Abstract
Ligaments and tendons are comprised of tough yet flexible connective tissue. Little is known, however, about the precise characteristics of the cells in ligaments and tendons due to the absence of specific markers and cell lines. We recently reported a periodontal ligament cell line, PDL-L2, with suppressed Runx2/Osf2 transcriptional activity and an inability to form mineralized nodules. The present study demonstrates that the homeobox protein Msx2 is a key factor in suppressing those two functions. Msx2 colocalizes with Runx2/Osf2 and suppresses its activity cooperatively, acting with another corepressor, TLE1, as a complex to recruit histone deacetylase 1 activity. Reverse transcription-PCR and in situ hybridization demonstrated that Msx2 expression is higher in periodontal ligament and tendon cells than in osteoblasts. Stable reduction of Msx2 expression in PDL-L2 cells induces osteoblastic differentiation, thereby causing matrix mineralization. Conversely, stable, forced Msx2 expression in MC3T3-E1 cells prevented osteoblast differentiation and matrix mineralization. Msx2-induced suppression of osteoblast differentiation was repressed by bone morphogenetic protein 2. In addition, Msx2 was downregulated in a symptom- and calcification-dependent manner at the affected region in patients with ossification of the posterior longitudinal ligament. Our findings indicate that Msx2 plays a central role in preventing ligaments and tendons from mineralizing.
Ligaments and tendons are comprised of tough yet flexible connective tissue. In contrast to other cells of mesenchymal origin, such as myocytes, chondrocytes, osteoblasts, and adipocytes, however, little is known about the precise characteristics of cells in ligaments and tendons due to the absence of specific markers and cell lines. To characterize the nature and function of ligament and tendon cells, we focused on cells within the periodontal ligament, given the highly cellular nature of this structure, and we recently established a cell line, PDL-L2, which is indistinguishable from periodontal ligament fibroblasts in terms of gene expression profile (34). PDL-L2 cells share with osteoblasts the expression of genes for type I collagen, Runx2/Cbfa1/Osf2, and periostin but not the genes for bone sialoprotein (BSP) or osteocalcin (OCN). This profile of gene expression in PDL-L2 cells exactly matches that of the majority of periodontal ligament fibroblastic cells in vivo (34). Unlike osteoblastic MC3T3-E1 cells, however, PDL-L2 cells failed to form mineralized nodules in the absence of recombinant human bone morphogenetic protein 2 (rhBMP-2) despite significant expression of the alkaline phosphatase gene (ALP) and the Runx2/Osf2 gene (34). As Runx2/Osf2 plays a critical role in osteoblast differentiation and bone formation (6, 17), regulatory mechanisms may exist to prevent osteoblastic differentiation of periodontal ligament fibroblasts, thereby suppressing matrix mineralization.
Evidence is presented herein indicating the activity of just such a molecular mechanism, both in vivo and in vitro. Under cDNA microarray analysis, high expression of Msx2 was observed in PDL-L2 cells compared with that in MC3T3-E1 cells. In addition, Msx2 expression was maintained in PDL-L2 cells throughout the culture period in differentiation medium but was gradually downregulated in MC3T3-E1 cells as mineralized nodules were produced. Msx2 is a transcription factor with a homeobox domain and has been implicated in bone development. A missense mutation of Msx2 has been documented in humans affected with Boston-type craniosynoptosis (12). This mutation stabilizes the DNA binding of Msx2, causing a gain of function (23). On the other hand, Msx2 deficiency in mice causes defects in skull ossification and persistent calvarial foramen, which results from defective proliferation of osteoprogenitors at the osteogenic front during calvarial morphogenesis (36). This phenotype closely resembles that associated with human Msx2 haploinsufficiency in parietal foramina (43).
In contrast to these observations, ectopic Msx2 overexpression inhibits calvarial osteoblast differentiation and in vitro mineralization of osteoblasts, whereas Msx2 antisense RNA delivered with a retrovirus vector is stimulatory (4). It has also been shown that Msx2 suppresses the osteocalcin promoter (29, 30, 42). Furthermore, it was recently shown that Msx2 suppresses Runx2 transcriptional activity and mineralization in C2C12 cells (38). Using transgenic mice overexpressing the Msx2 gene under the control of a 5.2-kb segment of the Msx2 promoter which closely approximates the expression pattern of the endogenous Msx2 gene, Liu et al. provided evidence that Msx2 promotes proliferation of osteoprogenitors, which may be a mechanism of Msx2-mediated human craniosynostosis (22).
Although much of the attention has been focused on Msx2 function in early osteoblast differentiation, these observations suggest that Msx2 may have two different effects: one inhibiting osteoblast differentiation and promoting proliferation of osteoprogenitors in bone development and the other inhibiting mineralization of the extracellular matrix. The latter effect may not be appreciated in normal bone remodeling because Msx2 gene expression may be suppressed at the time of mineralization, as is seen in in vitro mineralization studies. However, this effect is the key to the function of ligaments and tendons, as is described in this study. We found that Msx2 interacts and colocalizes with Runx2/Osf2 and represses transcriptional activity by recruiting histone deacetylase 1 (HDAC1) activity. Msx2 repression was enhanced by TLE1, a human homolog of Drosophila Groucho protein and a repressor of Runx2. Moreover, stable expression of Msx2 antisense RNA in PDL-L2 cells induced matrix mineralization even in the absence of rhBMP-2, and expression of Msx2 was downregulated at the calcified region in patients with ossification of the posterior longitudinal ligament (OPLL). Taken together, these findings indicate that Msx2 plays a central role in preventing osteoblastic differentiation and mineralization of ligament fibroblasts by repressing Runx2/Osf2 transcriptional activity.
MATERIALS AND METHODS
Cell cultures.
The mouse periodontal ligament fibroblast cell line PDL-L2 (34) was cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Life Technologies Inc.) supplemented with 10% heat-inactivated FBS (Gibco-BRL) at 33°C and 5% CO2. C3H 10T1/2 (Human Science Research Resources Bank, Osaka, Japan), MC3T3-E1 (Riken Gene Bank, Wako, Japan), and ROS 17/2.8 (Yamanouchi Pharmaceutical Co. Ltd., Tokyo, Japan) cells were cultured in basal minimal essential medium (MEM) with 10% heat-inactivated fetal bovine serum (FBS), α-MEM with 10% FBS, and DMEM/F12 medium with 10% FBS, respectively. C2C12 (Riken Gene Bank) and COS-1 (Human Science Research Resources Bank) cells were cultured in DMEM with 10% FBS. For myogenic differentiation, C2C12 cells were cultured in DMEM with 2% horse serum (Sigma).
To obtain tendon fibroblasts, the Achilles tendon was aseptically removed from a 7-week-old C57BL/6J mouse and dissected longitudinally. Tissue explants were placed in 24-well tissue culture plates (Falcon, Nippon Becton Dickinson Co., Tokyo, Japan) containing DMEM supplemented with 10% heat-inactivated FBS. Explants were covered with coverslips (Nalge Nunc International) and incubated under conditions of 37°C and 5% CO2. Outgrown cells from the explants were used.
For stable cell lines, PDL-L2 and MC3T3-E1 cells were transfected with Msx2 antisense and sense expression plasmids, respectively. Cells were selected in 400-μg/ml G418 (Gibco-BRL), and single clones were chosen after 10 to 14 days and expanded.
Semiquantitative RT-PCR.
Extraction of cellular total RNA and reverse transcription-PCR (RT-PCR) were performed as previously described (34). Cycling conditions and primer sequences were as described previously (34), as follows. For the mouse Msx2, the conditions were 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s for 25 cycles and the primers were 5′-GCG CAA GTT CCG CCA GAA ACA GTA-3′ and 5′-GTG AGA GGA AAG GGG GCA TTT GGA-3′. For the mouse Osterix gene, the conditions were 94°C for 30 s, 62°C for 30 s, and 72°C for 75 s for 30 cycles, and the primers were 5′-GAA TTC CAC CAT GGC GTC CTC TCT GCT TG-3′ and 5′-CTC GAG TCA GAT CTC TAG CAG GTT GCT C-3′. For the mouse TLE1 gene, the conditions were 94°C for 30 s, 58°C for 30 s, and 72°C for 60 s for 26 cycles, and the primers were 5′-CCA CAT GCA AGC CCC ACA CAC-3′ and 5′-GCT TCC CCT CCC ACT ATG AGA GT-3′. For the human Msx2 gene, the conditions were 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s for 26 cycles, and the primers were 5′-GCG CAA GTT CCG TCA GAA ACA GTA-3′ and 5′-AAG AAA ACA GGG CTT GGT GCC TC-3′. For the human β-actin gene, the conditions were 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s for 13 cycles, and the primers were 5′-CAAGAG ATG GCC ACG GCT GCT-3′ and 5′-TCC TTC TGC ATC CTG TCG GCA-3′.
