Abstract
The intervertebral disc is the largest avascular organ in the human body. However, with the progress of intervertebral disc degeneration (IDD), the disc tends to be vascularized increasingly via angiogenesis. It is well established that both human nucleus pulposus (NP) cells and vascular endothelial cells express FasL and Fas. However, the issue remains open as to whether there are certain active mechanisms preventing angiogenesis in the disc via the FasL-Fas machinery. Here, we established a co-culture system of human NP cells and vascular endothelial (HMEC-1) cells. We found that normal NP cells were more capable of inducing apoptosis in HMEC-1 cells (14.2±3.4%) than degenerate NP cells (6.7±1.9%), p<0.05. By up-regulating the FasL expression in degenerate NP cells, we found that FasL played an essential role in the mediation of HMEC-1 cell apoptosis with the activation of downstream FADD and caspase-3. Furthermore, we found an increased Fas expression in HMEC-1 cells following co-cultured with NP cells, which might be closely linked with FasL produced by NP cells and enhance their interaction. Collectively, this is the first study showing FasL-Fas network might plays an important role in the molecular mechanisms of angiogenesis avoidance of human disc. Consequently, our findings might shed light on the pathogenesis in human IDD and provide a novel target for the treatment strategies for IDD.
Keywords: Intervertebral disc, nucleus pulposus, vascular endothelial cells, FasL, Fas, HMEC-1
Introduction
Intervertebral disc degeneration (IDD) is an important cause of low back pain, which affects about 80% of the population at least at one time during their lives [1]. The underlying mechanisms of IDD have been ascribed to various factors including abnormal mechanical forces [2], increased cell death [3], gene polymorphisms [4], immune privilege unbalance [5], and aberrant miRNAs expression [6]. Despite intensive basic and clinical pieces of studies aiming for addressing the issues, the pathological mechanisms of IDD remain yet defined.
Anatomically, normal human intervertebral disc consists of three parts: the nucleus pulposus (NP), the anulus fibrosus (AF) and the adjacent cartilaginous endplates. The dense fibrous of AF and extracellular matrix components of cartilaginous endplates have formed a unique structure and prevent blood vessels growing into the central NP. Actually, the disc is the largest avascular organ in the human body [7]. However, with the progress of IDD, the disc tends to be more vascularized. Subsequently, abnormal nerve fibers penetrate into the NP along with blood vessels and cause hyperpathia [8]. Meanwhile, various infiltrating immunocytes destruct the immune balance of the disc and induce inflammation. Therefore, vascularization plays an important role in IDD.
Accumulating evidence indicates various mechanisms of angiogenesis exist in IDD. Gross matrix alterations, including increased lamellar disorganization and fissures, are thought to destroy the physical barriers that suppress blood vessel ingrowth [9]. As well, degenerate NP cells express increased basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) [10,11] and platelet-derived growth factor (PDGF) [12], as well as related pro-inflammatory cytokines including IL-1β [13] and TNF-α [14]. Moreover, aberrant extracellular matrix changes act as an inducer in vessel ingrowth [15]. The available evidence indicates the interaction between degenerate NP tissue and endothelial cells with particular reference to the stimulation of endothelial cells ingrowth. Traditional structural hallmark of the disc is noted as a significant factor contributing to its avascular maintenance. However, whether there are active mechanisms preventing disc vascularization is still unknown.
It has been reported that FasL exists on normal NP cells and its expression is decreased with IDD [16,17]. As a Type II transmembrane protein of the tumor necrosis factor (TNF) family, FasL can mediate activation of cell death once it binds on Fas-bearing target cells [18]. In our previous studies, we showed that FasL from NP cells could induce apoptosis in immunocytes and play an important role in the immune privilege of human disc [19,20]. Given that Fas is expressed on a variety of cell types including endothelial cells, we assumed that FasL expressed by NP cells might be able to cause apoptosis in vascular endothelial cells and subsequently inhibit blood vessel infiltrating. To date, no studies have demonstrated apoptosis of vascular endothelial cells caused by FasL-expressing NP cells. Accordingly, the aim of this study was to address the role of FasL between NP cells and vascular endothelial cells interaction.
Materials and methods
Sample collection
The NP samples for experimental application were approved by the institutional Ethics Review Board of Xijing Hospital (No. 20090611-3, No. 20111103-7). Moreover, we obtained written informed consents from each patient or his/her relatives. The study groups consisted of NP from cadavers and patients as below: normal cadaveric donors as control (n=8; average age 41.8±3.6 (range 37-47)) and patients with IDD undergoing discectomy as degenerative NP samples (n=20; average age 43.4±5.4 (range 35-52)) (Table 1). The individuals included in control and experimental groups are matched for age, with no smoking, diabetes, hypertension and radicular symptoms, etc. The IDD degree of patients was graded by magnetic resonance imaging (MRI) according to the Pfirrmann grading system. As for the cadavers, MRI data in records were collected.
Table 1.
