Abstract
Introduction: Low-frequency noise (LFN) leads to the development of tissue fibrosis. We previously reported the development of myocardial and perivascular fibrosis and a reduction of cardiac connexin43 in rats, but data is lacking concerning the affected type of collagen as well as the ultrastructural myocardial modifications. Objectives: The aim of this study was to quantify cardiac collagens I and III and to evaluate myocardial ultrastructural changes in Wistar rats exposed to LFN. Methods: Two groups of rats were considered: A LFN-exposed group with 8 rats continuously submitted to LFN during 3 months and a control group with 8 rats. The hearts were sectioned and the mid-ventricular fragment was selected. After immunohistochemical evaluation, quantification of the collagens and muscle were performed using the image J software in the left ventricle, interventricular septum and right ventricle and the collagen I/muscle and collagen III/muscle ratios were calculated. Transmission electron microscopy (TEM) was used to analyze mid-ventricular samples taken from each group. Results: The collagen I/muscle and collagen III/muscle ratios increased in totum respectively 80% (p<0.001) and 57.4% (p<0.05) in LFN-exposed rats. TEM showed interstitial collagen deposits and changes in mitochondria and intercalated discs of the cardiomyocytes in LFN-exposed animals. Conclusions: LFN increases collagen I and III in the extracellular matrix and induces ultrastructural alterations in the cardiomyocytes. These new morphological data open new and promising paths for further experimental and clinical research regarding the cardiac effects of low-frequency noise.
Keywords: Low-frequency noise, collagen I and III, myocardial ultrastructure, immunohistochemistry, transmission electron microscopy
Introduction
Low-frequency noise (LFN) is characterized by large pressure amplitude (≥90 dB SPL) and low frequency bands (≤500 Hz) and leads to an abnormal proliferation of collagen and development of tissue fibrosis [1-6]. We previously reported a significant fibrotic development in ventricular myocardium of rats exposed to LFN [7] and an increase of perivascular fibrosis in the arterial coronary vessels after exposure to industrial noise [8]. Modifications on the electrophysiological milieu, characterized by a significant reduction of connexin43 in rats exposed to LFN were also observed in a recent study performed by our group [9]. From the results of our studies we put forward that the universal existence of LFN in modern societies can contribute to aggravate preexisting cardiac diseases or even explain some idiopathic cardiomyopathies and ventricular tachyarrhythmias. Consequently, looking into new morphological data, one can establish more bridges to experimental and clinical investigations.
In the heart, type I and III collagens represent respectively 85% and 11% of the extracellular matrix collagen composition and the effects of LFN on each one are not known. Also, the ultrastructural changes induced by LFN in the cardiomyocyte are not defined.
The aim of this study was to perform an immunohistochemical quantification of collagen I and III and to evaluate the ultrastructure of the ventricular myocardium in rats exposed to low-frequency noise.
Methods
Sixteen adult Wistar rats were studied. The animals were treated in accordance with the EU Commission on Animal Protection for Experimental and Scientific Purposes and with the Portuguese legislation for the same purpose. Eight rats were continuously exposed to LFN for a three-month period. The control group of 8 rats was kept in a silent environment. All the animals were kept in cages, fed standard rat food and had free access to water. The sound spectrum emanating from an analog noise generator was similar to the previously reported [10].
After the rats were sacrificed with an intraperitoneal injection of a lethal dose of sodium pentobarbital, the hearts were removed, fixed in 10% buffered formalin, transversely sectioned from the ventricular apex to the atria and the mid-ventricular fragment was selected for the study. The fragments were dehydrated with progressive graded ethanol series, cleared with xylene and embedded in paraffin. The paraffin blocks were sliced into sections with 3.5 micrometers and mounted in glass slides and after deparaffinization and rehydration the endogenous peroxidase activity was blocked with use of 3% H2O2 for 10 minutes. Then, sections were incubated overnight at room temperature with polyclonal antibodies to collagen I and III diluted 1:500 for immunohistochemical analysis. The slides were finally counterstained with hematoxylin, dehydrated and mounted.