PCR products were fractionated on 1% agarose gels, transferred to positively charged nylon membranes, and cross-linked with UV light. Membranes were hybridized with digoxigenin (Roche Diagnostics)-labeled DNA probes and detected with CDP-Star substrate (New England BioLabs, Inc.).
In situ hybridization.
In situ hybridization with digoxigenin-labeled RNA probes was performed as previously described (34). Digoxigenin-UTP-labeled and biotin-UTP-labeled single-stranded RNA probes were prepared with a digoxigenin RNA labeling kit and biotin RNA labeling mix (Roche Diagnostics). For Msx2, corresponding cDNA fragments were obtained by RT-PCR with the above primers. Hybridization was carried out at 58°C for 16 h. Specific transcripts were detected with an alkaline phosphatase-conjugated antidigoxigenin antibody (digoxigenin detection kit; Roche Diagnostics). Sections were counterstained with methyl green. For immunofluorescence detection, Alexa Fluor 488 donkey anti-sheep immunoglobulin G (heavy and light chains) (Molecular Probes) was used as the secondary antibody for the antidigoxigenin antibody. For double-label in situ hybridization, we detected biotin-UTP-labeled RNA probes by streptavidin-horseradish peroxidase and indocarbocyanine-conjugated tyramide (PerkinElmer Life & Analytical Sciences Inc.).
Expression and reporter plasmids.
For a mammalian expression plasmid encoding Msx2 and TLE1, the RT-PCR-amplified mouse Msx2 and TLE1 open reading frames were subcloned into the pcDNA3 (Invitrogen). To generate an Msx2 antisense expression plasmid, the Msx2 open reading frame was subcloned into pcDNA3 in the reverse direction. The expression plasmid pcDNA3-Runx2/Osf2 and the reporter plasmid p6OSE2-Luc were generated as described previously (34). For Flag-tagged proteins, Runx2/Osf2, Msx2, and TLE1 were subcloned into pFLAG-CMV-5a (C-terminal Flag expression vector; Sigma) and pcDNA3-Flag (N-terminal Flag expression vector) generated with a PCR-based approach. The Msx2 mutant Msx2 T147A, in which the threonine at position 147 was replaced by alanine, was similarly generated. For Myc-tagged Msx2 and mutant Msx2, Msx2 and Msx2 T147A were subcloned into pcDNA3.1/myc-His A (Invitrogen). For glutathione S-transferase (GST) fusion proteins, fragments of Msx2 and Runx2 were generated by PCR and cloned in frame into pGEX-2T (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). All PCR-generated constructs were verified by sequencing.
Luciferase reporter assays.
Cells (5 × 104 cells per 35-mm dish) were transfected with various plasmid DNAs with Fugene-6 (Roche Diagnostics). Either 24 or 48 h after transfection, cells were harvested with reporter lysis buffer (Promega) for analysis of luciferase and β-galactosidase activities. For inhibition of histone deacetylase (HDAC), cells were treated with 500 nM trichostatin A (Sigma) at 6 h after transfection and harvested after 18 h of trichostatin A treatment. The luciferase and β-galactosidase assays were performed with a PicaGene system and an Aurora Gal-XE kit, respectively (Wako Pure Chemical Industries). All luciferase activity values were normalized for transfection efficiency against the β-galactosidase activities from the cotransfected pCMV-SPORT-βgal plasmid (Gibco-BRL). Each assay was performed in triplicate, and the data shown are representative of at least two experiments. The total amount of DNA was kept constant in these transfection and reporter assays.
Immunoprecipitation and GST interaction assays.
GST fusion proteins were expressed in Escherichia coli and then purified by adsorption to glutathione-Sepharose 4B (Amersham Pharmacia Biotech). All lysates were processed at 4°C. Cells at 80 to 90% confluence in a 100-mm cell culture plate were transfected with the indicated plasmid with Lipofectamine 2000 (Invitrogen). Cells were washed with ice-cold phosphate-buffered saline (PBS) at 24 to 48 h after transfection, collected, and lysed in 1 ml of TNE buffer (10 mM Tris-HCl [pH 7.8], 1% NP-40, 0.15 M NaCl, 1 mM EDTA, 10 μg of aprotinin per ml) for 30 min and passed through a 22-gauge needle six times. After centrifugation at 15,000 × g for 30 min, supernatants were mixed with anti-Flag M2 affinity resin (Sigma) or GST-Msx2 or GST-Runx2/Osf2 affinity resin for 2 h. Affinity resins were collected by centrifugation at 2,000 × g for 30 s and washed five times with 0.5 ml of TNE buffer. Coprecipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before electroblotting onto polyvinylidene difluoride membranes for Western blotting with the indicated antibodies.
Western blotting.
Preparation of cell extracts and Western blotting were performed as described previously (34). The antibodies used comprised a mouse monoclonal anti-Myc antibody (Invitrogen) at 1:5,000, a mouse monoclonal anti-Flag M2 antibody (Sigma) at 1:2,500, a rabbit polyclonal anti-Msx2 antibody (49) at 1:4,000, and a rabbit polyclonal anti-HDAC1 antibody (Cell Signaling Technology Inc.) at 1:1,000. Immunoblotting was visualized by chemiluminescence with the ECL+ kit (Amersham Pharmacia Biotech).
Double-label immunofluorescence microscopy.
PDL-L2 and C2C12 cells grown on Lab-Tek II chamber slides (Nalge Nunc International) were transfected with the Runx2/Osf2 expression vector and Myc-tagged Msx2 expression vector. Cells were rinsed twice with ice-cold PBS at 24 h after transfection and then fixed in 3.7% formaldehyde in PBS for 10 min on ice. After being rinsed twice with PBS, the cells were permeabilized in 0.1% Triton X-100 in PBS, rinsed twice with 0.5% bovine serum albumin in PBS (PBSA), and antibody stained. Antibody staining was performed by incubation with a rabbit anti-Runx2/Osf2 antibody at 1:200 and a mouse anti-Myc antibody (Invitrogen) at 1:500 for 1 h at 37°C. The cells were rinsed four times with PBSA before addition of the secondary antibody. The secondary antibodies used were Alexa Fluor 488-goat anti-mouse immunoglobulin G (heavy and light chains) and Alexa Fluor 546-goat anti-rabbit immunoglobulin G (heavy and light chains) (Molecular Probes). Cells were incubated with the secondary antibodies at 1:800 for 1 h at 37°C and then washed four times with PBSA.
Mineralization assay.
For the mineralization study, cells at confluence were cultured for 3 days in differentiation medium (α-MEM containing 5% FBS supplemented with 1 μM dexamethasone, 10 mM β-glycerophosphate, and 50 μg of ascorbic acid per ml) in the presence or absence of rhBMP-2 (kindly provided by Yamanouchi Pharmaceutical Co. Ltd.) and then cultured for an additional 25 days. Incubation media were changed every 3 days without further addition of rhBMP-2. Cells were then subjected to alizarin red-S staining and quantification for calcium as described previously (34).
OPLL patients.
In our institution, preoperative computed tomography is performed for all cases of OPLL. Samples of OPLL tissues and pathologically unremarkable PLL tissues were obtained from three patients receiving surgical treatment. Each tissue specimen was divided into two pieces after resection. For RNA extraction, one sample was immediately frozen in liquid nitrogen at the time of surgery and stored at −80°C until extraction was performed. The other sample was processed for pathological examination. Informed consent was obtained from each subject.
RESULTS
High level of Msx2 gene expression detected in ligament and tendon fibroblasts in vitro and in vivo.
A cell line of periodontal ligament fibroblasts, PDL-L2, has recently been established in which matrix mineralization is suppressed, although osteoblastic potential was retained (34) (see Fig. 5B). As Runx2/Osf2 transcriptional activity is significantly reduced in PDL-L2 cells (34) (see Fig. 2A), a key factor (or factors) may prevent osteoblastic differentiation in periodontal ligament fibroblasts. To identify such factors and specific genes in ligament fibroblasts, cDNA microarray analysis was undertaken with PDL-L2 cells and undifferentiated osteoblastic MC3T3-E1 cells.
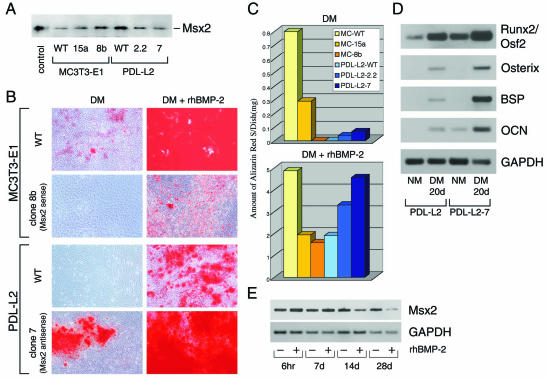
FIG. 5.