Demographic data of cadaveric donors and patients
| NO. | Age | Gender | Level | Degree* |
|---|---|---|---|---|
| Normal control | ||||
| 1 | 44 | M | L4/5 | I |
| 2 | 38 | M | L4/5 | I |
| 3 | 37 | F | L4/5 | I |
| 4 | 45 | M | L4/5 | I |
| 5 | 47 | M | L4/5 | I |
| 6 | 39 | F | L4/5 | I |
| 7 | 45 | M | L4/5 | I |
| 8 | 39 | M | L4/5 | I |
| IDD Group | ||||
| 9 | 47 | F | L4/5 | IV |
| 10 | 52 | M | L4/5 | IV |
| 11 | 43 | F | L4/5 | V |
| 12 | 36 | F | L4/5 | IV |
| 13 | 42 | M | L4/5 | V |
| 14 | 50 | F | L5/S1 | IV |
| 15 | 45 | F | L5/S1 | IV |
| 16 | 41 | M | L4/5 | IV |
| 17 | 38 | M | L4/5 | IV |
| 18 | 35 | F | L5/S1 | V |
| 19 | 39 | M | L4/5 | IV |
| 20 | 42 | F | L5/S1 | IV |
| 21 | 52 | M | L4/5 | IV |
| 22 | 42 | M | L4/5 | IV |
| 23 | 46 | M | L4/5 | IV |
| 24 | 38 | F | L4/5 | IV |
| 25 | 49 | F | L4/5 | IV |
| 26 | 36 | M | L5/S1 | IV |
| 27 | 43 | F | L4/5 | IV |
| 28 | 51 | M | L4/5 | IV |
Pfirrmann grading system.
Human NP cells isolation and culture
All specimens were obtained within 1 hour either after autopsy or surgery. Subsequently, the NP was separated from the AF using a stereotaxic microscope carefully and washed with Hank balance salt solution to eliminate contamination and blood. Specimens were digested for 40 minutes in 0.2% pronase, then washed with Hank balance salt solution and incubated in 0.25% type II collagenase at 37°C under gentle agitation. After 4 hours, remaining tissue debris was removed through a 40 μm cell strainer. Cells were centrifuged at 200 g for 8 min and seeded in culture flasks cultured with DMEM/F12-based culture medium, containing 15% fetal bovine serum (FBS; Gibco-BRL) and 1% penicillin/streptomycin (Invitrogen) in 5% CO2 and 20% oxygen incubator.
HMEC-1 cell cultures
HMEC-1 is an immortalized cell line of human microvascular endothelia cells. It retains the characteristic of normal human microvascular endothelial cells in morphology, phenotype and function [21]. In this study, HMEC-1 cells were seeded in culture flasks cultured with DMEM (containing 10% FBS, 1% penicillin/streptomycin) in 5% CO2 and 20% oxygen incubator. The culture medium was changed every three days.
Up-regulation of FasL in NP cells with lentiviral vector
The lentiviral vector (Genechem, Shanghai, China) encoding FasL labeled with green fluorescent protein (GFP) was used for FasL up-regulation. Scrambled sequence was used as control. Human NP cells were plated at a density of 1.5×105 cells/well in a 24-well plate in a final volume of 250 μl complete medium. Viral solutions at a multiplicity of infection (MOI) of 10 were added to NP cells. After incubation for 10 h at 37°C, the cells were allowed to recover over the ensuing 96 h in culture medium. To verify cell transfection, culture flasks were detected by fluorescent microscopy.
Quantification of FasL in the supernatant of NP cells
According to the manufacturer’s instructions, enzyme-linked immunosorbent assay (ELISA) kits with an antibody that recognizes human FasL (Rand D Systems, Minneapolis, USA) was used to determine the expression of FasL in the supernatant of cultured NP cells (1×106 cells/well). Natural human FasL provided by the supplier was used to construct a standard curve and to obtain absolute values for calibration. The concentration was determined in triplicate in each sample and the average measurement was considered to be the final concentration.
Indirect co-cultures of NP cells and HMEC-1 cells
The indirect co-culture system was established with Transwell inserts (0.4 μm pore-size) placing in six-well plates. The co-culture ratio is 50:50, consisted of NP cells plated into Transwell inserts (1.5×106 per well) and HMEC-1 cells in six-well plate (1.5×106 per well) with 10% DMEM/F12 culture medium mentioned above. HMEC-1 cells cultured without NP cells at the same condition were used as controls. After 2 days of culture, HMEC-1 cells were harvested.
Flow cytometry (FCM) analysis
To address apoptosis in HMEC-1 cells after co-cultures, we performed FCM with Annexin V-FITC/PI (BD Biosciences, San Diego, CA, USA) staining upon the aforementioned treated cells. In brief, 1×106 cells were re-suspended in binding buffer following washing twice with PBS. Then the cells were incubated in Annexin V-FITC and PI at room temperature for 15 min. The samples were then analyzed by FCM. Each experiment was repeated for at least three times.