The histological images were obtained with an optical microscope using 400 x magnifications. In each section the optical fields were selected from the left ventricle, the interventricular septum and the right ventricle. Criteria used to select each field were defined by the myocardial samples containing the highest visualization of immunostained collagen I and III. A total of 132 optical fields were selected from all the anatomical components, by three observers, under blinded assessment, and analyzed using the Image J software. The signal intensity threshold value of 140 on the 0 - 255 scale was identified to distinguish collagen from other structures and the collagen I/muscle and collagen III/muscle ratios were calculated.
Concerning the ultrastructure evaluation and as a preliminary illustrating purpose, samples from mid-ventricular segments of five rats were cut in fragments of less than 1 mm3 and fixed in 3% glutaraldehyde in 0.1 M cacodylate buffer pH 7.3, followed by 1% osmium tetroxide in the same buffer and uranyl acetate 0.5% in acetate acetic acid buffer 0.1 M, pH 5. After dehydration in ethanol and passage epoxypropane, the samples were embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and were examined and photographed in a transmission electron microscope.
Statistical analysis
Data are presented as mean ± SE. In order to assess the differences between LFN exposed and control animals concerning the collagen to muscle ratios in each anatomical region and in totum, MANOVA model was fit to the data. A p value <0.05 was considered statistically significant.
Results
The histological observations showed immunostained collagen I and III in the extracellular matrix. Examples from LFN-exposed and control rats are shown in Figures 1 and 2 respectively for collagen I and III. Histological observations showed diffuse interstitial deposits of collagen in LFN-exposed rats.
Figure 1.
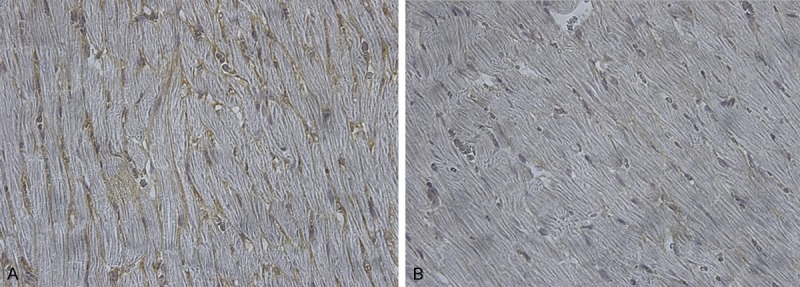
Immunostained collagen I in a section taken from the left ventricle of a LFN-exposed rat (A) and control rat (B). Immunoreactive type I collagen appears brown between the cardiomyocytes (x 400).
Figure 2.
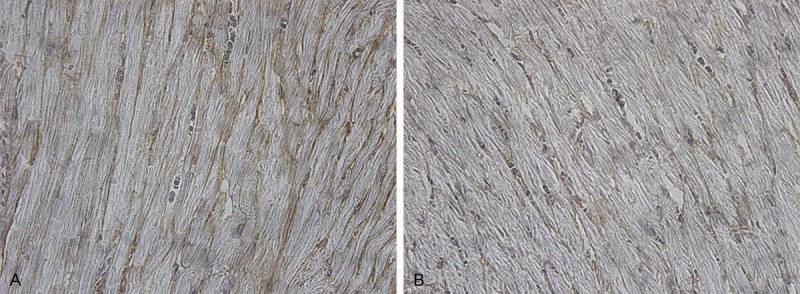
Immunostained collagen III in a section taken from the left ventricle of a LFN-exposed rat (A) and control rat (B). Immunoreactive type III collagen appears brown between the cardiomyocytes (x 400).
Prior to the analysis of the ratios, the assumption of homogeneous variance-covariance matrix was validated by the M-Box test (p=0.593). This multivariate approach to the data showed that there were marked differences between exposed and non-exposed animals for the selected anatomical regions and in totum, as expressed by the statistical significance achieved for the collagen I/muscle ratio (p=0.001) with an observed power of 99.6% and for the collagen III/muscle ratio (p=0.021) with an observed power of 79.6%.
The results obtained for the collagen I/muscle ratio for each of the anatomical regions and in totum, are showed in Table 1 and Figure 3. The percent increase with LFN exposure was 80% (p<0.001) in totum and 76% (p=0.055), 105% (p=0.046) and 84% (p=0.032) respectively for the left ventricle, interventricular septum and right ventricle.
Table 1.