Stable modification of Msx2 expression causes an alteration in matrix mineralizing ability. Msx2 antisense and sense expression plasmids were stably transfected into PDL-L2 and MC3T3-E1 cells, respectively. (A) Western blot analysis of Msx2 protein was performed with nuclear extracts (30 μg) of each wild-type (WT) and selected clone. For controls, cell lysates of C3H 10T1/2 cells transfected with the Msx2 expression plasmid were used. (B) For the mineralization assay in each of the wild-type and stable transformants, cells were cultured for 28 days in differentiation medium (DM) in the presence or absence of 250-ng/ml rhBMP-2 and then subjected to alizarin red-S (AR-S) staining. (C) For quantifying the degree of mineralization, each stained culture was subjected to extraction, and aliquots of the alizarin red-S extract were used. Note that PDL-L2-7 cells (reduced Msx2 expression stably) could form mineralized nodules even in differentiation medium (B and C). (D) Expression of osteoblast differentiation markers in PDL-L2 and PDL-L2-7 cells was determined by semiquantitative RT-PCR analysis. Both cell lines were cultured in normal medium (NM) until 80% confluent and then for an additional 20 days in differentiation medium. Total RNA extracts from each cell line were used for analysis. (E) The expression pattern of Msx2 in PDL-L2 cells throughout the mineralization assays was determined by semiquantitative RT-PCR analysis. Cells at confluence were cultured for 6 h, 7 days, 14 days, or 28 days in differentiation medium in the presence or absence of 250-ng/ml rhBMP-2. See the text and Materials and Methods for details.
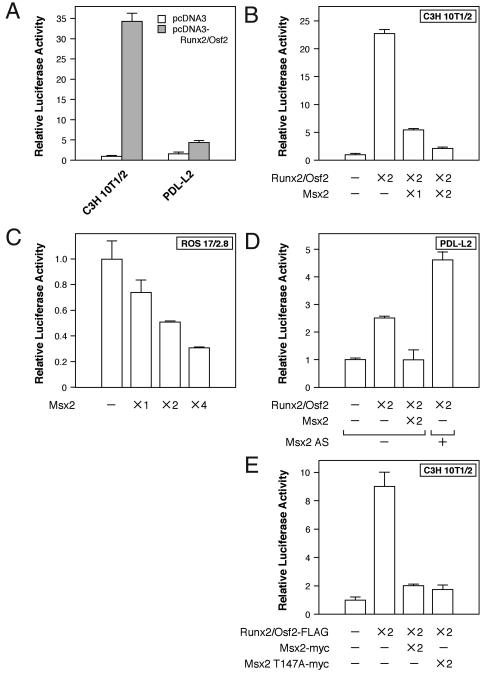
FIG. 2.
Msx2 repressed Runx2/Osf2 transcriptional activity. Transcriptional activity frome the OSE2 sequence of Runx2/Osf2 was assayed with luciferase reporter assays. Each cell line was transfected with p6OSE2-Luc, in which luciferase expression is controlled by six copies of the Runx2 binding OSE2 site of the OCN promoter, followed by the minimal promoter, and the indicated expression constructs. Luciferase activity was determined 24 h after transfection. The relative amount used for the transfection of each construct is indicated as ×N, where N is 1, 2, or 4. Note that the reduction of endogenous Msx2 by transfection with the Msx2 antisense (Msx2 AS) RNA expression plasmid (×2) enhanced Runx2/Osf2 transcriptional activity in PDL-L2 cells (D). See the text for details.
Microarray analysis revealed that the expression levels of about 100 genes differed between the cells. Among these differentially expressed genes, Msx2 demonstrated the greatest difference in expression levels between the two cell lines, being substantially increased in PDL-L2 cells. Expression of this gene was therefore examined in several cell lines and primary cultured cells by RT-PCR. Msx2 was highly expressed not only in PDL-L2 cells but also in a mixture of uncloned PDL cell and primary cultured mouse Achilles tendon cells (Fig. 1A). Expression of Msx2 was also detected in undifferentiated MC3T3-E1 cells, in the mesenchymal cell line C3H 10T1/2, and in the myoblastic C2C12 line. Interestingly, when MC3T3-E1 cells were cultured in differentiation medium, expression levels of Msx2 were dramatically suppressed (Fig. 1A). Msx2 expression was also downregulated when C2C12 cells were differentiated into myoblasts (Fig. 1A).
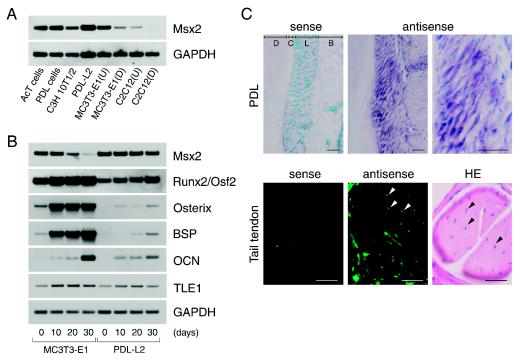
FIG. 1.
Expression of Msx2 gene was high and maintained in ligament and tendon fibroblasts. (A) Msx2 expression was determined by semiquantitative RT-PCR analysis. AcT cells, primary cultured Achilles tendon cells; PDL cells, culture of periodontal ligament cells containing a mixed cell population; MC3T3-E1(U), undifferentiated MC3T3-E1 cells; MC3T3-E1(D), fully differentiated osteoblastic MC3T3-E1 cells (mineralization stage); C2C12(U), undifferentiated C2C12 cells; C2C12(D), fully differentiated myoblastic C2C12 cells (myotube stage). AcT, PDL, C3H 10T1/2, PDL-L2, MC3T3-E1(U), and C2C12(U) cells were used at 80% confluence. (B) Expression patterns of Msx2, TLE1, and osteoblast differentiation markers throughout the mineralization experiments were determined by semiquantitative RT-PCR analysis in PDL-L2 and MC3T3-E1 cells. Cells at confluence (0 day) were cultured for 10, 20, or 30 days in differentiation medium (DM). The incubation medium was changed every 3 days. (C) In vivo Msx2 expression was detected in periodontal ligament and tail tendon fibroblasts from 7-week-old C57BL/6J mice by in situ hybridization. Immunofluorescence detection was performed on the tail tendon in order to increase detection sensitivity, as tendon fibroblasts (arrowhead) are sparse and thin in this tissue. Strong, diffuse fluorescent signals represent the sums of several signals coming from above and below the focal plane. D, dentin; C, cementum; L, ligament; B, alveolar bone; HE, hematoxylin-eosin stain. Bar, 40 μm.
To examine whether the downregulation of Msx2 expression represents a general feature of cell differentiation, further analysis of the expression patterns of Msx2 and osteoblast differentiation markers was undertaken in PDL-L2 and MC3T3-E1 cells throughout the period of culture in differentiation medium. Runx2/Osf2 was abundantly expressed in PDL-L2 cells, but the expression levels were lower than in MC3T3-E1 cells. Expression levels increased with time (Fig. 1B). In contrast, expression of the downstream genes Osterix (28), BSP, and OCN was undetectable at day 0 and remained markedly suppressed at day 30 (Fig. 1B). Interestingly, Msx2 expression levels were maintained in PDL-L2 cells throughout the experiment but were gradually downregulated concomitant with osteoblastic differentiation in MC3T3-E1 cells, becoming barely detectable by day 30, when the formation of mineralized nodule was peaking (Fig. 1B).
Further investigation into whether Msx2 was expressed in ligament and tendon fibroblasts was conducted in vivo. Msx2 was present in mouse periodontal ligament fibroblasts, tail tendon fibroblasts (Fig. 1C), and Achilles tendon fibroblasts (data not shown), as determined by in situ hybridization. Previous studies have demonstrated that Msx2 plays important roles in cell proliferation (4, 8, 11, 12, 22, 36, 43) and in repressing BSP (2) and OCN (29, 42, 47) expression in the early stages of osteoblastic differentiation in vitro. The phenotypes of Msx loss- and gain-of-function mutations are also consistent with roles for Msx genes in regulating cellular proliferation and differentiation in vivo (12, 13, 36, 43, 45). Taken as a whole, our findings indicate that Msx2 is a good candidate for the key factor repressing Runx2/Osf2 activity, thereby inhibiting matrix mineralization in ligament and tendon fibroblasts.
Endogenous Msx2 represses Runx2/Osf2 transcriptional activity in PDL-L2 cells.