Western blotting
To determine the Fas expression level of HMEC-1 cells following co-cultured with NP cells, the cultured HMEC-1 cells were trypsinized. Prepare total cell lysates by solubilizing cells in 2X SDS sample buffer. Following electrophoresed in 10% gel, proteins were transferred to PVDF membrane. The membranes were incubated for 1 h at room temperature with rabbit polyclonal antibodies against Fas (Abcam), FADD (Abcam) and caspase-3 (Abcam), and mouse monoclonal antibody specific to β-actin (Sigma). Antibody labeling was identified using IRDye 800 anti-rabbit or anti-mouse IgG antibody (LI-COR Biosciences, Nebraska, USA). Expression levels were analyzed by LI-COR Odyssey Imaging System.
Statistical analysis
Student’s t-test was used in the analysis of two-group parameters. ANOVA test was used in comparisons of multiple group data. The SPSS statistical package (SPSS, Chicago, IL, USA) for statistical analysis was used. A p value <0.05 was considered significant.
Results
NP cells can induce apoptosis of HMEC-1 cells
The apoptosis rate of HMEC-1 cells was increased following co-cultures with NP cells (both degenerate and normal group) compared with the controls. In addition, HMEC-1 cells showed more apoptosis percentage in the normal NP cells co-cultures (14.2±3.4%) than that in the degenerate NP cells co-cultures (6.7±1.9%) (Figure 1A, 1B). Schematic drawing of the co-culture system with the two types of cells was shown (Figure 1C).
Figure 1.
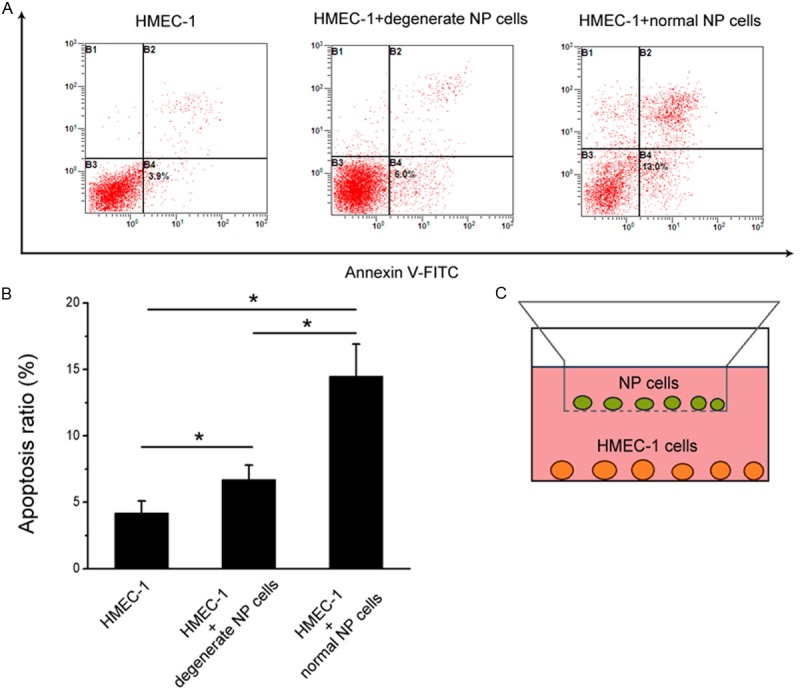
Flow cytometry apoptosis analysis of HMEC-1 cells after co-cultures with degenerate and normal NP cells. A. Contour diagram of Annexin V-FITC/PI FCM of HMEC-1 cells. The graphs stand for typical results of cell apoptosis; values represent the means for three experiments. B. Comparison of apoptotic cells in various groups. Data are representative of three experiments. Error bars represent SEM. *p<0.05. C. Schematic drawing of the co-culture system with the two types of cells.
Expression of FasL in NP cells supernatant
Studies have demonstrated that FasL expresses on NP cells. FasL produced by NP cells can act as an apoptosis inducer in immunocytes in our previous study [19]. As expected, we found an increased FasL expression in normal NP cells supernatant (67.4±5.2 pg/ml) than that in the degenerate NP cells supernatant (40.2±4.3 pg/ml). Meanwhile, after transfection of lentiviral vector encoding FasL, NP cells showed an increased FasL production (81.3±5.6 pg/ml). Transfection with lentiviral vector encoded scrambled sequence showed low FasL expression (45.1±3.9 pg/ml) (Figure 2).
Figure 2.
