Abstract
Background: Prostate carcinoma is a major cause of morbidity and mortality. The MAPK Signaling Pathway plays an important role in multiple tumors, including prostate carcinoma. MAPK signaling is mediated by ERK1/2, JNK and p38 MAPK, which are important in the control of cell proliferation, differentiation and apoptosis. However, relatively little is known about the regulatory mechanism of p38 MAPK in prostate cancers. NOB1 is among the most novel topic in MAPK studies currently. Recent studies found its vital role in tumor metastasis in glioblastoma proliferation, however, its expression profile and its prognostic value in prostate carcinoma have not been investigated. Methods: To determine the relationship between NOB1 and p38 MAPK expressions, a population-based study was conducted for immunohistochemical staining analysis of tumor tissues, in matched malignant and nonmalignant prostatectomy samples from 132 PCa patients. Moreover, Western blot analysis and NOB1 interference studies of prostate cancer cell lines. To evaluate the diagnostic and prognostic between NOB1 and p38 MAPK in prostate cancer (PCa) tissue after radical prostatectomy, the hypothesis that prostate cancers with NOB1 expression have distinct clinical, prognostic and molecular attributes was tested. Results: Among 132 prostate cancers, NOB1 expression was detected in 117 (88.7%) tumors by immunohistochemistry. NOB1 and p38 MAPK expression had significant positive correlation with carcinogenesis, tumor progression and patient survival. Immunohistochemically, NOB1 expression in prostate cancer was independently associated with p38 MAPK activation (P=0.0002). Furthermore, p38 MAPK expression was completely suppressed by NOB1 interference in the prostate cancer cell lines DU-145 and PC-3. Conclusions: NOB1 expression status was closely correlated with important histopathologic characteristics and the recurrence and metastasis of prostate carcinomas. These data support a potential link between NOB1 and p38 MAPK, and suggest that NOB1 may identify a subset of prostate cancer patients with a poor prognosis. This study proved that NOB1 in PCa tissue can be used, in combination with traditional clinicopathological factors, as promising diagnostic and prognostic tools.
Keywords: Prostate carcinoma, NOB1, p38 MAPK, histopathologic grade, prognostic markers
Introduction
Prostate cancer is the most common malignancy in USA and the second leading cause of deaths from cancer [1]. This disease in men with 382,000 new cases in Europe and with a predicted incidence of 241,470 for 2012 in the United States [2]. In the early stage, prostate cancer usually grows slowly and remains confined to the gland, initially producing few or no symptoms. As the cancer advances, it can spread beyond the prostate into the surrounding tissues and to other areas, such as the bones, lungs, and bladder. Therefore, symptoms often appear after the cancer has processed to an advanced stage [3]. However, there is an ongoing controversial debate on the use of prostate-specific antigen (PSA) testing for prostate cancer [4,5]. The limitation of PSA in discriminating between benign and malignant prostate disease and its inability to distinguish between aggressive and less aggressive tumors result in over-diagnosis and over-treatment of rather insignificant tumors with low potential morbidity or death. Consequently, current effort in urologic research has focused on the discovery of new molecular markers to improve both early prostate cancer detection and risk prediction for patients.
The MAPKs are serine/threonine protein kinases that participate in intracellular signaling during proliferation, differentiation, cellular stress responses, and apoptosis [6]. Activation of MAPKs, including extracellular signal-regulated kinase 1 and 2 (ERK1/2), p38 MAPK, and the stress activated protein kinase (SAPK)/c-Jun NH2-terminal kinase (JNK), has been implicated in the activity of numerous chemotherapy and genotoxic drugs [7]. MAPK can regulate apoptosis through specific phosphorylation of downstream mediators of apoptosis, including the tumor suppressor p53, thus linking cellular stress signaling and regulation of p53 activity. Notably, recent studies point at an important role of p38 MAPK in signaling regulation. This intracellular kinase was initially identified as a major pro-inflammatory mediator and was later found to be involved in multiple cellular processes and in diseases including cancer [8,9]. In fact, p38 MAPK has been implicated as a suppressor of malignant processes, like oncogene-mediated cell senescence and contact inhibition. Other studies, however, have shown pro-migratory and invasive effects of p38 MAPK activation in cancer cells and stromal cells [10]. Like other MAPKs, the functions of p38 are dependent on its down-stream kinases, including serine/threonine kinases such as p38-regulated/activated kinase (PRAK) and MAPK-activated kinase-2, and many different up-stream kinases, such as MKK3/6, MKK4, as well as MAPKK independent signals. Cell specific as well as subcellular localization specific effects add to the complexity of p38 function [11].
The human NOB1 gene (Nob1p), located on human chromosome 16q22.1, which plays a role in RNA metabolism, was also cloned and characterized [12]. It is composed of nine exons and the Nob1 cDNA is 1,749 bp long and contains a putative open reading frame of 1,239 bp. Nob1 mRNA is expressed mainly in liver, lung and spleen. Nob1 protein is mainly localized in the nucleus of the mammalian cells [13]. However, the physiological and pathological functions of NOB1 remain unclear, and its relationship with p38 MAPK has not been examined to date. According to latest reports that NOB1 increased the tumorigenesis of glioma cells in vivo and in vitro through the modulation of the MAPK pathway [14]. Therefore, it was determined that studies on NOB1 and p38 MAPK using human prostate tissues would provide an important direction for chemotherapy using MAPK regulators in the future. We evaluated the immunohistochemical expression level or pattern of NOB1 and p38 according to several clinicopathological factors using prostate cancer tissues, and analyzed the mutual correlation of NOB1 and p38. To confirm the validity of the result of immunohistochemical study perfomed on the prostate cancer tissues, molecular pathological studies using prostate cancer cell lines were performed.