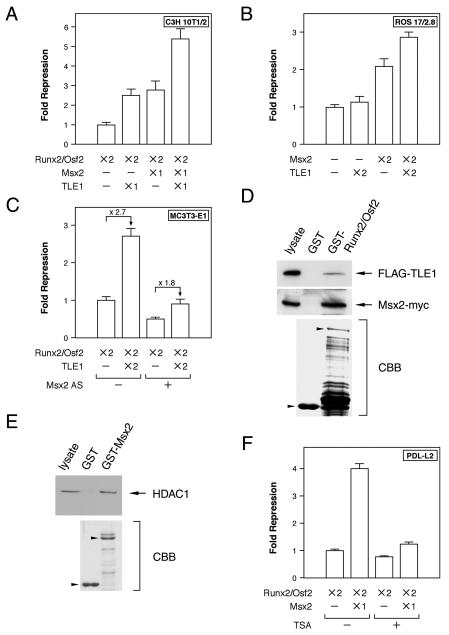
The luciferase reporter assay and Runx2/Osf2-binding OSE2 sites (3) were next utilized to investigate whether Msx2 represses Runx2/Osf2 transcriptional activity. Initial tests were conducted in C3H 10T1/2 cells, which have moderate Msx2 expression and are devoid of significant amounts of Runx2/Osf2. Forced expression of Runx2/Osf2 markedly induced transcription of Runx2 through OSE2 sites in C3H 10T1/2 cells (Fig. 2A), while Msx2 repressed transcriptional activation in a dose-dependent manner (Fig. 2B). A similar result was obtained for osteoblastic ROS 17/2.8 (Fig. 2C) and PDL-L2 (Fig. 2D) cells, but substantially more Msx2 was required to repress endogenous Runx2/Osf2 transcriptional activation in ROS 17/2.8 cells (Fig. 2C). This was probably due to the combination of large amounts of Runx2/Osf2 and only small amounts of Msx2 present in the ROS 17/2.8 cells (data not shown). These findings suggest that exogenous Msx2 represses Runx2/Osf2 activity through OSE2 sites, with the efficiency depending on the ratio of Msx2 and Runx2/Osf2 expressed in each cell line. Our observation is in good agreement with the recent study of Shirakabe et al. demonstrating that Msx2 represses Runx2 activity in vitro (38).
Next, to confirm whether endogenous Msx2 actually represses Runx2/Osf2 transcriptional activity, an Msx2 antisense RNA expression plasmid was introduced into PDL-L2 cells, to reduce the amount of endogenous Msx2. Transcriptional activity was then determined. As expected, Msx2 antisense RNA in PDL-L2 cells enhanced Runx2/Osf2 transcriptional activation (Fig. 2D). Previous studies have reported Msx2 suppression of OCN transcription independent of homeodomain DNA binding, acting instead via protein-protein interactions (29, 30, 47). We therefore tested whether Msx2 could repress Runx2/Osf2 transcriptional activation from OSE2 sites without binding to DNA. An Msx2 point mutant (Msx2 T147A) that completely lacks DNA binding activity (30) was found to be indistinguishable from wild-type Msx2 as a repressor of Runx2/Osf2 (Fig. 2E). These results suggest that endogenous Msx2 in PDL-L2 cells represses Runx2/Osf2 activity via protein-protein interactions. Similar observations have been reported earlier (38).
Msx2 interacts and colocalizes with Runx2/Osf2.
To confirm the protein-protein interactions between Msx2 and Runx2/Osf2 in cells, we first investigated their physical association by combining Flag-tagged Runx2/Osf2-expressing C3H10T1/2 cell extracts with GST-Msx2 affinity resin. We found a robust and specific interaction between Runx2/Osf2 and the GST-Msx2 resin (Fig. 3A). Next, a coimmunoprecipitation analysis was performed. Coexpression of Myc-tagged Msx2 and Flag-tagged Runx2/Osf2 in C2C12 cells and immunoprecipitation with a monoclonal anti-Flag antibody indicated a physical interaction of these proteins in vivo (Fig. 3B, upper panel). Conversely, no protein was detected in immunoprecipitates from cells transfected with an empty vector (Fig. 3B, middle panel), but similar amounts of Myc-tagged Msx2 protein were present in each transfected cell population (Fig. 3B, lower panel). These data indicate that Msx2 forms a complex with Runx2/Osf2.
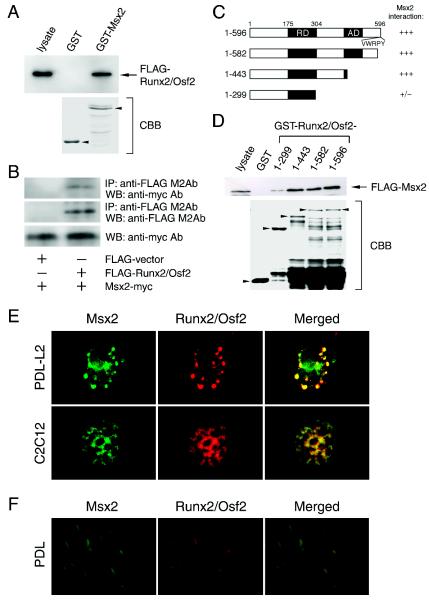
FIG. 3.
Msx2 interacts and colocalizes with Runx2/Osf2. (A) The interaction of Msx2 with Runx2/Osf2 was assessed by GST interaction. Flag-tagged Runx2/Osf2-expressing C3H10T1/2 cell lysates were combined with a GST-Msx2 fusion protein and tested for the presence of Runx2/Osf2 with a Flag antibody. (B) The interaction of Msx2 with Runx2/Osf2 was assessed in coimmunoprecipitation assays. Cell lysates from transfected C2C12 cells expressing the indicated tagged proteins were used for immunoprecipitations (IP), followed by Western blotting (WB) with the indicated antibody (Ab). (C) Schematic representations of Runx2/Osf2 deletion mutants expressed as GST fusion proteins. (D) Flag-tagged Msx2-expressing C3H10T1/2 cell lysates were combined with the various GST-Runx2/Osf2 protein indicated in C. An anti-Flag antibody was used to detect the presence of Msx2. Photographs of Coomassie brilliant blue (CBB)-stained gels are shown to confirm the integrity and nearly equal loading of the fusion proteins. (E) Colocalization of Msx2 with Runx2/Osf2 was assessed by double-label immunofluorescence microscopy. Runx2/Osf2 was cotransfected with Myc-tagged Msx2 into PDL-L2 and C2C12 cells, and the cells were stained with anti-Runx2/Osf2 and anti-Myc antibodies. (F) In vivo expression of Msx2 and Runx2/Osf2 was detected in periodontal ligament fibroblasts of 7-week-old C57BL/6J mice by double-label in situ hybridization. See the text for details.
Runx2/Osf2 proteins reside in unique subnuclear foci, colocalizing with transcriptional coregulators that activate or repress Runx-dependent genes (13, 46). To assess this possibility, double-label immunofluorescence microscopy was performed with PDL-L2 and C2C12 cells (Fig. 3E, upper and lower panels, respectively). Furthermore, the coexpression of Runx2/Osf2 and Msx2 in the same periodontal ligament cells was shown by double-label in situ hybridization (Fig. 3F). These results indicate that Msx2 forms a complex with Runx2/Osf2, thereby repressing transcriptional activity.
Msx2 and TLE1 cooperatively repress Runx2/Osf2 transcriptional activity by recruiting HDAC complex.
Since other Runx2-inhibitory cofactors have also been identified (7), we next examined whether Msx2-induced repression of Runx2/Osf2 activity also involved these cofactors. TLE, a mammalian homologue of the Drosophila Groucho protein, is expressed in osteoblasts and inhibits the transactivation function of Runx2, mainly through interaction with the VWRPY motif (1, 20, 39, 41). We observed that TLE1 expression was maintained in MC3T3-E1 and PDL-L2 cells throughout the culture period in mineralization medium (Fig. 1B), whereas TLE2 was barely expressed in PDL-L2 cells (data not shown). Therefore, we first examined the repressive effects of Msx2 and TLE1 on Runx2 transcriptional activity in the luciferase reporter assay. Msx2 and TLE1 caused equivalent repression of Runx2 when either was cotransfected with Runx2 into C3H10T1/2 cells (Fig. 4A), and their repressive activities were additive (Fig. 4A). When ROS 17/2.8 cells, which express only small amounts of Msx2, were similarly treated, TLE1 alone failed to repress Runx2/Osf2 activity, whereas the effect of Msx2 was similar to that in C3H 10T1/2 cells. Furthermore, TLE1 further enhanced the Msx2-mediated repression of Runx2 activity (Fig. 4B). Moreover, a transient reduction of Msx2 expression in MC3T3-E1 cells decreased the efficiency of TLE1-mediated repression (Fig. 4C). These data suggest that Msx2 is a key regulator of Msx2-TLE1-mediated repression of Runx2/Osf2 activity.
FIG. 4.
Msx2 and TLE1 cooperatively repress Runx2/Osf2 transcriptional activity by recruiting the HDAC complex. (A to C and F) Transcriptional activity from the OSE2 sequence of Runx2/Osf2 was assayed as in Fig. 2. Each cell line was transfected with p6OSE2-Luc and the indicated expression constructs. As for Msx2 antisense, the same amount (×2) was used for transfection. (D) Flag-TLE1 and Msx2-Myc expression constructs were cotransfected into COS-1 cells. Lysates of these cells were combined with a GST-Runx2/Osf2 affinity resin and tested for the presence of TLE1 and Msx2 with an anti-Flag and an anti-Myc antibody, respectively. (E) Interaction of Msx2 and HDAC1 was assessed by a GST interaction assay with GST-Msx2 and C3H10T1/2 cell lysates, followed by Western blotting with an anti-HDAC1 antibody. (F) PDL-L2 cells were treated with 500 nM trichostatin A (TSA) at 6 h after transfection and harvested after 18 h of trichostatin A treatment for luciferase and β-galactosidase assays.