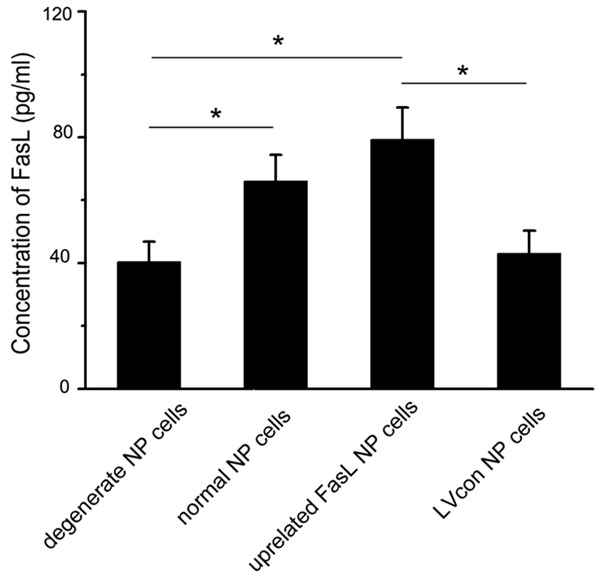
Levels of FasL in NP cells culture supernatants. FasL expression in normal NP cells supernatant is higher than that in the degenerate NP cells supernatant. After transfection of lentiviral vector encoding FasL, the FasL production of NP cells increases. Transfection with lentiviral vector encoded scrambled sequence shows low FasL expression. Data are representative of three experiments; error bars represent SEM. *p<0.05.
Up-regulated FasL of NP cells results in increased HMEC-1 cell apoptosis after co-cultures
The GFP-expressing lentiviral vector transfection at a MOI of 10 encoding FasL resulted in high-level GFP expression in NP cells (Figure 3). FCM detection revealed the apoptosis of HME-1 cells in different groups (Figure 4A). As for HMEC-1 cells without co-cultures, the apoptosis rate was 3.7±1.0%. Approximately 6.4±1.1% HMEC-1 cells were apoptotic when cultured with degenerate NP cells. It was noteworthy that NP cells with up-regulated FasL resulted in about 21.3±3.2% apoptosis rate of HMEC-1 cells (p<0.05). In the lentiviral-control group with scrambled sequence, the apoptosis rate of HMEC-1 was 5.8±.9% (Figure 4B).
Figure 3.
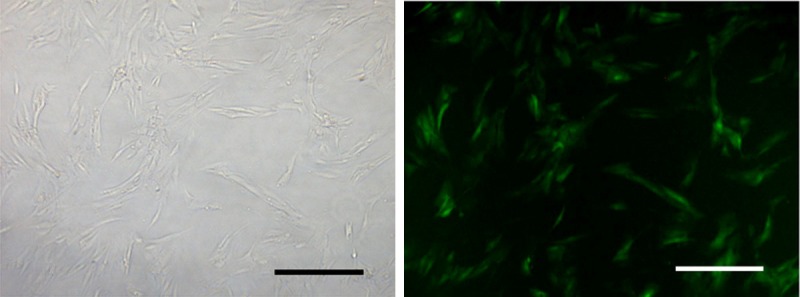
Up-regulation of FasL in NP cells. Bright-field (left) and fluorescent (right) microscopy of human NP cells 96 h following transfection with lentivirus encoding FasL labelled with GFP. Bar=30 μm.
Figure 4.
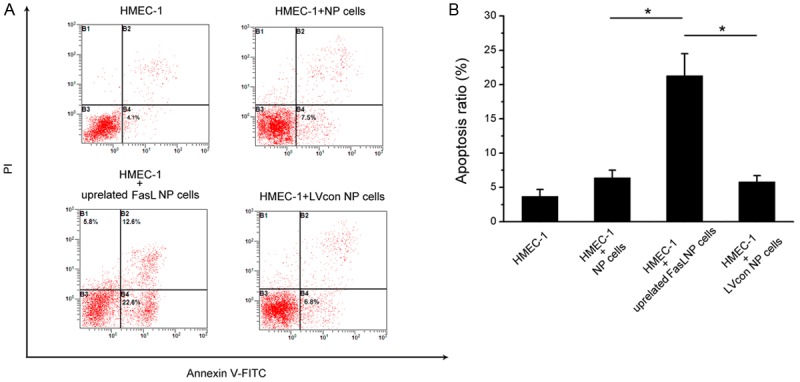
Flow cytometry apoptosis analysis of HMEC-1 cells after co-cultures with up-regulated FasL NP cells. A. Contour diagram of Annexin V-FITC/PI FCM of HMEC-1 cells. The graphs stand for typical results of cell apoptosis; values represent the means for three experiments. B. Comparison of apoptotic cells in various groups. Data are representative of three experiments. Error bars represent SEM. *p<0.05.
Exposure to NP cells increased the expression of Fas in HMEC-1 cell
Western blot analysis demonstrated the expression of Fas in the HMEC-1 cells. The expression of Fas-associated death domain-containing protein (FADD) and caspase-3 in HMEC-1 cells was increased after co-cultured with normal NP cells or up-regulated FasL degenerate NP cells. Moreover, Fas expression was increased following co-cultured with NP cells. Notably, NP cells with up-regulated FasL resulted in an increased Fas expression in HMEC-1 cell, which indicated FasL might be a key factor in the regulation of Fas expression (Figure 5).
Figure 5.