Materials and methods
Case selection and tissue sampling
For this retrospective study, tumor tissue and matched normal adjacent tissue were taken from prostate specimens after radical prostatectomy performed between January 1999 and February 2006 on 132 men with untreated prostate carcinoma before surgery, at the Department of Urology, Tongji University Affiliated Shanghai Tenth People’s Hospital in China. The various clinicopathological parameters of the patients were confirmed by a review of the patient charts and pathology files. Patient survival was confirmed through telephone calls and follow-up visit by doctor in charge. Informed consent was obtained from each subject according to the institutional guidelines, and the research protocols were approved by the IRB of our hospital.
A full frontal tissue slice of 2-4 mM thickness that was suspicious for malignancy was immediately cryopreserved in liquid nitrogen. This tissue slice was only used for research if not necessary for additional diagnostic purposes. To obtain pure tumor tissue (>90%) and matched adjacent normal tissue completely free of tumor filtrates and without signs of inflammation or atrophy, cryosections stained with hematoxylin-eosin were made to identify these regions of interest supported by the stained sections from the adjacent in buffered formaldehyde-fixed and in paraffin-embedded tissue blocks. The selection of patients was made according to the availability of tissue samples and the completeness of follow-up data of the patients on the basis of sample size calculation as mentioned below. For each patient, clinicopathological information on age, prostate volume, preoperative PSA, tumor classification according to the UICC 2002 TNM System,18 tumor Gleason grade based on the whole specimen and PSA concentrations during postoperative follow-up were compiled (Table 1).
Table 1.
Clinical characteristics of the study group1
| Characteristics | Total | No recurrence | Recurrence | P2 |
|---|---|---|---|---|
| Number | 132 | 98 | 34 | |
| Age (y) | 0.642 | |||
| Median (95% CI) | 67 (65.5-68) | 65 (63.1-66) | 70 (67.6-71) | |
| Range | 47-79 | 47-79 | 49-76 | |
| Preoperative PSA (ng/mL) | 0.893 | |||
| Median (95% CI) | 7.7 (6.9-8.8) | 7.7 (6.7-9.2) | 7.8 (6.1-12.7) | |
| Range | 1.8-43.7 | 1.9-43.7 | 1.8-37.2 | |
| Preoperative % fPSA | 0.517 | |||
| Median (95% CI) | 8.9 (7.6-10.2) | 8.4 (7.2-10.5) | 9.4 (7.2-14.3) | |
| Range | 3.1-47.7 | 3.1-47.7 | 3.7-19.8 | |
| Prostate volume (ml) | 0.954 | |||
| Median (95% CI) | 36 (34-39) | 36 (30-41) | 39 (28-46) | |
| Range | 14-102 | 18-102 | 14-97 | |
| Pathological stage (%) | <0.0003 | |||
| pT2a | 11 (8.3) | 11 (11.6) | - | |
| pT2b | 23 (17.4) | 16 (16.8) | 7 (18.9 | |
| pT2c | 49 (37.1) | 44 (46.3) | 5 (13.5) | |
| pT3a | 36 (27.3) | 19 (20.0) | 17 (45.9) | |
| pT3b | 13 (9.8) | 5(5.3) | 8 (21.6) | |
| Gleason score (%) | <0.0001 | |||
| 5-6 | 37 (28.0) | 33 (31.7) | 4 (14.3) | |
| 7 | 49 (37.1) | 41 (39.4) | 8 (28.6) | |
| 8 | 25 (18.9) | 19 (18.3) | 6 (21.4) | |
| 9 | 18 (13.6) | 11 (10.6) | 7 (25.0) | |
| 10 | 3 (2.3) | - | 3 (10.7) | |
| Follow-up (m) | <0.0001 | |||
| Median (95% CI) | 46.1 (40.1-52.6) | 49.5 (46.7-62.2) | 19.3 (9.8-40.3) | |
| Range | 0.5-107 | 7.3-107 | 0.5-89.7 |
Values are given as medians with 95% CI in parentheses and ranges or numbers of patients with percentages in parentheses.
Significances between no recurrence and recurrence tested by Mann-Whitney U test or x2 test.
Histopathological analysis
Microscopic examination
Each tumor was re-evaluated by retrospect analysis of the medical records and the tissue slide file of the pathology department, and age, tumor size, histological subtype and the degree of differentiation, the depth of tumor invasion, the status of lymph node metastasis, lymphovascular invasion and distant metastasis, were assessed. The stage was defined according to the TNM staging system of the AJCC (8). Tissues to be examined were fixed in 10% neutral formalin, and the prepared paraffin-embedded tissues were sectioned 4-5 μm in thickness, and hematoxylin and eosin staining was performed. The cells were examined under a light microscope, the representative area suitable to the study purpose was selected, and slides were prepared for immunohistochemical analysis.
Immunohistochemical staining
Immunohistochemical staining for NOB1 and p38 MAPK protein were performed on the validating set of Pca patients. Briefly, the 4-μm sections that were obtained after formalin fixation and paraffin embedding were deparaffinized in xylene and were rehydrated with distilled water through a graded series of ethanol solutions. The sections were then placed in a glass jar with 10 mM citrate buffer (pH 6.0) and irradiated in a microwave oven for 15 min, and were allowed to cool down in the jar at room temperature for 20 min. The slides were then rinsed with PBS. Blocking reagent was added for 10 min after quenching the endogenous peroxidase activity in 0.3% hydrogen peroxide for 10 min. The slides were then washed as before, and they were subsequently subjected to the primary antibody reaction. Mouse monoclonal p38 MAPK (Santa Cruz Biotechnology, Santa Cruz, CA, USA, dilution 1:1000) and rabbit polyclonal NOB1 (Abcam, dilution 1:1000) applied to the tissue section and they were allowed to incubate in a moist chamber for 1 h at room temperature. The slides were washed with PBS, incubated with biotinylated secondary antibody (Abcam), and treated with Immunopure Metal enhanced DAB substrate kit (Pierce, Rockford IL) according to the manufacturer’s instructions. Counterstaining was performed with Mayer’s hematoxylin.