We next determined whether Runx2/Osf2 was able to form a complex with Msx2 and TLE1. A GST interaction assay with deletion mutants of Msx2 demonstrated that Msx2 and TLE1 were isolated together on GST-Runx2/Osf2 affinity resin (Fig. 4D) and that the region between amino acid 299 and 443 of Runx2/Osf2, which differs from the TLE interaction region containing the VWRPY motif, was required for Msx2 interaction (Fig. 3C and D). HES1, a basic helix-loop-helix protein, also binds to the repressor domain of the Runx2 and inhibits Runx2 activity. Interestingly, HES-1 and TLE compete for binding to Runx2 (26, 39). Thus, Msx2 is the first example of a molecule that acts as a repressor as well as a corepressor of Runx2.
Previous reports have shown that Msx3, a member of the Msx family, can recruit and associate with HDAC1 to form a complex that contains no cyclic AMP response element-binding protein-binding protein (CBP). This complex, in turn, suppresses Msx1 promoter activity (27). We therefore tested whether HDAC1 activity was also required for Msx2-mediated repression of Runx2/Osf2 activity. C3H10T1/2 cell lysates were combined with GST-Msx2 affinity resin and tested for the presence of HDAC1. It was found that Msx2 can recruit HDAC1 (Fig. 4E) and that trichostatin A, an HDAC inhibitor, relieved the Msx2-induced repression (Fig. 4F). On the basis of these data, we conclude that Msx2 and TLE1 cooperatively repress Runx2/Osf2 transcriptional activity by recruiting the HDAC complex.
Stable reduction of Msx2 expression in PDL-L2 cells induces osteoblastic differentiation, thereby causing matrix mineralization.
PDL-L2 cells fail to form mineralized nodules if rhBMP-2 is absent from the differentiation medium (34). If this is due to the maintenance of high levels of Msx2 mRNA expression throughout the culture period (Fig. 1B), stable reduction of Msx2 expression should induce matrix mineralization in PDL-L2 cells in the absence of rhBMP-2 in the differentiation medium. To test this possibility, sense and antisense Msx2 RNAs were stably transfected into the MC3T3-E1 and PDL-L2 cell lines, respectively. Expression of Msx2 in each clone was verified by Western blot analysis. Expression of Msx2 was enhanced in MC3T3-E1-15a and MC3T3-E1-8b cells but was reduced in PDL-L2-2.2 and PDL-L2-7 cells (Fig. 5A).
Mineralization assays were subsequently performed with these clones. As predicted, PDL-L2-7 cells with reduced expression of Msx2 formed mineralized nodules in differentiation medium lacking rhBMP-2 (Fig. 5B and C). In contrast, MC3T3-E1-8b cells were unable to produce mineralized nodules in the differentiation medium (Fig. 5B and C). This mutant cell line, like the PDL-L2 cells, was still able to produce mineralized nodules in the presence of rhBMP-2. The degree of mineralization in the PDL-L2 and MC3T3-E1 mutants was inversely proportional to the level of Msx2 expression (Fig. 5A and C). To exclude the possibility that manipulation of recloning in the PDL-L2-7 cell line had altered the cell characteristics in such a way as to allow formation of mineralized nodules, a stable lacZ transformant was obtained simultaneously as a control. As with PDL-L2 cells, no mineralized nodules were formed in PDL-L2-lacZ cells cultured in differentiation medium (data not shown).
Furthermore, we investigated whether the expression of Runx2/Osf2 and its downstream genes in PDL-L2-7 cells was upregulated compared to that in the wild type. In PDL-L2-7 cells, expression of Runx2/Osf2, Osterix, BSP, and OCN was strikingly enhanced in differentiation medium at 20 days (Fig. 5D). Basal levels of Runx2/Osf2 and OCN expression were also increased (Fig. 5D). The increased basal level of Runx2 expression in PDL-L2-7 cells can be explained by an autoregulatory mechanism: when antisense Msx2 cancels Msx2-mediated repression of Runx2 activity, Runx2 becomes activated. Once activated, Runx2 immediately induces upregulation of its own expression. Such a mechanism has been reported previously (7). Stable reduction of Msx2 expression thus enables ligament fibroblasts to undergo osteoblastic differentiation, subsequently inducing matrix mineralization. In addition, PDL-L2-7 cells acquired the potential for adipocytic differentiation, as determined by Oil-Red O staining and expression of PPARγ2 (data not shown).
rhBMP-2 enhances matrix mineralization in PDL-L2 cells by suppressing Msx2 gene expression.
As described above, the maintenance of Msx2 expression was shown to be important for preventing matrix mineralization in ligament fibroblasts (Fig. 5A to D). The potential for repression of Msx2 gene expression by rhBMP-2, which can induce matrix mineralization even in PDL-L2 cells, was then demonstrated (Fig. 5B and C). Previous reports, however, revealed that BMP enhances Msx2 gene expression in the limb bud (43). The effect of rhBMP-2 on Msx2 gene expression was therefore examined in PDL-L2 cells. Expression of Msx2 mRNA was gradually reduced over 14 days in differentiation medium (Fig. 5E), exactly as had been observed in MC3T3-E1 cells (Fig. 1B). These results indicate that Msx2 mRNA should be downregulated following the middle stage of differentiation for osteoblasts to mature fully and to allow maximal mineralization of the extracellular matrix. If insufficient downregulation of Msx2 occurs after the middle stage of osteoblastic differentiation, as in the case of rhBMP-2-treated PDL-L2 cells, only a limited number of mineralized nodules are formed (Fig. 5B and C). Stable expression of Msx2 reduces the amount of mineralized nodules by about two-thirds, even in rhBMP-2-treated MC3T3-E1 cells (Fig. 5B and C, compare MC3T3-E1 with MC3T3-E1-7B). Our results are consistent with a previous report that retrovirus-mediated Msx2 overexpression inhibits chicken calvarial osteoblasts (4) but are in contrast to the observation that retrovirus-mediated Msx2 overexpression stimulates osteogenesis and mineralization of mesenchymal C3H10T1/2 cells (3).
Expression of Msx2 gene downregulated in calcified regions of patients with OPLL of the spine.
The importance of Msx2 to ligament fibroblasts was demonstrated in vitro, as described above. As the periodontal ligament never calcifies in vivo, we were inclined to believe that the same mechanisms worked in vivo. To test this thesis, we investigated whether an alteration of Msx2 expression was associated with the onset and/or progression of human diseases such as ossification of the posterior longitudinal ligament (OPLL). This disease causes ectopic ossification of the ligament attached to the dorsal surface of the vertebral bodies and in severe cases can result in paralysis through compression of the spinal cord. OPLL is not uncommon in Japan, with a reported prevalence of 1.5 to 2.4% in adults (24). OPLL is also closely associated with diffuse idiopathic skeletal hyperostosis in European populations, occurring in 43 to 50% of individuals with diffuse idiopathic skeletal hyperostosis (24, 33). OPLL may therefore also not be a rare disease in European populations. Since the clinical features of OPLL are variable, the disease has been considered the collective result of heterogenous factors. To understand the mechanisms of OPLL onset and to identify ways to treat patients, knowledge of the common cellular mechanisms that result in the pathology is absolutely essential, irrespective of the factors involved.
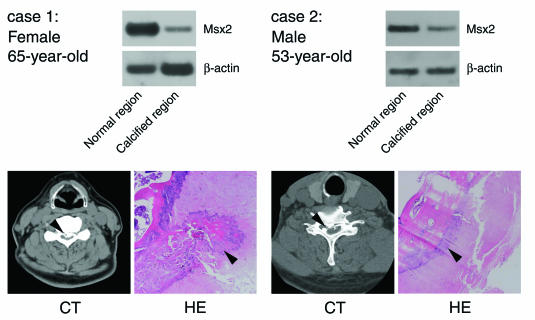
To investigate the expression of Msx2 mRNA in OPLL patients, ligament tissues were obtained from the calcified regions and adjacent normal regions of three OPLL patients during orthopedic operations. Case 1 displayed numbness of the upper and lower limbs, dysesthesia, and difficulty in walking. Cases 2 and 3 exhibited difficulty in walking only. As shown by computed tomography and in histological sections (Fig. 6), the posterior longitudinal ligament of the spine had undergone ossification in these patients. Of note is the fact that expression of Msx2 mRNA in the calcified region of the ligament was significantly reduced compared to that in the adjacent normal region in all cases (Fig. 6), although the degree of ossification was much higher in case 1. A strong correlation was observed between the degree of calcification, symptoms, and suppression of Msx2 expression. These results confirm that reduced Msx2 expression is commonly associated with OPLL. As a whole, our results indicate that Msx2 represents a key regulator for the inhibition of matrix mineralization in ligament fibroblasts, both in vitro and in vivo.