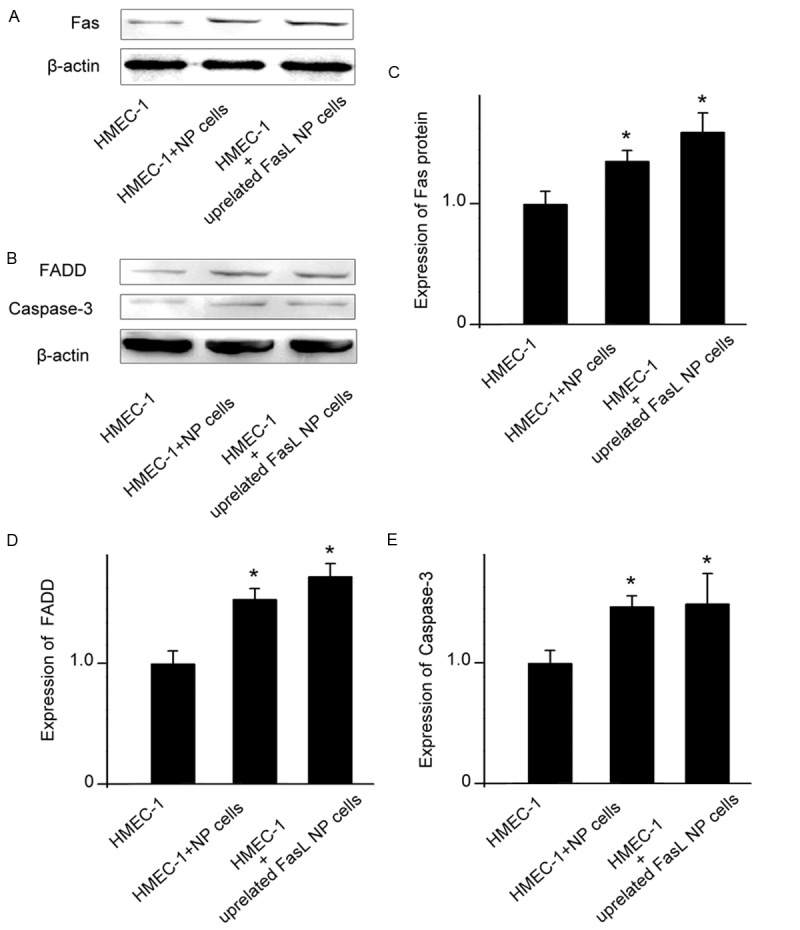
Western blotting analyses. A. Fas positive expression in HMEC-1 cells. The degree of Fas expression in HMEC-1 cells (lanes 3 and 4) is up-regulated following co-cultures with normal NP cells or degenerate FasL transfection NP cells. β-actin expression is almost the same in all samples. B. The degree of FADD and caspase-3 in HMEC-1 cells is up-regulated following co-cultures with normal NP cells or degenerate FasL transfection NP cells. β-actin expression is almost the same in all samples. C-E. Quantitative examination confirms the results. Error bars represent SEM. *p<0.05.
Discussion
Many pieces of evidence identify the expression of FasL on NP cells [17,22,23]. As one of the most important pathways of apoptosis, Fas-FasL caspases signaling pathway could result in the recruitment and activation of several key proteins and caspases, the chief of which are Fas-associated death domain-containing protein (FADD) and caspase-3 [24]. In immune privilege organs such as the eye and brain, as well as the intervertebral disc, FasL has been shown an immune cell death inducer and play an essential role in immune balance [19,25]. On the other hand, normal human intervertebral disc is characterized by avascularity with restricted blood vessels present in the outer few millimeters of the AF. With the degeneration of the disc, the blood vessels penetrate into NP tissue and cause downstream pathological alternations [9]. In this aspect, endothelial cells migration is the first important step of angiogenesis [26]. Available evidence has only shown an inducing function of degenerate NP to attract angiogenesis of blood vessel endothelial cells ingrowth. However, how normal NP prevents angiogenesis with molecular mechanisms remains largely unknown.
In this study, we established a co-culture system of NP cells and HMEC-1 cells. We found normal NP cells could induce more HMEC-1 cells apoptosis than degenerate NP cells. By up-regulating FasL expression in degenerate NP cells, we noted FasL as an essential factor in the mediation of HMEC-1 cell apoptosis. Furthermore, we found an increased Fas expression in HMEC-1 cells following co-cultured with NP cells, which might be closely related with FasL produced by NP cells.
Accumulating evidence has indicated that FasL is a key factor in the maintenance of the immune privilege of the disc by inducing apoptosis of immune cells [16,19]. As well, studies have shown Fas-mediated apoptosis plays essential roles in angiogenesis prevention in other tissues [27,28]. In addition, the studies of Park and colleagues showed disc cells could mediate Fas-bearing cancer cells undergo apoptosis by the production of FasL [29,30]. However, the relationship between FasL and blood vessel as one important channel of these types of cells infiltration has been ignored. Normal human NP is well encapsulated by the dense fibrous tissue of the AF and sandwiched by the cartilaginous endplates with restricted blood vessels. In the early stage of disc formation, FasL expression develops to a high degree with the blood vessels in the disc receding [31,32]. However, as the disc degenerates, FasL expression decreases along with angiogenesis and other pathological process in the disc [33].
