunological reactions because collagen is one of the most common proteins in the human body [20]. The degree of disintegration of the membrane, then, is secured by the degree of membrane crosslinking.
The non-absorbable membranes require a second procedure for their extraction, which involves renewed displacing of the periosteum and with it bone loss at the separation site [9]; exposure to the oral environment is also more complicated when the membrane is not absorbable. In fact, the study by Gotfredsen [21] showed that when the membrane was exposed, there was less bone formation than in defects where no membrane was used. Additionally, when the non-absorbable membrane was kept below the periosteum, the bone formation was greater than in the control group. Our results corroborate the efficiency of both membrane systems, there being similarities in the outcomes and better results than the defect without membrane.
Other biological membrane systems have been successfully applied, where the fibrin-rich plasma has been used as a membrane to cover access windows to the maxillary sinus, proving equally as efficient as the collagen membranes [22].
Was reported that guided bone regeneration depends on the appropriate use of the membrane, soft tissue stability, creation and maintenance of the space, close adaptation between the membrane and the adjacent bone and adequate recovery time [23]. In addition, was concluded that fenestration defects created in canine models regenerated with new bone in contact with the implant when collagen membranes were used; despite some exposure, regeneration only underwent minor volumetric changes. For other hand, the use of collagen membrane and a hydrogel membrane applied in a spray on defects linked to implants in humans was reported showing vertical bone formation of 5.6 mm in the defect with collagen membrane and 4.25 mm in the defect area treated with hydrogel, which was considered satisfactory in terms of regeneration [24].
Considerations about the membrane-protected bone defect with no use of bone graft
The results found by Hürzeler [9] in post-extraction dental alveoli showed that the absence of a bone graft meant a decrease in the volume of bone repair, probably due to the collapse of the membrane on the cavity. Other research showed that bone formation in the defect filled with bone graft was almost twice as great as the control group (defect treated without filling) and was almost three times greater when a biological collagen membrane was incorporated, demonstrating the contribution of the membrane in bone regeneration of the sector [3]. Another study on dogs conducted by Schwars [7] installed implants with a created fenestration defect covered by hydroxyapatite plus beta tricalcium phosphate or collagen-coated natural bone mineral with a collagen membrane, obtaining total cover of the bone defect with the formation of new bone tissue at 9 weeks post-surgery, which confirmed the adequate osteoconduction capacity of the materials together with the membrane that stabilized the bone graft; this may be responsible for the success of the membrane-covered bone graft since the stability of the installed material and the maintenance of a metabolism almost exclusively associated with bone could justify the best response when the grafted material (autogenous or biomaterial) is covered by a membrane. This is why the use of biomaterials in conjunction with collagen membranes has been successful in other clinical studies, demonstrating new bone formation and bone stability in the filled defects [4].
Finally, we can conclude that in this animal model, bone regeneration in fenestration defects associated with implants can be filled with bone when they are protected with biological absorbable or non-absorbable membranes.
Disclosure of conflict of interest
None.
References
- 1.Farso Nielsen F, Karring T, Gogolewski S. Biodegradable guide for bone regeneration. Polyurethane membranes tested in rabbit radius defects. Acta Orthop Scand. 1992;63:66–69. doi: 10.3109/17453679209154853. [DOI] [PubMed] [Google Scholar]
- 2.Cortellini P, Bartolucci E, Clauser C, Pini Prato GP. Localized ridge augmentation using guided tissue regeneration in humans. Clin Oral Implants Res. 1993;4:203–209. [Google Scholar]
- 3.Lee TS, Ko SH, Kim YT, Jung UW, Choi AH. Guided bone regeneration using cyanocrylate-combined calcium phasphate in a dehisecnce defect: a histologic study in dogs. J Oral Maxillofac Surg. 2012;70:2070–2079. doi: 10.1016/j.joms.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 4.Kim YK, Kim SG, Lim SC, Lee HJ, Yun PY. A clinical study on bone formation using a demineralized bone matrix and resorbable membrane. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2012;109:e6–11. doi: 10.1016/j.tripleo.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira GR, Olate S, Cavalieri-Pereira L, Pozzer L, Asprino L, de Moraes M, de Albergaría-Barbosa JR. Maxillary sinus floor augmentation using blood without graft material. Preliminary results in 10 patients. J Oral Maxillofac Surg. 2013 Oct;71:1670–5. doi: 10.1016/j.joms.