FIG. 6.
Expression of Msx2 mRNA in calcified regions of ligament was reduced compared to expression in normal regions in patients with ossification of the posterior longitudinal ligament (PLL). Semiquantitative RT-PCR analysis of Msx2 expression was performed with ligament tissues at the calcified and adjacent normal region of the PLL from the spine of patients with OPLL. Computed tomography (CT) and histological sections stained with hematoxylin and eosin (HE) revealed that a portion of the PLL from each patient had been ossified.
DISCUSSION
The present report demonstrates the crucial role played by Msx2 in preventing osteoblastic differentiation of ligament fibroblasts by repressing Runx2/Osf2 transcriptional activity. Moreover, the results suggest that factors capable of repressing Msx2 expression may be a cause of pathological ossification of ligaments in diseases such as OPLL.
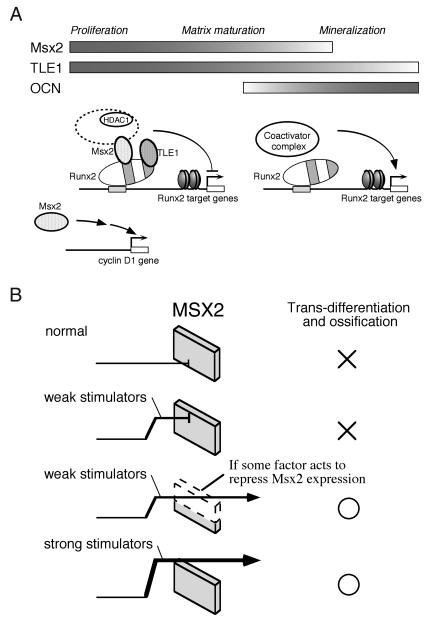
Msx loss- and gain-of-function mutations cause profound defects in mammary gland and cranial development that have been interpreted as arrested differentiation (12, 21, 36, 43, 45). Hu et al. demonstrated that Msx maintains cyclin D1 expression and prevents exit from the cell cycle (Fig. 7A), thereby inhibiting terminal differentiation of progenitor cells (11). This model, first proposed by Dodig et al. (4), not only provides a basis for reconciling the observed similarities between the phenotypes of Msx loss- and gain-of-function mutations (11, 12, 21, 36, 43, 45) but also explains the most seemingly contradictory observation to the present study, i.e., premature suture closure and ectopic cranial bone formation in mice expressing Msx2 transgenes in the developing skull (21), suggesting that Msx2 actually promotes bone formation.
FIG. 7.
Model for Msx2 function in osteoblasts and ligament fibroblasts. (A) Runx2/Osf2 transcriptional activity is cooperatively repressed by two corepressors, Msx2 and TLE1, recruiting the HDAC complex. Attenuation of Msx2 expression after the middle stage of osteoblast differentiation is important for further Runx2/Osf2 activation, which induces differentiation and maximal matrix mineralization. Msx2 also has a direct function on the cell cycle by regulating target genes such as cyclin D1. (B) In ligament fibroblasts, Msx2 acts as a molecular defense mechanism for preventing ossification, as expression of the Msx2 gene is highly maintained. See the Discussion for details.
When overexpression of the Msx2 gene was produced by means of a 5.2-kb segment of the Msx2 promoter, the pattern and time course of expression of overexpressed Msx2 closely approximated that of the endogenous Msx2 gene, and the promoter activity was reduced postnatally. This means that the promoter worked sufficiently well perinatally to enhance osteoblastic cell differentiation to a point just before terminal differentiation and that, as Msx2 expression decreased, the osteoblastic cells became fully differentiated and ready to mineralize bone, resulting in premature fusion of the suture (22). This process exactly matches the present observation that a deficiency in Msx2 expression induced differentiation of PDL-L2 cells, leading to mineralization (Fig. 5).
In addition to its function in the cell cycle, previous studies have shown that Msx2 directly represses gene expression of differentiation markers, such as OCN (20, 26, 41). Here, we have provided evidence that Runx2/Osf2 transcriptional activity is repressed cooperatively by two corepressors, Msx2 and TLE1, and that HDAC activity is required for the repression (Fig. 7A). Msx2-mediated repression of Runx2/Osf2 may thus be attributable to a modulation of chromatin structure. An inducible cofactor, Msx2-interacting protein (Mint), may also be involved in Msx2-induced repression, as reported by Newberry et al. (31) and Shi et al. (37). In osteoblasts, Msx2 attenuation after the middle stage of its differentiation is important for maximal matrix mineralization (Fig. 5 and 7A). This is consistent with previous data in which retrovirus-mediated overexpression of sense and antisense Msx2 prevented and stimulated, respectively, the mineralization of chicken primary osteoblast cultures (4). In a somewhat contradictory study, Cheng et al. showed that retrovirus-induced overexpression of Msx2 suppressed adipogenic differentiation and promoted osteoblastic differentiation of the mesenchymal progenitor C3H10T1/2 cells, leading to matrix mineralization (3). It is possible that the remaining nontransfected cells (10%) were responsible for the mineralization via some agent(s) secreted into the medium in that study (3). Alternatively, a different mechanism may be working in PDL-L2 and C3H10T1/2 cells, because Msx2 T147A, a mutant lacking DNA binding ability, retained its suppressive effect on Runx2 activity in our system, whereas it lost its osteogenesis-promoting effect in the study of Cheng et al. (3).
These observations suggest the intriguing possibility that Msx2 may promote osteoblast differentiation by directly binding to DNA and may inhibit mineralization by binding to Runx2 through protein-protein interaction. Specific key factors may be involved in each case, and they are certainly the targets for future studies. Although we showed that Msx2 directly associates with and suppresses the transcriptional activity of Runx2, it is possible that Msx2 also inhibits the Runx2 and Ku-Tbdn100 transcription complex, as reported by Willis et al. (44). It has also been demonstrated that Stat1 in the cytoplasm binds to and prevents the nuclear localization of Runx2, thereby attenuating Runx2 activity (14). This observation is in accord with the fact that Runx2 activity must be suppressed for mineralization to occur (16). We do not know at present if Msx2 suppression of mineralization is related to this interesting observation. It is unlikely, however, that the same mechanism is working in ligament and tendon cells, because these cells are not mineralized under normal physiological conditions during life and because Msx2 suppression of Runx2 takes place in the cells where the two factors colocalize in the nuclei (Fig. 3E).
In the present study, we have shown that maintenance of Msx2 at a higher level prevents PDL-L2 cells from mineralizing and that forced downregulation of Msx2 expression blocks this mechanism, allowing the extracellular matrix to be mineralized. Conversely, Msx2 expression decreased as mineralization of the osteoblast culture progressed, and forced overexpression of Msx2 in osteoblasts prevented mineralization. Therefore, we conclude that Msx2 must be downregulated to allow the extracellular matrix to be mineralized. Thus, a transcription factor or cofactor must exist to suppress Msx2 expression at the middle stage of osteoblast differentiation and thereafter, possibly representing a third key factor acting downstream of Runx2 and Osterix for the differentiation and maturation of osteoblasts. The identification of molecules capable of maintaining Msx2 expression in ligament fibroblasts represents a valuable goal for future studies.
Based on our data, two categories of factors that enable ligament fibroblasts to generate ossification appear to exist. One category comprises intrinsic stimulators of osteoblastic differentiation, such as BMP-2. The other comprises factors that reduce Msx2 expression. Moreover, we propose that Msx2 in ligament fibroblasts acts as a molecular defense mechanism, suppressing signals from stimulators that would otherwise induce osteoblastic differentiation. In the ordinary in vitro situation or in the normal in vivo microenvironment, this molecular defense mechanism works perfectly (Fig. 7B). Weak stimulators such as differentiation medium are unable to overcome this mechanism, and thus ligament fibroblasts fail to form mineralized nodules due to the high expression level of Msx2 (Fig. 7B). If some factor acts to repress Msx2 expression, however, even weak stimulators can induce ligament fibroblasts to form mineralized nodules (Fig. 5 and 7B). That is, the level of Msx2 seems to decide the threshold for ossification in the ligament. In contrast, if ligament fibroblasts are exposed to strong stimulators such as BMP-2, the defense mechanism is overridden (Fig. 7B). Previous reports have shown that signal transducers of the transforming growth factor beta-BMP superfamily receptors (Smads) interact with Runx2 in vitro and in vivo and enhance the transactivation ability of this factor (10, 19, 32, 48). BMP-induced Runx2-Smad interactions may thus relieve Runx2 from the Msx2-dependent repression complex. Further analysis is obviously required to fully elucidate the relationships between Runx2, Msx2, and the BMP signaling pathways.