To the best of our knowledge, this is the first study indicating that the avascular status of NP is not only due to the simple physiological barrier, but Fas-FasL network as active molecular mechanisms. It has been noted that focal proteoglycan loss of AF results in a matrix alternation that is conducive to nerve and blood vessel ingrowth [34]. Moreover, increased blood vessel ingrowth is associated with proteoglycan depletion AF lesion [35]. AF injury has the potential to initiate angiogenesis/nerve ingrowth and an inflammatory reaction through the interactions of AF and neural tissues [36]. Meanwhile, disc aggrecan is inhibitory to endothelial cell migration [37]. Moreover, mechanical stimulation influences the capacity of the disc to stimulate endothelial migration [38]. Nevertheless, these studies demonstrated an important role of passive physical barrier in preventing disc angiogenesis. Supplementary to previous studies, our findings indicate that besides the traditional barrier, FasL might be a molecular monitor in the status of disc avascular maintenance by inducing vascular endothelial cell apoptosis.
Consistent with previous studies that Fas is most conserved in HMEC-1 cell lines [39], our study noted the positive Fas expression in HMEC-1 cells. In addition, FasL could elevate the Fas expression of vascular endothelial cells, which might strengthen its impact on apoptosis by increasing FasL-Fas interaction. At this point, our findings are consistent with Han and colleagues, who noted an up-regulated Fas expression on NP cells following treatment of FasL and indicated a double role of FasL in the apoptosis of NP cells [40]. Therefore, FasL might play a more complex role in disc vascularization pathology. Further studies are needed to address this issue.
Notwithstanding our study deepen our understandings of vascular endothelial cells and FasL interaction in the disc; we acknowledge that there are several limitations in the study. For one, the in vitro co-culture environment is different from that in vivo with the feature of hypoxia, mechanical forces and diverse biological factors. For another, the co-culture condition of NP cells and HMEC-1 cells might be different from local vascular endothelial cells. However, as an immortalized human vascular endothelial cell line, HMEC-1 retains the morphologic, phenotypic, and functional characteristics of normal human vascular endothelial cells.
In conclusion, this study is the first successfully establishing an in vitro model addressing the interaction of human NP cells and vascular endothelial cells. Our findings show FasL-Fas network might play an important role in the molecular mechanisms of angiogenesis prevention of human disc. Consequently, our findings might shed light on the pathogenesis in human disc degeneration and provide a novel target for the treatment strategies for IDD.
Acknowledgements
This work was supported by Chinese National Natural Science Foundation Grants (No. 30901509, 81270028, No. 81171747, No. 81271342). We thank Jin-Tao Hu for the assistance in FCM analysis; Dan Li for helping obtain cadaver specimens.
Disclosure of conflict of interest
The authors have declared that no competing interest exists.
Abbreviations
- AF
annulus fibrosus
- bFGF
basic fibroblast growth factor
- FADD
Fas-associated death domain-containing protein
- FCM
Flow cytometry
- HMEC-1
human vascular endothelial cells
- IDD
intervertebral disc degeneration
- NP
nucleus pulposus
- PDGF
platelet-derived growth factor
- VEGF
vascular endothelial growth factor
References
- 1.Waddell G. Low back pain: a twentieth century health care enigma. Spine (Phila Pa 1976) 1996;21:2820–2825. doi: 10.1097/00007632-199612150-00002. [DOI] [PubMed] [Google Scholar]
- 2.Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine (Phila Pa 1976) 2004;29:2724–2732. doi: 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tschoeke SK, Hellmuth M, Hostmann A, Robinson Y, Ertel W, Oberholzer A, Heyde CE. Apoptosis of human intervertebral discs after trauma compares to degenerated discs involving both receptor-mediated and mitochondrial-dependent pathways. J Orthop Res. 2008;26:999–1006. doi: 10.1002/jor.20601. [DOI] [PubMed] [Google Scholar]