Analysis and interpretation of the staining
A pathologist who did not know the clinical course of the subjects in order to exclude subjectivity evaluated the staining results. Scoring of p38 was based upon the distribution of p38 within the cell membrane (0-1), cytoplasm (0-2), and nuclei (0-2), with a total score of 0 reflecting cell membrane staining only, similar to that seen in normal prostate tissue, up to an aggregate score of 5 for tumors with strong nuclear staining (2), diffuse cytoplasmic staining (2), and loss of cell membrane staining (1). Total scores were then collapsed into two grades (inactive, 0-2; active, 3-5). NOB1 expression was determined by assessing the level of immunohistochemical staining in tumor cell nuclei. Initially, the overall staining intensity was scored as none, weak, moderate or strong. Cases categorized as positive were those characterized by weak, moderate or strong staining, while cases categorized as negative were those with no nuclear staining [15].
Western blot analysis of NOB1 and p38 MAPK
Cell culture
Human prostate cancer cell lines PC-3, DU-145 and LNCaP were obtained from the American Type Culture Collection (ATCC, Manassas, VA), which seeded with 1.5x106 cells in a 10-cm petri dish and maintained in RPMI-1640 medium (Invitrogen) containing 10% FBS (Gibco BRL, Grand Island, NY, USA) with 100 μ/ml penicillin and 100 μg/ml streptomycin at 37°C in 5% CO2 and the medium was renewed every 2-3 days.
Reagents
Polyclonal rabbit anti-human NOB1, monoclonal anti-p38 and ECL system were purchased from Santa Cruz Biotechnology and monoclonal mouse anti-actin was purchased from Sigma (St. Louis, MO, USA). Unless specified, drugs were purchased from Gibco BRL.
Western blotting analysis
Electrophoretically separated proteins were transferred to an NC membrane, blocked in 5% skim-milk/TBST, and incubated with primary antibodies to NOB1, p38 and β-actin. Then, the membranes were incubated with HRP-conjugated secondary antibody and visualized with Super Signal chemiluminescence kit (Pierce Biotechnology), and finally the signals were acquired by image analyzer (Image station 4000 MN, Kodak).
RNA interference
Small interfering RNA (siRNA) targeting NOB1 sequence (AAGGTTAAGGTGAGCTCATCG) and non-silencing sequence (AATTCTCCGAACGTGTCACGT) were transformed into short hairpin RNA (shRNA) and were cloned into pLV-THM-lentiviral vectors with BamHI/EcoRI sites. Then, the recombined pLVTHM-lentiviral vector and two-helper vector system (GeneChem Co. LTD, Shanghai, China) were transfected into HEK293T cells via Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) to generate lentivirus. After 3 days of incubation, the lentivirus from culture medium was collected and concentrated with Centricon-plus-20 (Millipore, Billerica, MA, USA). For lentivirus infection, PC-3, LNCaP and DU-145 cells were cultured in 6-well plates. Then, NOB1 shRNA-expressing lentivirus (sh-NOB1) and nontargeting shRNA-expressing lentivirus (control) was added, with a multiplicity of infection of 10 in PC-3, LNCaP and DU-145 cells. Then cells were grown for 48 h prior to total protein extraction.
Statistical analysis
Computerized statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 13.0 Clinical and histopathologic information and the results from the immunohistochemical studies were entered into a database. Stat View software package (Abacus Concepts, Berkeley, CA, USA) was used for statistical analysis. x2 test, Fisher’s exact test, ANOVA, and logistic regression analysis were used to determine the correlation between clinicopathologic parameters and expression patterns of NOB1 and p38 MAPK. Log-rank test was used for the analysis of Kaplan-Meier survival. Statistical significance was determined at P<0.05.
Results
Patient characteristics and clinicopathological findings
A total of 132 prostate cancer patients were included in this retrospective study with matched-paired tissue samples after radical prostatectomy and complete follow-up data. The survival to February 31, 2006, of 127 cases examined by immunohistochemical analysis in our study was assessed by telephone and follow-up visit. The survival record of the longest survival 154 months (average follow-up period: 67.9±55.6 months) could be confirmed. 132 cases showed T disease stage, the presence or absence of regional lymph node metastasis, and the presence or absence of distant metastasis as shown in Table 2.
Table 2.