Similar to the in vitro situation, ligaments and tendons display prominent expression of Msx2 (Fig. 1C) and do not ossify in vivo under normal circumstances. However, if the expression of Msx2 is decreased, the defense mechanism is compromised and the tissues become susceptible to signals inducing ossification. Indeed, this turned out to be the case with OPLL. Expression of Msx2 was downregulated in the pathologically calcified regions of all three cases of OPLL examined in the present study (Fig. 6). Unfortunately, when or at what stage Msx2 expression was repressed in these affected ligaments is both unclear and undeterminable. However, once Msx2 expression is reduced, suppression of matrix mineralization ceases, which in turn reduces the threshold for OPLL onset. Thus, factors actually modulating Msx2 expression in addition to strong stimulators of osteoblast differentiation represent good candidates for OPLL risk factors. In addition, OPLL displays a strong genetic background, although the mean age of onset is about 50 years. Several OPLL genetic backgrounds have been analyzed in case-control association studies, and the contribution of genetic factors is likely to differ with sex, age, and severity of ossification (9, 15, 18, 25, 35, 40). Further study of the potential relationships between OPLL and genetic polymorphisms of Msx2 and related genes is thus warranted.
It would have been interesting to see how ligaments were affected in Msx2-deficient mice. Unfortunately, no data are available yet. Because Msx2 is necessary for recruiting osteoprogenitors, as described above, it may not be possible to examine the ossification of mesenchymally derived cells other than osteoblasts in Msx2-deficient mice. Conditional knockout or knockin experiments will be necessary to confirm our hypothesis, and we, of course, would like to do such experiments. However, that is well beyond the scope of the present study.
In conclusion, the present study demonstrated that endogenous Msx2 prevents ligament fibroblasts from undergoing osteoblastic differentiation by repressing Runx2/Osf2 transcriptional activity and that reduced Msx2 expression can be associated with human pathologies such as OPLL. To the best of our knowledge, this represents the first report of the molecular mechanisms by which ligament fibroblasts prevent the onset of ossification despite having osteoblastic potential.
Acknowledgments
We are grateful to Paul Denny and Gerard Karsenty for generous gifts of the antibodies for Msx2 and Runx2/Osf2, respectively, and to Masato Kobori of Yamanouchi Pharmaceutical Co., Ltd., for invaluable technical advice and discussion.
This work was supported in part by the Novartis Foundation (Japan) for the Promotion of Science (T.Y.), by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (nos. 10470388, 12470389, and 14370591 to H.K.), a grant from Ground Research for Space Utilization promoted by the National Space Development Agency and Japan Space Forum, and by grants from Yamanouchi Pharmaceutical Co., Ltd., and Novartis Pharma Japan.
REFERENCES
- 1.Aronson, B. D., A. L. Fisher, K. Blechman, M. Caudy, and J. P. Gergen. 1997. Groucho-dependent and -independent repression activities of Runt domain proteins. Mol. Cell. Biol. 17:5581-5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, G. L., A. Javed, S. M. Walker, M. H. Kamel, K. E. Herbert, M. Q. Hassan, A. Bellhcene, A. J. Wijnen, M. F. Young, J. B. Lian, G. S. Stein, and L. C. Gerstenfeld. 2003. Osteoblast-related transcription facter Runx2(Cbfa1/AML-3) and msx2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 63:2631-2636. [PubMed] [Google Scholar]
- 3.Cheng, S.-L., J.-S. Chao, N. Karlton-Kachigian, A. P. Loewy, and D. A. Towler. 2003. Msx2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J. Biol. Chem. 45969-45977. 278: [DOI] [PubMed]
- 4.Dodig, M., T. Tadic, M. S. Kronenberg, S. Dacic, Y. H. Liu, R. Maxson, D. W. Rowe, and A. C. Lichtler. 1999. Ectopic Msx2 overexpression inhibits and Msx2 antisense stimulates calvarial osteoblast differentiation. Dev. Biol. 209:298-307. [DOI] [PubMed] [Google Scholar]
- 5.Ducy, P., and G. Karsenty. 1995. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 15:1858-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ducy, P., R. Zhang, V. Geoffroy, A. L. Ridall, and G. Karsenty. 1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747-754. [DOI] [PubMed] [Google Scholar]
- 7.Ducy, P. 2000. Cbfa1: a molecular switch in osteoblast biology. Dev. Dynam. 219:461-471. [DOI] [PubMed] [Google Scholar]
- 8.Gotoh, M., K. Notoya, Y. Ienaga, M. Kawase, and H. Makino. 2002. Enhancement of osteogenesis in vitro by a novel osteoblast differentiation-promoting compound, TAK-778, partly through the expression of Msx2. Eur. J. Pharmacol. 451:19-25. [DOI] [PubMed] [Google Scholar]
- 9.Hamanishi, C., A. Tan, T. Yamane, M. Tomihara, K. Fukuda, and S. Tanaka. 1995. Ossification of the posterior longitudinal ligament. Autosomal recessive trait. Spine 20:205-207. [DOI] [PubMed] [Google Scholar]
- 10.Hanai, J., L. F. Chen, T. Kanno, N. Ohtani-Fujita, W. Y. Kim, W. H. Guo, T. Imamura, Y. Ishidou, M. Fukuchi, M. J. Shi, J. Stavnezer, M. Kawabata, K. Miyazono, and Y. Ito. 1999. Interaction and functional cooperation of PEBP2/CBF with Smads. Synergistic induction of the immunoglobulin germline Cα promoter. J. Biol. Chem. 274:31577-31582. [DOI] [PubMed] [Google Scholar]
- 11.Hu, G., H. Lee, S. M. Pricel, M. M. Shenl, and C. Abate-Shen. 2001. Msx homeobox genes inhibit differentiation through upregulation of cyclin D1. Development 128:2373-2384. [DOI] [PubMed] [Google Scholar]
- 12.Jabs, E. W., U. Muller, X. Li, L. Ma, W. Luo, I. S. Haworth, I. Klisak, R. Sparkes, M. L. Warman, and J. B. Mulliken. 1993. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell 75:443-450. [DOI] [PubMed] [Google Scholar]
- 13.Javed, A., B. Guo, S. Hiebert, J.-Y. Choi, J. Green, S.-C. Zhao, M. A. Osborne, S. Stifani, J. L. Stein, J. B. Lian, A. J. van Wijnen, and G. S. Stein. 2000. Groucho/TLE/R-Esp proteins associate with the nuclear matrix and repress RUNX (CBFα/AML/PEBP2α) dependent activation of tissue-specific gene transcription. J. Cell Sci. 113:2221-2231. [DOI] [PubMed] [Google Scholar]
- 14.Kim, S., T. Koga, M. Isobe, B. F. Kern, T. Yokochi, Y. F. Chin, G. Karsenty, T. Taniguchi, and H. Takayanagi. 2003. Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev. 17:1979-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koga, H., T. Sakou, E. Taketomi, K. Hayashi, T. Numasawa, S. Harata, K. Yone, S. Matsunaga, B. Otterud, I. Inoue, and M. Leppert. 1998. Genetic mapping of ossification of the posterior longitudinal ligament of the spine. Am. J. Hum. Genet. 62:1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komori, T. 2002. Runx2, a multifunctional transcription factor in skeletal development. J. Cell Biochem. 87:1-8. [DOI] [PubMed] [Google Scholar]
- 17.Komori, T., H. Yagi, S. Nomura, A. Yamaguchi, K. Sasaki, K. Deguchi, Y. Shimizu, R. T. Bronson, Y. H. Gao, M. Inada, M. Sato, R. Okamoto, Y. Kitamura, S. Yoshiki, and T. Kishimoto. 1997. Targeted disruption of Cbfa1 results in complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755-764. [DOI] [PubMed] [Google Scholar]
- 18.Koshizuka, Y., H. Kawaguchi, N. Ogata, T. Ikeda, A. Mabuchi, A. Seichi, Y. Nakanura, K. Nakanura, and S. Ikegawa. 2002. Nucleotide pyrophosphatase gene polymorphism associated with ossification of the posterior longitudinal ligament of the spine. J. Bone Miner. Res. 17:138-144. [DOI] [PubMed] [Google Scholar]
- 19.Lee, K.-S., H. J. Kim, Q. L. Li, X. Z. Chi, C. Ueta, T. Komori, J. M. Wozney, E. G. Kim, J. Y. Choi, H. M. Ryoo, and S. C. Bae. 2000. Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 20:8783-8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levanon, D., R. E. Goldstein, Y. Bernstein, H. Tang, D. Goldenberg, S. Stifani, Z. Paroush, and Y. Groner. 1998. Transcriptional repression by AML1 and LEF-1 is mediated by the TLE/Groucho corepressors. Proc. Natl. Acad. Sci. USA 95:11590-11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, Y. H., R. Kundu, L. Wu, W. Luo, M. A. Ignelzi, Jr., M. L. Snead, and R. E. Maxson, Jr. 1995. Premature suture closure and ectopic cranial bone in mice expressing Msx2 transgenes in the developing skull. Proc. Natl. Acad. Sci. USA 92:6137-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, Y. H., Z. Tang, R. K. Kundu, L. Wu, W. Luo, D. Zhu, F. Sangiorgi, M. L. Snead, and R. E. Maxson. 1999. Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: a possible mechanism for MSX2-mediated craniosynostosis in humans. Dev. Biol. 205:260-274. [DOI] [PubMed] [Google Scholar]
- 23.Ma, I., S. Golden, L. Wu, and R. Maxson. 1996. The molecular basis of Boston-type craniosynoptosis: the Pro148His mutation in the N-terminal arm of the Msx2 preferences. Hum. Mol. Genet. 9:1915-1920. [DOI] [PubMed] [Google Scholar]
- 24.Matsunaga, S., and T. Sakou. 1997. Epidemiology of ossification of the posterior longitudinal ligament, p. 3-17. In K. Yonenobu, T. Sakou, and K. Ono (ed.), Ossification of the posterior longitudinal ligament. Springer-Verlag Press, Tokyo, Japan.