Collagen I/muscle ratio in each anatomical region and in totum in LFN-exposed (n=8) and control (n=8) animals
| Anatomical region | Group | Mean | Std. error | Percent increase with LFN exposure | p |
|---|---|---|---|---|---|
| LV | Exposed | 0.044 | 0.006 | 76% | 0.055 |
| Control | 0.025 | 0.006 | |||
| IVS | Exposed | 0.037 | 0.006 | 105% | 0.046 |
| Control | 0.018 | 0.006 | |||
| RV | Exposed | 0.059 | 0.008 | 84% | 0.032 |
| Control | 0.032 | 0.008 | |||
| in totum | Exposed | 0.045 | 0.002 | 80% | <0.001 |
| Control | 0.025 | 0.002 |
LV=Left Ventricle; IVS=Interventricular Septum; RV=Right Ventricle; LFN=Low-Frequency Noise.
Figure 3.
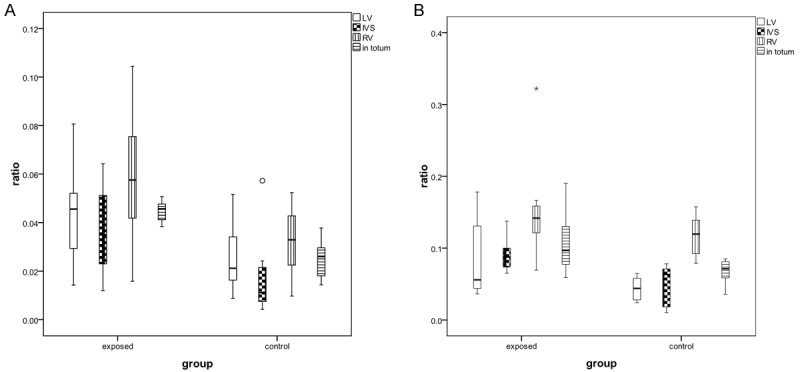
Collagen I/muscle ratio (A) and collagen III/muscle ratio (B) in the left ventricle (LV), interventricular septum (IVS), right ventricle (RV) and in totum in LFN-exposed and control animals.
For the collagen III/muscle ratio the results obtained are presented in Table 2 and Figure 3. In this case differences were detected at the interventricular septum and in totum. The percent increase with LFN exposure was 57.4% (p=0.027) in totum and 90.9% (p=0.063), 85.7% (p=0.006) and 31.6% (p=0.206) respectively for the left ventricle, interventricular septum and right ventricle.
Table 2.
Collagen III/muscle ratio in each anatomical region and in totum in LFN-exposed (n=8) and control (n=8) animals
| Anatomical region | Group | Mean | Std. error | Percent increase with LFN exposure | p |
|---|---|---|---|---|---|
| LV | Exposed | 0.084 | 0.014 | 90.9% | 0.063 |
| Control | 0.044 | 0.014 | |||
| IVS | Exposed | 0.091 | 0.009 | 85.7% | 0.006 |
| Control | 0.049 | 0.009 | |||
| RV | Exposed | 0.154 | 0.020 | 31.6% | 0.206 |
| Control | 0.117 | 0.020 | |||
| in totum | Exposed | 0.107 | 0.011 | 57.4% | 0.027 |
| Control | 0.068 | 0.011 |
LV=Left Ventricle; IVS=Interventricular Septum; RV=Right Ventricle; LFN=Low-Frequency Noise.
Examples of the ultrastructural analysis by TEM are shown in Figures 4, 5 and 6. In LFN-exposed rats the ultrastructural evaluation confirmed extracellular matrix alterations, characterized by accumulation of collagen. The cardiomyocytes are pushed apart with no visible gap junctions at the interplicate regions of the intercalated discs. Additionally, a considerable number of enlarged mitochondria and some lipofuscin granules were observed.
Figure 4.