Frequency of NOB1 expression in prostate carcinoma according to clinical and pathologic features (%)
| Clinical and pathologic feature | Total No. | NOB1 | P |
|---|---|---|---|
| All cases | 132 (100) | 117 (88.7) | |
| T stage | |||
| pT2a | 11 (8.3) | 7 (63.6) | |
| pT2b | 23 (17.4) | 19 (82.6) | <0.005 |
| pT2c | 49 (37.1) | 45 (91.8) | |
| pT3a | 36 (27.3) | 34 (94.4) | <0.005 |
| pT3b | 13 (9.8) | 12 (92.3) | |
| Gleason score (%) | |||
| 5-6 | 37 (28) | 31 (83.8) | <0.01 |
| 7-8 | 74 (56.1) | 67 (90.5) | <0.005 |
| 9 | 18 (13.6) | 16 (88.9) | <0.01 |
| 10 | 3 (2.3) | 3 (100) | <0.0005 |
| Lymph node | <0.005 | ||
| - | 77 (58.3) | 53 (68.8) | |
| + | 55 (41.7) | 49 (89.1) | |
| Distant metastasis | <0.0005 | ||
| - | 130 (98.5) | 115 (88.6) | |
| + | 2 (1.5) | 2 (100) | |
| P38, nuclear | |||
| - | 53 (40.2) | 38 (71.7) | Referent |
| + | 47 (35.6) | 41 (87.2) | <0.05 |
| ++ | 32 (24.2) | 29 (90.6) | <0.05 |
| P38, cytoplasmic | |||
| - | 63 (47.7) | 52 (82.5) | Referent |
| + | 44 (33.3) | 34 (77.3) | <0.01 |
| ++ | 25 (18.9) | 23 (92) | <0.005 |
| P38, membrane | <0.05 | ||
| Expressed | 59 (44.7) | 42 (71.2) | |
| Lost (1+) | 73 (55.3) | 61 (83.6) | |
| P38, overall score1 | <0.0005 | ||
| 0-2 (inactive) | 79 (59.8) | 48 (60.8) | |
| 3-5 (active) | 53 (40.2) | 43 (81.2) |
Only significant p-values are described.
P38 score was calculated as the sum of nuclear (0-2), cytoplasmic (0-2) and membrane (0-1) scores as described in Materials and Methods.
Clinicopathological significance of NOB1
Interrelation between NOB1 and Gleason score
Immunohistochemical staining was performed on 132 cases of prostate cancer. Among them, 117 cases (88.7%) showed positive expression of NOB1 in the nucleus (Figure 1). In the cases that performed the test, examining the rate of the positive expression of NOB1 according to Gleason score, 31 patients of 37 Gleason score 5-6 patients (83.8%), 67 patients of 74 Gleason score 7-8 patients (90.5%) and 16 patients of 18 Gleason score 9 patients (88.9%), 3 patients of 3 Gleason score 10 patients (100%) were positive, the higher score showed a higher positive rate, and it was statistically significant (P<0.005).
Figure 1.
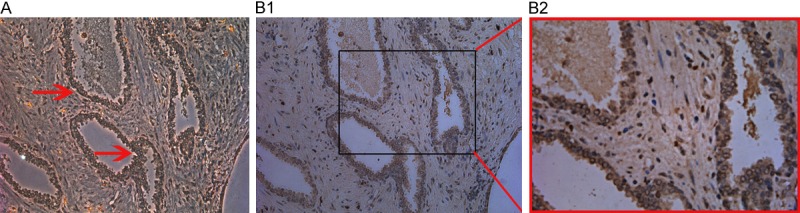
Immunohistochemical staining of prostate carcinoma for NOB1. Tumor cells showed diffuse strong positive nuclear staining. (A: ×200; B1: ×200; B2: ×400).
Interrelation between NOB1 and T stage
The change of NOB1 according to the T stage was analyzed. The disease T stage was pT2a-pT3b. In the pT1 stage patients were not included in the analysis subjects, and thus its interpretation is limited. Nonetheless, as the T stage became higher, the expression of NOB1 was elevated. The expression of NOB1 was statistically significantly elevated in the pT3 stage in comparison with pT2 stage (P<0.005).
Interrelation between NOB1 and lymph node metastasis
Among 55 cases with lymph node metastasis, 49 cases (89.1%) expressed NOB1. Among 77 cases without lymph node metastasis, 53 cases (68.8%) expressed NOB1. The expression of NOB1 correlated to lymph node metastasis, and it was statistically significant (P<0.005).
Interrelation between NOB1 and distant metastasis
Only 2 cases with distant metastasis were included in the analysis, and thus it was very limited to interpret statistical significance. Nonetheless, among 130 cases without distant metastasis, 115 cases (88.6%) showed NOB1 positivity. 2 cases with distant metastasis were NOB1 positive, and thus the expression of NOB1 significantly correlated to distant metastasis (P<0.0005).
Mutual relation of immunohistochemical expression of P38 and NOB1
Analysis by intranuclear staining of p38
Nuclear staining was observed in 79 prostate cancer cases (59.8%). 47 cases (35.6%) showed weak expression, and 32 cases (24.2%) showed moderate/strong expression (Figure 2). In 53 cases (40.2%) there was no expression of p38, 38 cases (71.7%) showed positive expression of NOB1. In the cases that showed positive expression of p38 within the nucleus, 87.2-90.6% cases showed positive expression of NOB1, and thus the positive expression of p38 in the nucleus correlated to the positive expression of NOB1, and it was statistically significant (P<0.05).
Figure 2.
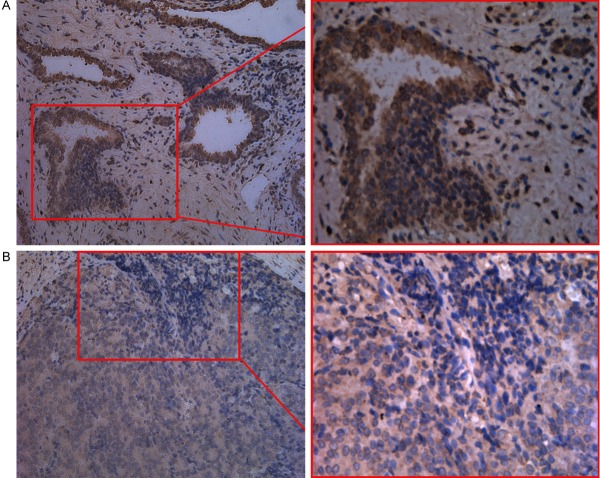
Immunohistochemical staining of prostate carcinoma for p38 MAPK. A: Tumor cells showed diffuse positive nuclear staining and some membranous staining. B: Tumor cells showed diffuse positive cytoplasmic staining. (Left: ×200; Right: ×400).