- 25.Matsunaga, S., M. Yamaguchi, K. Hayashi, and T. Sakou. 1999. Genetic analysis of ossification of the posterior longitudinal ligament. Spine 24:937-938. [DOI] [PubMed] [Google Scholar]
- 26.McLarren, K. W., R. Lo, D. Grvabec, K. Thirunavukkarasu, G. Karsenty, and S. Stifani. 2000. The mammalian basic helix loop helix protein HES-1 binds to and modulates the transactivating function of the runt-related factor Cbfa1. J. Biol. Chem. 275:530-538. [DOI] [PubMed] [Google Scholar]
- 27.Mehra-Chaudhary, R., H. Matsui, and R. Raghow. 2001. Msx3 protein recruits histone deacetylase to downregulate the Msx1 promoter. Biochem. J. 353:13-22. [PMC free article] [PubMed] [Google Scholar]
- 28.Nakashima, K., X. Zhou, G. Kunkel, Z. Zhang, J. M. Deng, R. R. Behringer, and B. de Crombrugghe. 2002. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell 108:17-29. [DOI] [PubMed] [Google Scholar]
- 29.Newberry, E. P., J. M. Boudreaux, and D. A. Towler. 1997. Stimulus-selective inhibition of rat osteocalcin promoter induction and protein-DNA interactions by the homeodomain repressor Msx2. J. Biol. Chem. 272:29607-29613. [DOI] [PubMed] [Google Scholar]
- 30.Newberry, E. P., T. Latifi, J. T. Battaile, and D. A. Towler. 1997. Structure-function analysis of Msx2-mediated transcriptional suppression. Biochemistry 36:10451-10462. [DOI] [PubMed] [Google Scholar]
- 31.Newberry, E. P., T. Latifi, and D. A. Rowler. 1999. The RRM domain of MINT, a novel Msx2 binding protein, recognizes and regulates the rat osteocalcin promoter. Biochemistry 38:10678-10690. [DOI] [PubMed] [Google Scholar]
- 32.Pardali, E., X. Q. Xie, P. Tsapogas, S. Itoh, K. Arvanitidis, C. H. Heldin, P. ten Dijke, T. Grundstrom, and P. Sideras. 2000. Smad and AML proteins synergistically confer transforming growth factor β1 responsiveness to human germ-line IgA genes. J. Biol. Chem. 275:3552-3560. [DOI] [PubMed] [Google Scholar]
- 33.Resnick, D., J. Guerra, Jr., C. A. Robinson, and V. C. Vint. 1978. Association of diffuse idiopathic skeletal hyperostosis (DISH) and ossification of the posterior longitudinal ligament. Am. J. Roentgenol. 131:1049-1053. [DOI] [PubMed] [Google Scholar]
- 34.Saito, Y., T. Yoshizawa, F. Takizawa, M. Ikegame, O. Ishibashi, K. Okuda, K. Hara, K. Ishibashi, M. Obinata, and H. Kawashima. 2002. A cell line with characteristics of the periodontal ligament fibroblasts is negatively regulated for mineralization and Runx2/Cbfa1/Osf2 activity, part of which can be overcome by bone morphogenetic protein-2. J. Cell Sci. 115:4191-4200. [DOI] [PubMed] [Google Scholar]
- 35.Sakou, T., E. Taketomi, S. Matsunaga, M. Yamaguchi, S. Sonoda, and S. Yashiki. 1991. Genetic study of ossification of the posterior longitudinal ligament in the cervical spine with human leukocyte antigen haplotype. Spine 16:1249-1252. [DOI] [PubMed] [Google Scholar]
- 36.Satokata, I., L. Ma, H. Ohshima, M. Bei, I. Woo, K. Nishizawa, T. Maeda, Y. Takano, M. Uchiyama, S. Heaney, H. Peters, Z. Tang, R. Maxson, and R. Maas. 2000. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat. Genet. 24:391-395. [DOI] [PubMed] [Google Scholar]
- 37.Shi, Y., M. Downes, W. Xie, H.-Y. Kao, P. Ordentlich, C.-C. Tsai, M. Hon, and R. M. Evans. 2001. Sharp, an inducible cofactor that integrates nuclear receptor repression and activation. Genes Dev. 15:1140-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shirakabe, K., K. Terasawa, K. Miyama, H. Shibuya, and E. Nishida. 2001. Regulation of the transcription factor Runx2 by two homeobox proteins, Msx2 and Dlx5. Genes Cells 6:851-856. [DOI] [PubMed] [Google Scholar]
- 39.Stifani, S., C. M. Blanumueller, N. J. Redhead, R. E. Hill, and S. Artavanis-Tsakonas. 1992. Human homologs of a Drosophila Enhancer of split gene product define a novel family of nuclear proteins. Nat. Genet. 2:119-127. [DOI] [PubMed] [Google Scholar]
- 40.Terayama, K. 1989. Genetic studies on ossification of the posterior longitudinal ligament of the spine. Spine 14:1184-1191. [DOI] [PubMed] [Google Scholar]
- 41.Thirunavukkarasu, K., M. Mahajan, K. W. McLarren, S. Stifani, and G. Karsenty. 1998. Two domains unique to osteoblast-specific transcription factor Osf2/Cbfa1 contribute to its transactivation function and its inability to heterodimerize with CBFβ. Mol. Cell. Biol. 18:4197-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Towler, D. A., S. J. Rutledge, and G. A. Rodan. 1994. Msx-2/Hox8.1: a transcriptional regulator of the rat osteocalcin promoter. Mol. Endocrinol. 8:1484-1493. [DOI] [PubMed] [Google Scholar]
- 43.Wikie, A. O., Z. Tang, N. Elanko, S. Walsh, S. R. Twigg, J. A. Hurst, S. A. Wall, K. H. Chrzanowska, and R. E. Maxson, Jr. 2000. Functional haploinsufficiency of the human homeobox gene Msx2 causes defects in skull ossification. Nat. Genet. 24:387-390. [DOI] [PubMed] [Google Scholar]
- 44.Willis, D. M., A. P. Lowey, N. Karlton-Kachigian, J.-S. Shao, D. M. Ornitz, and D. A. Towler. 2002. Regulation of osteocalcin gene expression by a novel Ku antigen transcription factor complex. J. Biol. Chem. 277:37280-37291. [DOI] [PubMed] [Google Scholar]
- 45.Winograd, J., M. P. Reilly, R. Roe, J. Lutz, E. Laughner, X. Xu, L. Hu, T. Asakura, C. van der Kolk, J. D. Strandberg, and G. L. Semenza. 1997. Perinatal lethality and multiple craniofacial malformations in MSX2 transgenic mice. Hum. Mol. Genet. 6:369-379. [DOI] [PubMed] [Google Scholar]
- 46.Zeng, C., S. McNeil, S. Pockwinse, J. Nickerson, L. Shopland, J. B. Lawrence, S. Penman, S. Hiebert, J. B. Lian, A. J. van Wijnen, J. L. Stein, and G. S. Stein. 1998. Intranuclear targeting of AML/CBFα regulatory factors to nuclear matrix-associated transcriptional domains. Proc. Natl. Acad. Sci. USA 95:1585-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, H., G. Hu, H. Wang, P. Sciavolino, N. Iler, M. M. Shen, and C. Abate-Shen. 1997. Heterodimerization of Msx2 and Dlx homeoproteins results in functional antagonism. Mol. Cell. Biol. 17:2920-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Y.-W., N. Yasui, K. Ito, G. Huang, M. Fujii, J. Hanai, H. Nogami, T. Ochi, K. Miyazono, and Y. Ito. 2000. A RUNX2/PEBP2αA/CBFA1 mutation displaying impaired transactivation and Smad interaction in cleidocranial dysplasia. Proc. Natl. Acad. Sci. USA 97:10549-10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou, Y. L., Y. Lei, and M. L. Snead. 2000. Functional antagonism between Msx2 and CCAAT/Enhancer-binding protein α in regulating the mouse amelogenin gene expression is mediated by protein-protein interaction. J. Biol. Chem. 275:29066-29075. [DOI] [PubMed] [Google Scholar]