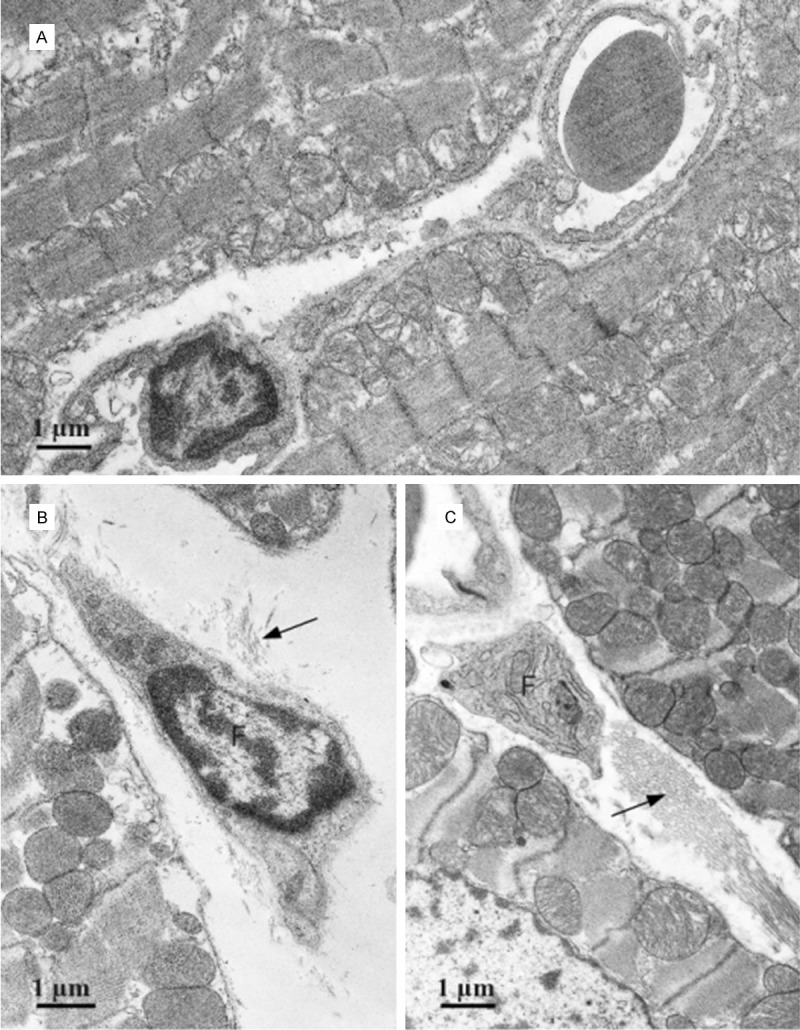
Electron micrograph (TEM) of a section of ventricular muscle. A. Control rat; B and C. LFN-exposed rats. Fibroblasts (F) surrounded by collagen (arrows) are observed in the interstitium of the exposed rats.
Figure 5.

Electron micrograph (TEM) of a longitudinal section of ventricular muscle. A. Control rat; B. LFN-exposed rat. Numerous enlarged mitochondria surrounding the sarcomeres are observed in the LFN-exposed rat (M). Lipofuscin granules are also observed in LFN exposed rats (L).
Figure 6.
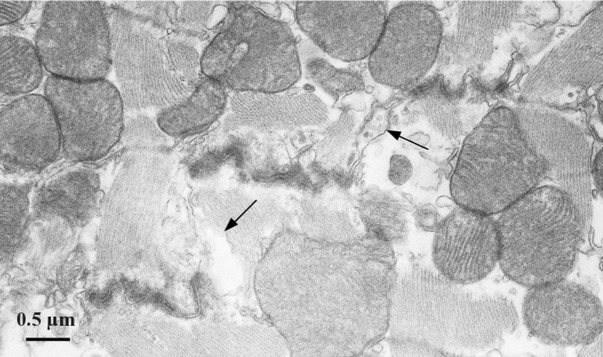
Electron micrograph (TEM) section of a LFN-exposed ventricular myocardium, showing a typical steplike intercalated disc and numerous mitochondria surrounding the sarcomeres. Cell membrane separation (arrows) is observed in the interplicate region of the intercalated disc.
Discussion
Our study analyzes the cardiac type I and III collagens and the ultrastructure of the ventricular myocardium in rats exposed to LFN. Type I collagen is responsible for the tensile strength, while type III contributes to the elasticity of the myocardium. A correct balance between the synthesis and degradation of these collagens is necessary to maintain myocardial structure and function. Fibrosis represents an abnormal deposition of collagen and can lead to myocardial stiffness and diastolic dysfunction [11,12] and in addition, modifies the electrophysiological myocardium properties facilitating the occurrence of ventricular arrhythmias [13,14].
In our study, a significant increase of collagen I and III was observed. An increase was shown in all the studied anatomical areas but some did not reach statistical significance.