Analysis by intracytoplasmic staining of P38
Staining in the cytoplasm was observed in 69 cases of prostate cancer (52.3%). 44 cases (33.3%) showed weak expression, and 25 cases (18.9%) showed moderate/strong expression (Figure 2). Among 63 cases (47.7%) without the expression of p38 within the cytoplasm, 52 cases (82.5%) showed positive expression of NOB1. In the cases that showed positive expression of p38 in the cytoplasm, in regard to the staining intensity, the positive expression of NOB1 was shown in 77.3%. In cases showing moderate/strong expression, 92% showed positive expression of NOB1, and thus the expression of p38 within the cytoplasm correlated to the positive expression of NOB1, and it was statistically significant (P<0.01 and P<0.005, respectively).
Analysis by cytoplasmic membrane staining of P38
The staining of p38 of cell membrane was lost in 73 cases (55.3%) of prostate cancer (Figure 3), and the staining of cell membrane was maintained in 59 cases (44.7%). Among the cases that lost the staining of cell membrane, in 61 cases (83.6%), the expression of NOB1 was observed, and in the cases maintaining the staining of cell membrane, the expression of NOB1 was observed only in 42 cases (71.2%). Therefore the expression of NOB1 correlated to the loss of the membranous staining of p38, and it was statistically significant (P<0.05).
Figure 3.
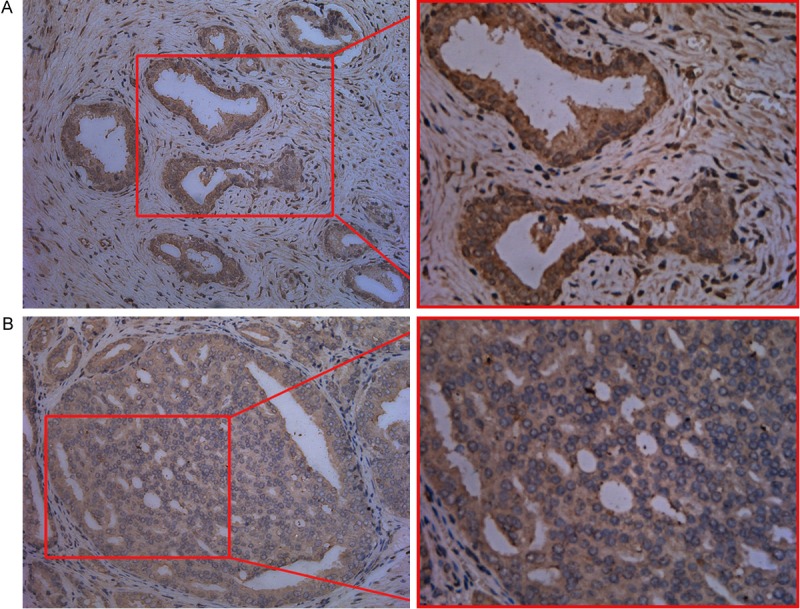
Immunohistochemical staining of prostate carcinoma for p38 MAPK. A: Tumor cells showed diffuse positive membranous staining. B: Tumor cells showed negative membranous staining. (Left: ×200; Right: ×400).
Analysis by P38 total score
In regard to the above mentioned p38 staining, the cases with p38 inactivation corresponding to 0-2 points of the score summing of the evaluation of the nucleus, the cytoplasm, and cell membrane consisted of 79 of the entire prostate cases (59.8%). The cases with p38 activation that corresponds to 3-5 points consisted of 53 of the entire prostate cancer cases (40.2%). In the cases with p38 activation, the rate of the expression of NOB1 was 81.2%, on the other hand, in the cases with p38 inactivation, the rate of the expression of NOB1 was 60.8%, the expression of NOB1 correlated to p38 activity, and it was statistically significant (P<0.0005).
Prostate cancer cell lines study
Western blot analysis - expression of NOB1
Prostate cancer cell lines were cultured, and NOB1 was measured. It was strongly expressed in PC-3, DU-145 and LNCaP cell lines (Figure 4). This suggests the involvement of NOB1 in prostate cancer as shown in the results of immunohistochemical staining.
Figure 4.
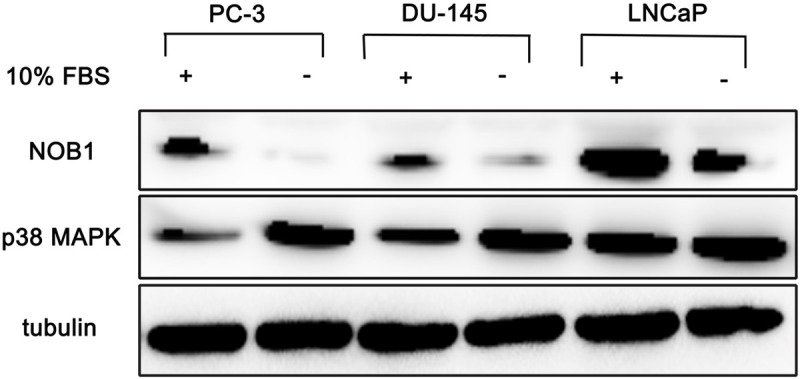
Western blotting analysis of prostate cancer cell lines for NOB1 and p38 MAPK. Each cell line expressed NOB1 and p38 MAPK intensely. After serum starvation for 24 h, NOB1 level was decreased in each cell line nevertheless, p38 level was increased in each cell line.