- 4.Williams FM, Bansal AT, van Meurs JB, Bell JT, Meulenbelt I, Suri P, Rivadeneira F, Sambrook PN, Hofman A, Bierma-Zeinstra S, Menni C, Kloppenburg M, Slagboom PE, Hunter DJ, Macgregor AJ, Uitterlinden AG, Spector TD. Novel genetic variants associated with lumbar disc degeneration in northern Europeans: a meta-analysis of 4600 subjects. Ann Rheum Dis. 2013;72:1141–1148. doi: 10.1136/annrheumdis-2012-201551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Z, Zhang M, Zhao XH, Liu ZH, Gao Y, Samartzis D, Wang HQ, Luo ZJ. Immune cascades in human intervertebral disc: the pros and cons. Int J Clin Exp Pathol. 2013;6:1009–1014. [PMC free article] [PubMed] [Google Scholar]
- 6.Wang HQ, Yu XD, Liu ZH, Cheng X, Samartzis D, Jia LT, Wu SX, Huang J, Chen J, Luo ZJ. Deregulated miR-155 promotes Fas-mediated apoptosis in human intervertebral disc degeneration by targeting FADD and caspase-3. J Pathol. 2011;225:232–242. doi: 10.1002/path.2931. [DOI] [PubMed] [Google Scholar]
- 7.Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94:1298–1304. doi: 10.1302/0301-620X.94B10.28986. [DOI] [PubMed] [Google Scholar]
- 8.Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, Ross ER, O’Brien JP, Hoyland JA. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–292. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 9.Mirza SK, White AA 3rd. Anatomy of intervertebral disc and pathophysiology of herniated disc disease. J Clin Laser Med Surg. 1995;13:131–142. doi: 10.1089/clm.1995.13.131. [DOI] [PubMed] [Google Scholar]
- 10.Fujita N, Imai J, Suzuki T, Yamada M, Ninomiya K, Miyamoto K, Iwasaki R, Morioka H, Matsumoto M, Chiba K, Watanabe S, Suda T, Toyama Y, Miyamoto T. Vascular endothelial growth factor-A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem Biophys Res Commun. 2008;372:367–372. doi: 10.1016/j.bbrc.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 11.Salo J, Kaigle Holm A, Indahl A, Mackiewicz Z, Sukura A, Holm S, Jamsen E, Konttinen YT. Expression of vascular endothelial growth factor receptors coincide with blood vessel in-growth and reactive bone remodelling in experimental intervertebral disc degeneration. Clin Exp Rheumatol. 2008;26:1018–1026. [PubMed] [Google Scholar]
- 12.Tolonen J, Gronblad M, Virri J, Seitsalo S, Rytomaa T, Karaharju EO. Platelet-derived growth factor and vascular endothelial growth factor expression in disc herniation tissue: and immunohistochemical study. Eur Spine J. 1997;6:63–69. doi: 10.1007/BF01676576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JM, Song JY, Baek M, Jung HY, Kang H, Han IB, Kwon YD, Shin DE. Interleukin-1beta induces angiogenesis and innervation in human intervertebral disc degeneration. J Orthop Res. 2011;29:265–269. doi: 10.1002/jor.21210. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholz B, Kinzelmann C, Benz K, Mollenhauer J, Wurst H, Schlosshauer B. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur Cell Mater. 2010;20:24–36. doi: 10.22203/ecm.v020a03. discussion 36-27. [DOI] [PubMed] [Google Scholar]
- 16.Takada T, Nishida K, Doita M, Kurosaka M. Fas ligand exists on intervertebral disc cells: a potential molecular mechanism for immune privilege of the disc. Spine (Phila Pa 1976) 2002;27:1526–1530. doi: 10.1097/00007632-200207150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Kaneyama S, Nishida K, Takada T, Suzuki T, Shimomura T, Maeno K, Kurosaka M, Doita M. Fas ligand expression on human nucleus pulposus cells decreases with disc degeneration processes. J Orthop Sci. 2008;13:130–135. doi: 10.1007/s00776-007-1204-4. [DOI] [PubMed] [Google Scholar]
- 18.Depraetere V, Golstein P. Fas and other cell death signaling pathways. Semin Immunol. 1997;9:93–107. doi: 10.1006/smim.1997.0062. [DOI] [PubMed] [Google Scholar]
- 19.Liu ZH, Sun Z, Wang HQ, Ge J, Jiang TS, Chen YF, Ma Y, Wang C, Hu S, Samartzis D, Luo ZJ. FasL Expression on Human Nucleus Pulposus Cells Contributes to the Immune Privilege of Intervertebral Disc by Interacting with Immunocytes. Int J Med Sci. 2013;10:1053–1060. doi: 10.7150/ijms.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Z, Wan ZY, Liu ZH, Guo YS, Yin JB, Duan CG, Gao Y, Li T, Wang HQ, Luo ZJ. Expression of soluble Fas and soluble FasL in human nucleus pulposus cells. Int J Clin Exp Pathol. 2013;6:1567–1573. [PMC free article] [PubMed] [Google Scholar]
- 21.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 22.Park JB, Chang H, Kim KW. Expression of Fas ligand and apoptosis of disc cells in herniated lumbar disc tissue. Spine (Phila Pa 1976) 2001;26:618–621. doi: 10.1097/00007632-200103150-00011. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto J, Maeno K, Takada T, Kakutani K, Yurube T, Zhang Z, Hirata H, Kurakawa T, Sakai D, Mochida J, Doita M, Kurosaka M, Nishida K. Fas ligand plays an important role for the production of pro-inflammatory cytokines in intervertebral disc nucleus pulposus cells. J Orthop Res. 2013;31:608–615. doi: 10.1002/jor.22274. [DOI] [PubMed] [Google Scholar]