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Netto HD, Olate S, Kluppel LE, do Carmo AMR, Vasquez B, Albergaria-Barbosa JR. Histomtric analyses of cancellous and cortical interface in autogenous bone grafting. Int J Clin Exp Pathol. 2013;6:1532–1537. [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz F, Herten M, Ferrari D, Wieland M, Schmitz L, Engelhardt E, Becker J. Guided bone regeneration at dehiscence-type defects using biphasic hydroxyapatite + beta tricalcium phosphate (Bone Ceramic) or a collagen-coated natural bone mineral (BioOss Collagen): an inminohistochemical study in dogs. Int J Oral Maxillofac Surg. 2007;36:1198–1206. doi: 10.1016/j.ijom.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Pineda LM, Büsing M, Meinig RP, Gogolewski S. Bone regeneration with resorbable polymeric membranes. III. Effect of poly (L-lactide) membranes pore size on the bone healing process in large defects. J Biomed Mater Res. 1996;31:385–394. doi: 10.1002/(SICI)1097-4636(199607)31:3<385::AID-JBM13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Hürzeler MB, Kohal RJ, Naghshbandi J, Mota LF, Conradt J, Hutmacher D, Caffesse RG. Evaluation of a new bioresorbable barrier to facilitate guided bone regeneration around exposed implant threads. An experimental study in the monkey. Int J Oral Maxillofac Surg. 1998;27:315–20. doi: 10.1016/s0901-5027(05)80623-x. [DOI] [PubMed] [Google Scholar]
- 10.Gerstenfeld LC, Wronski TJ, Hollinger JO, Einhorn TA. Application of histomorphometric methods to the study of bone. J Bone Miner Res. 2005;20:1715–1722. doi: 10.1359/JBMR.050702. [DOI] [PubMed] [Google Scholar]
- 11.Chaves Netto HDM, Olate S, Chaves MMGA, Barbosa AJR, Mazzonetto R. Histological analyses of osseous repair defetcs: recognized of critic defects. Int J Morphol. 2009;27:1121–1127. [Google Scholar]
- 12.Becker W, Becker BE, Berg L, Prichard J, Caffesse R, Rosenberg E. New attachment after treatment with root isolation procedures: report for treated class III and class II furcations and vertical osseous defects. Int J Periodontics Restorative Dent. 1988;8:8–23. [PubMed] [Google Scholar]
- 13.Schneider D, Weber FE, Grunder U, Andreoni C, Burkhardt R, Jung RE. A randomized controlled clinical multicenter trial comparing the clinical and histological performance of a new, modified polylactide-co-glycolide acid membrane to an expanded polytetrafluorethylene membrane in guided bone regeneration procedures. Clin Oral Implants Res. 2013 doi: 10.1111/clr.12132. doi: 10.1111/clr.12132. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Chiapasco M, Zaniboni M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: a systemic review. Clin Oral Implants Res. 2009;20(Suppl 4):113–123. doi: 10.1111/j.1600-0501.2009.01781.x. [DOI] [PubMed] [Google Scholar]
- 15.Lundgren D, Nyman S, Mathisen T, Isaksson S, Klinge B. Guided bone regeneration of cranial defects using biodegradable barriers: an experimental pilot study in the rabbit. J Craniomaxillofac Surg. 1992;20:257–60. doi: 10.1016/s1010-5182(05)80438-x. [DOI] [PubMed] [Google Scholar]
- 16.Dahlin C, Andersson L, Lindhe A. Bone augmentation at fenestration implants by an osteopromotive membrane technique. A controlled clinical study. Clin Oral Implants Res. 1991;2:159–165. doi: 10.1034/j.1600-0501.1991.020401.x. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi Y, Amizuka N, Nakadate M, Ohnishi H, Fujii N, Oda K, Nomura S, Maeda T. A histological evaluation for guided bone regeneration induced by collagenous membrana. Biomaterials. 2005;26:6158–6166. doi: 10.1016/j.biomaterials.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Sevor JJ, Meffert RM, Cassingham RJ. Regeneration of dehisced alveolar bone adjacent to endosseous dental implants utilizing a resorbable collagem membrane: clinical and histologic results. Int J Periodontics Restorative Dent. 1993;13:71–83. [PubMed] [Google Scholar]
- 19.Taylor SR, Gibbons DF. Effects of surface texture on the soft tissue response ti polymer implants. J Biomed Mater Res. 1983;17:205–227. doi: 10.1002/jbm.820170202. [DOI] [PubMed] [Google Scholar]
- 20.Greenstein G, Caton JG. Biodegradable barriers and guided tissue regeneration. Periodontol 2000. 1993 Feb;1:36–45. [PubMed] [Google Scholar]
- 21.Gotfredsen K, Nimb L, Buser D, Hjorting-Hansen E. Evaluation of guided bone generation around implants placed into fresh extraction sockets: an experimental study in dogs. J Oral Maxillofac Surg. 1993;51:879–884. doi: 10.1016/s0278-2391(10)80108-9. [DOI] [PubMed] [Google Scholar]
- 22.Gassling V, Purcz N, Braesen JH, Will M, Gierloff M, Behrens E, Açil Y, Wiltfang J. Comparison of two different absorbable membranes for the coverage of lateral osteotomy sites in maxillary sinus augmentation: a preliminary study. J Craniomaxillofac Surg. 2013;41:76–82. doi: 10.1016/j.jcms.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Oh TJ, Meraw SJ, Lee EJ, Giannobile WV, Wang HL. Comparative analysis of collagen membranes for the treatment of implant dehiscencia defects. Clin Oral Implants Res. 2003;14:80–90. doi: 10.1034/j.1600-0501.2003.140111.x. [DOI] [PubMed] [Google Scholar]
- 24.Joung RE, Hälg G, Thoma DS, Hämmerle CH. A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants. Clin Oral Implants Res. 2009;20:162–168. doi: 10.1111/j.1600-0501.2008.01634.x. [DOI] [PubMed] [Google Scholar]