Expression of P38
Prostate cancer cell lines were cultured and the expression of p38 was assessed. It was shown to be expressed strongly in PC-3, DU-145 and LNCaP cell lines. However, in LNCaP cells, the expression of P38 was slightly weaker than PC-3 and DU-145 (Figure 4). This suggests that p38 could be expressed by the involvement of NOB1 in prostate cancer as shown in the result of immunohistochemical staining.
Experiments in the absence of serum
To elucidate the relationship of NOB1 and p38, prostate cancer cell lines were cultured for 24 h in serum-free medium. In the absence of serum factors, the expression of NOB1 was reduced slightly in PC-3, DU-145 and LNCaP cell lines. However, p38 showed a tendency to be expressed more strongly in the medium without serum factors (Figure 4). In other words, under the condition where serum factors were omitted and thus NOB1 was reduced, p38 showed a tendency to be increased.
Expression of P38 after treatment of NOB1 siRNA
NOB1 interference was generated, and the expression of p38 in the prostate cancer cell lines, PC-3, DU-145 and LNCaP, was assessed. The expression of P38 was decreased noticeably in PC-3 and DU-145; nonetheless, it could not be suppressed completely. In addition, in LNCaP cases, reduction of the expression of P38 was not observed (Figure 5).
Figure 5.
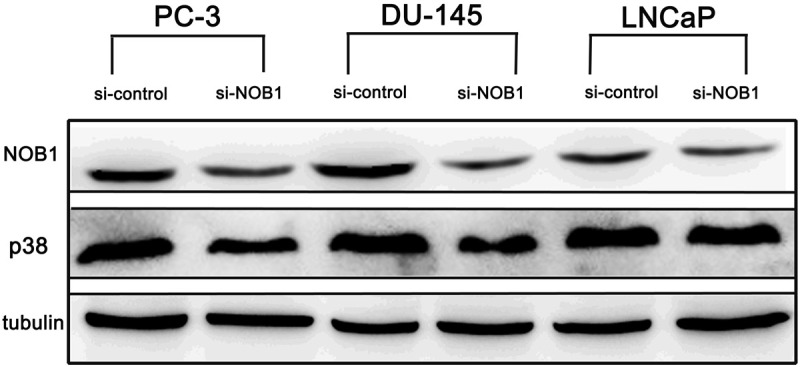
Effect of NOB1 interference on p38 MAPK expression in prostate cancer cell lines. Significant suppression of p38 MAPK was identified in PC-3 and DU-145, but was not significant in LNCaP. (5 μg siRNA/48 h).
Survival analysis by NOB1 expression
To analyze the outcome of patients according to the expression of NOB1, the effect of NOB1 on the survival of prostate cancer patients was analyzed. The overall 5-year survival rate of patients was 72.6%. At the time of examination, among 132 patients (average follow-up period: 5.1 years), 79 patients survived, and 59.8% survival rate was shown. Among the NOB1 negative group (15 patients), 11 patients (73.3%) survived. In the NOB1 positive group (117 patients), 63 patients (53.8%) survived (log-rank: P=0.024) (Figure 6). Hence, it was found that the expression of NOB1 was closely associated with the high mortality of prostate carcinoma patients.
Figure 6.
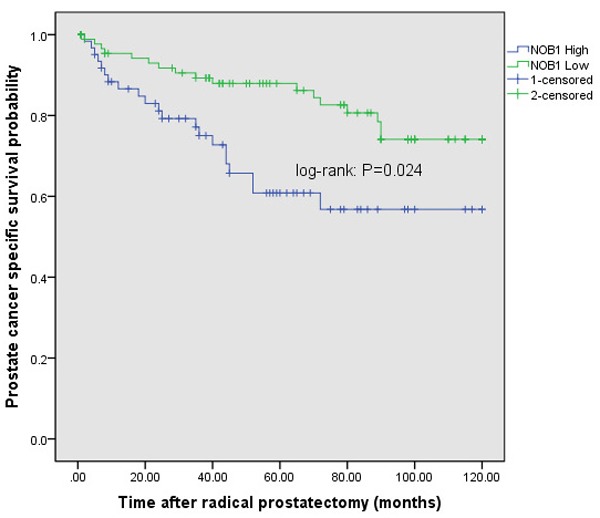
Cumulative survival curves of prostate carcinoma patients by NOB1 expression. NOB1 expression group showed significantly lower survival compared to NOB1 negative group.
Discussion
Novel strategies for the prevention of prostate cancer are highly desirable because prostate cancer continues to be the leading cause of cancer-related deaths among men in the United States [16]. Prostate cancer is usually diagnosed in the sixth or seventh decade of life, which allows a large window of opportunity for intervention to prevent or slow the progression of the disease [17,18]. In addition to conventional therapeutic strategies, targeted therapies are currently being developed to interfere with the transduction of key signaling pathways or to inhibit the function of tumor specific molecules in prostate cancer [19,20]. It is widely accepted that the future treatment options for Pca will greatly benefit from our improved understanding of the complex molecular mechanism in prostate cancer. Among them cancer therapy at the gene level through un derstanding of the molecular functions of cancer cell survival has gained more attention [20]. This study was focused to identification of an oncogenic target in prostate cancer cells and investigation of the effects of silencing the respective gene on prostate cancer cell proliferation. Our results showed that NOB1 is highly expressed in prostate cell lines and tissues, whereas its expression is decreased in normal prostate tissue. These findings suggest the therapeutic potential of NOB1 inhibition for Pca. Moreover, the expression of NOB1 might be associated with tumor grades as well as the prognosis of prostate cancer patients.