- 24.Zhao CQ, Jiang LS, Dai LY. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11:2079–2088. doi: 10.1007/s10495-006-0290-7. [DOI] [PubMed] [Google Scholar]
- 25.Lettau M, Paulsen M, Schmidt H, Janssen O. Insights into the molecular regulation of FasL (CD178) biology. Eur J Cell Biol. 2011;90:456–466. doi: 10.1016/j.ejcb.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Marcelo KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and specification. Circ Res. 2013;112:1272–1287. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang JH, Yang JS, Lu CC, Hour MJ, Chang SJ, Lee TH, Chung JG. Newly synthesized quinazolinone HMJ-38 suppresses angiogenetic responses and triggers human umbilical vein endothelial cell apoptosis through p53-modulated Fas/death receptor signaling. Toxicol Appl Pharmacol. 2013;269:150–162. doi: 10.1016/j.taap.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Mannam VK, Lewis RE, Cruse JM. The fate of renal allografts hinges on responses of the microvascular endothelium. Exp Mol Pathol. 2013;94:398–411. doi: 10.1016/j.yexmp.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Park JB, Lee JK, Cho ST, Park EY, Riew KD. A biochemical mechanism for resistance of intervertebral discs to metastatic cancer: Fas ligand produced by disc cells induces apoptotic cell death of cancer cells. Eur Spine J. 2007;16:1319–1324. doi: 10.1007/s00586-007-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JB, Lee JK, Park EY, Riew KD. Fas/FasL interaction of nucleus pulposus and cancer cells with the activation of caspases. Int Orthop. 2008;32:835–840. doi: 10.1007/s00264-007-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inui Y, Nishida K, Doita M, Takada T, Miyamoto H, Yoshiya S, Kurosaka M. Fas-ligand expression on nucleus pulposus begins in developing embryo. Spine (Phila Pa 1976) 2004;29:2365–2369. doi: 10.1097/01.brs.0000143172.07771.fc. [DOI] [PubMed] [Google Scholar]
- 32.Smith LJ, Elliott DM. Formation of lamellar cross bridges in the annulus fibrosus of the intervertebral disc is a consequence of vascular regression. Matrix Biol. 2011;30:267–274. doi: 10.1016/j.matbio.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rätsep T, Minajeva A, Asser T. Relationship between neovascularization and degenerative changes in herniated lumbar intervertebral discs. Eur Spine J. 2013 Jun 5; doi: 10.1007/s00586-013-2842-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefanakis M, Al-Abbasi M, Harding I, Pollintine P, Dolan P, Tarlton J, Adams MA. Annulus fissures are mechanically and chemically conducive to the ingrowth of nerves and blood vessels. Spine (Phila Pa 1976) 2012;37:1883–1891. doi: 10.1097/BRS.0b013e318263ba59. [DOI] [PubMed] [Google Scholar]
- 35.Melrose J, Roberts S, Smith S, Menage J, Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine (Phila Pa 1976) 2002;27:1278–1285. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- 36.Moon HJ, Kim JH, Lee HS, Chotai S, Kang JD, Suh JK, Park YK. Annulus fibrosus cells interact with neuron-like cells to modulate production of growth factors and cytokines in symptomatic disc degeneration. Spine (Phila Pa 1976) 2012;37:2–9. doi: 10.1097/BRS.0b013e31820cd2d8. [DOI] [PubMed] [Google Scholar]
- 37.Johnson WE, Caterson B, Eisenstein SM, Roberts S. Human intervertebral disc aggrecan inhibits endothelial cell adhesion and cell migration in vitro. Spine (Phila Pa 1976) 2005;30:1139–1147. doi: 10.1097/01.brs.0000162624.95262.73. [DOI] [PubMed] [Google Scholar]
- 38.Neidlinger-Wilke C, Liedert A, Wuertz K, Buser Z, Rinkler C, Kafer W, Ignatius A, Claes L, Roberts S, Johnson WE. Mechanical stimulation alters pleiotrophin and aggrecan expression by human intervertebral disc cells and influences their capacity to stimulate endothelial migration. Spine (Phila Pa 1976) 2009;34:663–669. doi: 10.1097/BRS.0b013e318194e20c. [DOI] [PubMed] [Google Scholar]
- 39.Lidington EA, Moyes DL, McCormack AM, Rose ML. A comparison of primary endothelial cells and endothelial cell lines for studies of immune interactions. Transpl Immunol. 1999;7:239–246. doi: 10.1016/s0966-3274(99)80008-2. [DOI] [PubMed] [Google Scholar]
- 40.Han D, Ding Y, Liu SL, Wang G, Si IC, Wang X, Cui L, Huang D. Double role of Fas ligand in the apoptosis of intervertebral disc cells in vitro. Acta Biochim Biophys Sin (Shanghai) 2009;41:938–947. doi: 10.1093/abbs/gmp087. [DOI] [PubMed] [Google Scholar]