The ultrastructural observation denoted high concentration of collagen in the extracellular matrix next to fibroblasts, confirming the pronounced effect of LFN on the connective tissue. Another relevant modification was observed in the cardiomyocytes by the presence of numerous enlarged mitochondria. In a previous study performed in rats submitted to infrasound [15], swelled mitochondria were observed, explained by a possible cellular membranous direct damage, leading to an overload of Ca2+ and consequent inhibition of the oxidative phosphorylation, followed by a reduction of the cardiomyocyte energy. In contrast, in our study, an increase of the mitochondria number and size was observed, being the main organelle alteration in response to LFN. This new data may suggest high-energy activity in cardiomyocytes and suggests that cardiac morphological changes induced by LFN may not be confined to the extracellular matrix.
Corroborating the hypothesis of a direct deleterious LFN action on the cardiomyocyte are the results we previously reported showing a reduction of cardiac connexin43 after LFN exposure [9]. However, the TEM observations in the present study, of cardiomyocyte separated cell membranes, puts forward the hypothesis of gap junction disruption, possibly caused by an interstitial fibrotic development. Further studies are needed to confirm these data.
Meanwhile, the immunohistochemical demonstration of an augmented collagen as well as the ultrastructural evidence of relevant collagen deposits, follow the line of our previous morphological studies showing a significant myocardial fibrotic development induced by LFN [7]. We believe that this acquired experimental knowledge on extracellular matrix modification should constitute the support for clinical research applied to people exposed to LFN.
In fact, myocardial fibrosis can lead to clinical consequences such as reduced coronary flow reserve, ventricular diastolic dysfunction and ventricular tachyarrhythmias. Coronary perivascular fibrosis can limit vessel distensibility and impair coronary blood flow [16,17]. Interstitial myocardial fibrosis has deleterious effects on diastolic function through an increased stiffness and reduction of elasticity of the ventricular myocardium [11,12,18]. Additionally, severe ventricular tachyarrhythmias may develop through reentrant phenomena induced by the electrophysiological heterogeneities in consequence of myocardial fibrosis [19-21].
Considering our previous and these present results, we can stress the importance of the clinical diagnosis and characterization of myocardial fibrosis as well as the study of its possible arrhythmic consequences. These issues deserve further clinical research in populations exposed to LFN.
One possible way could be the use of biochemical markers for myocardial fibrosis. In fact, high serum levels of procollagen type III aminoterminal peptide were shown in hypertensive patients [12], in dilated cardiomyopathy [22] in hypertrophic cardiomyopathy [23] in congenital heart disease [24] and in heart failure [25,26]. Excessive levels of procollagen type I carboxyterminal peptide was found in hypertensive patients [12,27]. The relation however between these biomarkers and the myocardial fibrosis induced by LFN is not known. Nevertheless, and taking into account the significant increase of collagen I and III found in our study, we do not discard a potential clinical application of applying specific fibrosis biomarkers to evaluate populations exposed to low-frequency noise.
Bearing in mind a possible link between modifications of biochemical markers for myocardial fibrosis and ventricular arrhythmogenesis [28] and hypothesizing that LFN can induce a morphological arrhythmogenic substrate [9] the use of specific biomarkers to identify LFN-exposed individuals or patients more prone to develop ventricular arrhythmias should be considered.
Another relevant clinical implication can be the use of echocardiography to evaluate the ventricular diastolic function in people exposed to LFN. In this regard a study performed in aerospace maintenance workers showed significant alterations of the E/A ratio reflecting changes in the ventricular diastolic function. The use of the echocardiographic parameter E/A ratio was suggested to evaluate the ventricular diastolic function and to check the health condition of people exposed to LFN [29].
In addition, cardiac magnetic resonance can also possibly be used to quantify cardiac fibrosis [30,31]. Nevertheless, its application for diffuse interstitial myocardial fibrosis showed some limitations [32].
In conclusion, LFN induces cardiac morphological changes in the extracellular matrix and in the cardiomyocyte ultrastructure. The significant increase of collagen I and III and the alteration detected at the cardiomyocyte intercalated disc together with the reduction of connexin43 reported in one of our previous studies, reinforce the hypothesis of an inducible morphological arrhythmogenic substrate. The new morphological data observed in this study open new and promising paths for experimental and clinical research regarding the cardiac effects of low-frequency noise.
Disclosure of conflict of interest
None.