NOB1 gene was found as an oncogene responsible for the higher rates of proliferation in cancer cells [21]. In humans NOB1 gene is located on chromosome 16q22.1, composed of nine exons and 1,749 bp long. The translation product of NOB1, NOB1 protein is mainly localized in the nucleus of the mammalian cells. It has been found that NOB1 plays essential roles in proteasome biogenesis [22]. And several recent studies have reported that repression of NOB1 gene inhibit the growth of ovarian cancer and hepatocellular carcinoma [14,21]. Therefore, this study the level of NOB1 proliferation in highly malignant prostate cancer cell models PC-3 and DU-145 were investigated and the results showed that both cell lines expressed significantly high levels of NOB1. These significant higher levels of NOB1 expression was down regulated by transfecting with NOB1 siRNA in order to find whether NOB1 down regulation has any effect on the prostate cancer cell survival.
The MAPK signaling is mediated by p38 MAPK, ERK1/2 and JNK, which are important in the control of cell proliferation, differentiation and apoptosis [23]. Accumulating evidences reveal that the roles of MAPKs in these functions are controversial and complicated, that depend on the stimuli, intensity, and duration, as well as cell types [24]. For instance, under stimulation of wound healing, p38 and ERK1/2 coordinate the dynamics of the processes through inducing migration by p38 and enhancing proliferation by ERK1/2 activation in corneal epithelial cells [25]. JNK plays opposite roles in carcinogenesis, which is involved in induction of apoptosis, but also implicated in promotion of cell survival and proliferation [26]. Recently, the dual role of p38 MAPK in response to different stimulations has been demonstrated in fibroblast cells. Exposure to anisomycin causes cellular stress and induces strong and persistent p38 activation, leading to cell cycle arrest. In contrast, mitogenic stimulation by serum results in weaker and transient p38 activation to induce cyclin D1 and inactivate Rb by phosphorylation, leading to enhancing cancer cell proliferation [27].
To confirm the relationship of NOB1 and p38 based on prostate cancer tissues, immunnohistochemical studies were performed. We report NOB1 was significantly associated with the expression pattern of p38, it positively correlated to the expression of p38 in the nucleus or in the cytoplasm and inversely correlated to the staining of p38 on cell membrane, and thus the possibility whether MAPKs signal pathway could be controlled through NOB1 became of interest. It is thought that the activation of p38 through the regulation of NOB1 may widen the understanding of the development of prostate cancer and also the understanding of the progression of prostate cancer.
The experiments using prostate cancer cell lines performed to assess the relationship of NOB1 and p38 MAPK comprehensively showed the results slightly lower than expectation, and thus it is considered that the relation of the regulation of p38 by NOB1 assessed through immunohistochemical staining may require slightly different interpretation. NOB1 inference was induced in the prostate cancer cell lines expressing p38 strongly by the application of siRNA, and the alteration of the expression of p38 was examined. Noticeable reduction was observed in some cases; nonetheless, complete suppression was not shown, and in some cases, the expression of p38 was only slightly reduced, and thus it is considered to imply that in the expression of p38, factors other than NOB1 are also involved. In addition, in the Western blotting analysis performed in the absence of serum factors, NOB1 was reduced but the expression of p38 was rather elevated, which renders the hypothesis that the expression of p38 is influenced by NOB1 primarily doubtful.
For all this, it is thought that to accept directly the result of in vitro experiments as the theory of the development and progression of general tumors without additional validation procedures may be very limited. Nevertheless, based on our study performed on tumor tissues, it was confirmed that NOB1 and p38 MAPK play a very important role in the development, progression and metastasis process of prostate cancer [28]. Moreover, it could be confirmed that the effective regulation of the expression of p38 through NOB1 is the proposal applicable only to some limited cases of prostate cancer, and not applicable to all prostate cancer. Therefore, it was understood that also the theory concerning the development of the treatment method for prostate cancer through the regulation of NOB1 is applicable to certain very limited prostate cancer. In conclusion through this study identifies NOB1 as a critical gene in the prostate cancer prognosis. Collectively, this study showed potential future prospects of using NOB1 gene therapy as an effective prostate cancer treatment method.
Acknowledgements
This work was partially supported by grants from the National Natural Science Foundation of China (No. 81000311 and No. 81270831), and Shanghai Health Bureau Foundation (No. 03.02.12.009).