References
- 1.Oliveira P, Brito J, Mendes J, da Fonseca J, Águas A, Martins dos Santos J. Effects of large pressure amplitude low frequency noise in the parotid gland perivascular-ductal connective tissue. Acta Med Port. 2013;26:237–242. [PubMed] [Google Scholar]
- 2.de Sousa Pereira A, Águas AP, Grande NR, Mirones J, Monteiro E, Castelo Branco NA. The effect of chronic exposure to low frequency noise on rat tracheal epithelia. Aviat Space Environ Med. 1999;70:A86–A90. [PubMed] [Google Scholar]
- 3.Grande NR, Águas AP, Sousa Pereira A, Monteiro E, Castelo Branco NAA. Morphological changes in the rat lung parenchyma exposed to low frequency noise. Aviat Space Environ Med. 1999;70:A70–A77. [PubMed] [Google Scholar]
- 4.Martins dos Santos J, Grande NR, Castelo Branco NAA, Zagalo C, Oliveira P, Alves-Pereira M. Lymphatic lesions and vibroacoustic disease. Euro J Lymphology. 2004;12:17–20. [Google Scholar]
- 5.Fonseca J, Martins dos Santos J, Oliveira P, Laranjeira N, Aguas A, Castelo-Branco N. Noise-induced gastric lesions: a light and electron microscopy study of the rat gastric wall exposed to low frequency noise. Arq Gastroenterol. 2012;49:82–88. doi: 10.1590/s0004-28032012000100014. [DOI] [PubMed] [Google Scholar]
- 6.Dos Santos JM, Grande NR, Castelo Branco NA, Zagalo C, Oliveira P. Vascular lesions and vibroacoustic disease. Eur J Anat. 2002;6:17–21. [Google Scholar]
- 7.Antunes E, Oliveira P, Borrecho G, Oliveira MJR, Brito J, Águas A, Martins dos Santos J. Myocardial fibrosis in rats exposed to low-frequency noise. Acta Cardiol. 2013;68:241–245. doi: 10.1080/ac.68.3.2983417. [DOI] [PubMed] [Google Scholar]
- 8.Antunes E, Oliveira P, Oliveira MJR, Brito J, Águas A, Martins dos Santos J. Histomorphometric evaluation of the coronary artery vessels in rats submitted to industrial noise. Acta Cardiol. 2013;68:285–289. doi: 10.1080/ac.68.3.2983423. [DOI] [PubMed] [Google Scholar]
- 9.Antunes E, Borrecho G, Oliveira P, Brito J, Águas A, Martins dos Santos J. Immunohistochemical evaluation of cardiac connexin43 in rats exposed to low-frequency noise. Int J Clin Exp Pathol. 2013;6:1874–1879. [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira PM, Pereira da Mata AD, Martins dos Santos JA, da Silva Marques DN, Branco NC, Silveira JM, Correia da Fonseca JC. Low-frequency noise effects on the parotid gland of the Wistar rat. Oral Dis. 2007;13:468–473. doi: 10.1111/j.1601-0825.2006.01322.x. [DOI] [PubMed] [Google Scholar]
- 11.Janicki JS, Brower GL. The role of myocardial fibrillar collagen in ventricular remodeling and function. J Card Fail. 2002;8(Suppl):S319–S325. doi: 10.1054/jcaf.2002.129260. [DOI] [PubMed] [Google Scholar]
- 12.Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure: evidence of increased myocardial turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 13.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 14.De Baker JM, von Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Slow conduction in the infarted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 15.Pei Z, Sang H, Li R, Xiao P, He J, Zhuang Z, Zhu M, Chen J, Ma H. Infrasound-induced hemodynamics, ultrastructure, and molecular changes in the rat myocardium. Environ Toxicol. 2007;22:169–175. doi: 10.1002/tox.20244. [DOI] [PubMed] [Google Scholar]
- 16.Isoyama S, Ito N, Satoh K, Takishima T. Collagen deposition and the reversal of coronary reserve in cardiac hypertrophy. Hypertension. 1992;20:491–500. doi: 10.1161/01.hyp.20.4.491. [DOI] [PubMed] [Google Scholar]