Disclosure of conflict of interest
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
Abbreviations
- Pca
Prostate carcinoma
- NOB1
Nin one binding protein 1
- ERK1/2
Extracellular signal-regulated kinase 1/2
- JNK
c-Jun NH2-terminal kinase
- PSA
Prostate-specific antigen
- f-PSA
Free prostate specific antigen
- IRB
Institutional Review Board
- IHC
Immunohistochemistry
- CI
Confidence interval
- RNAi
RNA interfere
- FBS
Fetal bovine serum
- PBS
Phosphate Buffered Saline
- AJCC
American Joint Committee on Cancer
- ATCC
American Type Culture Collection
- DU-145
a type of frequently-used prostate cancer cell line
- PC-3
a type of frequently-used prostate cancer cell line
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Klotz L. Re: editorial comment on focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study (lancet oncol 2012; 13: 622-632) J Urol. 2013;189:1601–1602. doi: 10.1016/j.juro.2012.11.087. [DOI] [PubMed] [Google Scholar]
- 4.Catalona WJ, D’Amico AV, Fitzgibbons WF, Kosoko-Lasaki O, Leslie SW, Lynch HT, Moul JW, Rendell MS, Walsh PC. What the U. S. Preventive Services Task Force missed in its prostate cancer screening recommendation. Ann Intern Med. 2012;157:137–138. doi: 10.7326/0003-4819-157-2-201207170-00463. [DOI] [PubMed] [Google Scholar]
- 5.Moyer VA U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 6.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 8.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 9.Chung LY, Tang SJ, Sun GH, Chou TY, Yeh TS, Yu SL, Sun KH. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res. 2012;18:4037–4047. doi: 10.1158/1078-0432.CCR-11-3348. [DOI] [PubMed] [Google Scholar]
- 10.Li XD, Liu ZY, Chang B, Liu DX, Chen B, Guo C, Wang YG, Xu JK, Huang DY, Du SX. Panax notoginseng saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK and P38 MAPK signaling pathways. Cell Physiol Biochem. 2011;28:367–376. doi: 10.1159/000331753. [DOI] [PubMed] [Google Scholar]
- 11.Jagielska J, Kapopara PR, Salguero G, Scherr M, Schutt H, Grote K, Schieffer B, Bavendiek U. Interleukin-1 assembles a proangiogenic signaling module consisting of caveolin-1, tumor necrosis factor receptor-associated factor 6, p38-mitogen-activated protein kinase (MAPK), and MAPK-activated protein kinase 2 in endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:1280–1288. doi: 10.1161/ATVBAHA.111.243477. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Ni J, Zhou G, Yuan J, Ren W, Shan Y, Tang W, Yu L, Zhao S. Cloning, expression and characterization of the human NOB1 gene. Mol Biol Rep. 2005;32:185–189. doi: 10.1007/s11033-005-3141-7. [DOI] [PubMed] [Google Scholar]
- 13.Veith T, Martin R, Wurm JP, Weis BL, Duchardt-Ferner E, Safferthal C, Hennig R, Mirus O, Bohnsack MT, Wohnert J, Schleiff E. Structural and functional analysis of the archaeal endonuclease Nob1. Nucleic Acids Res. 2012;40:3259–3274. doi: 10.1093/nar/gkr1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Xu T, Yan Y, Qin R, Wang H, Zhang X, Huang Y, Wang Y, Lu Y, Fu D, Chen J. MicroRNA-326 Functions as a Tumor Suppressor in Glioma by Targeting the Nin One Binding Protein (NOB1) PLoS One. 2013;8:e68469. doi: 10.1371/journal.pone.0068469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MT, Ouyang B, Ho SM, Leung YK. Differential expression of estrogen receptor beta isoforms in prostate cancer through interplay between transcriptional and translational regulation. Mol Cell Endocrinol. 2013;376:125–135. doi: 10.1016/j.mce.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 17.Choy B, Gordetsky J, Miyamoto H. Clinicopathologic features of prostate cancer in patients diagnosed by age 45 who underwent radical prostatectomy. Eur Urol. 2012;62:354–355. doi: 10.1016/j.eururo.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 18.van Vugt HA, Roobol MJ, van der Poel HG, van Muilekom EH, Busstra M, Kil P, Oomens EH, Leliveld A, Bangma CH, Korfage I, Steyerberg EW. Selecting men diagnosed with prostate cancer for active surveillance using a risk calculator: a prospective impact study. BJU Int. 2012;110:180–187. doi: 10.1111/j.1464-410X.2011.10679.x. [DOI] [PubMed] [Google Scholar]
- 19.Rajpar S, Fizazi K. Bone targeted therapies in metastatic castration-resistant prostate cancer. Cancer J. 2013;19:66–70. doi: 10.1097/PPO.0b013e31827f123e. [DOI] [PubMed] [Google Scholar]
- 20.Khatri A, Russell PJ. Targeted, gene-directed enzyme prodrug therapies to tackle diversity and aggression of late stage prostate cancer. Discov Med. 2007;7:39–45. [PubMed] [Google Scholar]
- 21.Lin Y, Peng S, Yu H, Teng H, Cui M. RNAi-mediated downregulation of NOB1 suppresses the growth and colony-formation ability of human ovarian cancer cells. Med Oncol. 2012;29:311–317. doi: 10.1007/s12032-010-9808-5. [DOI] [PubMed] [Google Scholar]
- 22.Pertschy B, Schneider C, Gnadig M, Schafer T, Tollervey D, Hurt E. RNA helicase Prp43 and its co-factor Pfa1 promote 20 to 18 S rRNA processing catalyzed by the endonuclease Nob1. J Biol Chem. 2009;284:35079–35091. doi: 10.1074/jbc.M109.040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turjanski AG, Vaque JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- 24.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 25.Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003;278:21989–21997. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- 26.Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinog. 2007;46:591–598. doi: 10.1002/mc.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faust D, Schmitt C, Oesch F, Oesch-Bartlomowicz B, Schreck I, Weiss C, Dietrich C. Differential p38-dependent signalling in response to cellular stress and mitogenic stimulation in fibroblasts. Cell Commun Signal. 2012;10:6. doi: 10.1186/1478-811X-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun B, Zhang X, Yonz C, Cummings BS. Inhibition of calcium-independent phospholipase A2 activates p38 MAPK signaling pathways during cytostasis in prostate cancer cells. Biochem Pharmacol. 2010;79:1727–1735. doi: 10.1016/j.bcp.2010.02.005. [DOI] [PubMed] [Google Scholar]