- 17.Dai Z, Aoki T, Fukumoto Y, Shimokaxa H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol. 2012;60:416–421. doi: 10.1016/j.jjcc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis. Functional significance and regulatory factors. Cardiovasc Res. 1993;27:341–8. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 19.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;97:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 20.Kawara T, Derksen R, de Groot JR, Coronel R, Tasseron S, Linnenbank AC, Hauer RN, Kirkels H, Janse MJ, de Bakker JM. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation. 2001;104:3069–3075. doi: 10.1161/hc5001.100833. [DOI] [PubMed] [Google Scholar]
- 21.La Vecchia L, Ometto R, Bedogni F, Finocchi G, Mosele GM, Bozzola L, Bevilacqua P, Vincenzi M. Ventricular late potentials, interstitial fibrosis, and right ventricular function in patients with ventricular tachycardia and normal left ventricular function. Am J Cardiol. 1998;81:790–792. doi: 10.1016/s0002-9149(97)01012-6. [DOI] [PubMed] [Google Scholar]
- 22.Klappacher G, Franzen P, Haab D, Mehrabi M, Binder M, Plesch K, Pacher R, Grimm M, Pribill I, Eichler HG. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol. 1995;75:913–918. doi: 10.1016/s0002-9149(99)80686-9. [DOI] [PubMed] [Google Scholar]
- 23.Lombardi R, Betocchi S, Losi MA, Tocchetti CG, Aversa M, Miranda M, D’Alessandro G, Cacace A, Ciampi Q, Chiariello M. Myocardial collagen turnover in hypertrophic cardiomyopathy. Circulation. 2003;108:1455–1460. doi: 10.1161/01.CIR.0000090687.97972.10. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto M, Masutani S, Seki M, Kajino H, Fujieda K, Senzaki H. High serum levels of procollagen type III N-terminal amino peptide in patients with congenital heart disease. Heart. 2009;95:2023–2028. doi: 10.1136/hrt.2009.170241. [DOI] [PubMed] [Google Scholar]
- 25.Barasch E, Gottdiener JS, Aurigemma G, Kitzman DW, Han J, Kop WJ, Tracy RP. Association between elevated fibrosis markers and heart failure in the elderly. The cardiovascular health study. Circ Heart Fail. 2009;2:303–310. doi: 10.1161/CIRCHEARTFAILURE.108.828343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cicoira M, Rossi A, Bonapace S, Zanolla L, Golia G, Franceschini L, Caruso B, Marino PN, Zardini P. Independent and additional prognostic value of aminoterminal propeptide of type III procollagen circulating levels in patients with chronic heart failure. J Card Fail. 2004;10:403–411. doi: 10.1016/j.cardfail.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Querejeta R, López B, González A, Sánchez E, Larman M, Martínez Ubago JL, Díez J. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: Relation to myocardial fibrosis. Circulation. 2004;110:1263–1268. doi: 10.1161/01.CIR.0000140973.60992.9A. [DOI] [PubMed] [Google Scholar]
- 28.Flevari P, Theodorakis G, Leftheriotis D, Kroupis C, Kolokathis F, Dima K, Anastasiou-Nana M, Kremastinos D. Serum markers of deranged myocardial collagen turnover: their relation to malignant ventricular arrhythmias in cardioverter-defibrillator recipients with heart failure. Am Heart J. 2012;164:530–537. doi: 10.1016/j.ahj.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Chao PC, Yeh CY, Juang YJ, Hu CY, Chen CJ. Effect of low-frequency noise on the echocardiographic parameter E/A ratio. Noise Health. 2012;14:155–158. doi: 10.4103/1463-1741.99881. [DOI] [PubMed] [Google Scholar]
- 30.Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561–1567. doi: 10.1016/s0735-1097(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 31.Tandri H, Saranathan M, Rodriguez ER, Martinez C, Bomma C, Nasir K, Rosen B, Lima JA, Calkins H, Bluemke DA. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45:98–103. doi: 10.1016/j.jacc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 32.Schalla S, Bekkers SC, Dennert R, van Suylen RJ, Waltenberger J, Leiner T, Wildberger J, Crijns HJ, Heymans S. Replacement and reactive myocardial fibrosis in idiopathic dilated cardiomyopathy: comparison of magnetic resonance imaging with right ventricular biopsy. Eur J Heart Fail. 2010;12:227–231. doi: 10.1093/eurjhf/hfq004. [DOI] [PubMed] [Google Scholar]


