Abstract
Background: We investigated a series of pancreaticoduodenectomy and duodenal biopsies with a panel of immunohistochemical markers to identify duodenal mucosal invasion by pancreatic ductal adenocarcinoma (PDAC), including markers of poor prognosis and targets of promising novel immunotherapies. Materials and Methods: Eighteen consecutive pancreaticoduodenectomy specimens with duodenal mucosal invasion by PDAC were examined for expression of MUC1, MUC4, MUC5AC, MUC6, mesothelin, MUC2, CDX2, and DPC4 on formalin-fixed, paraffin-embedded sections of duodenal-ampullary-pancreatic junctions. Expression of all but MUC6 was also assessed in duodenal biopsies from 12 patients with duodenal mucosal invasion by PDAC. Results: The duodenal mucosa expressed MUC1 (crypts), MUC2 (goblet cells), MUC6 (Brunner glands), CDX2, and DPC4. PDACs in the duodenal mucosa from the resection (n=16-18) and biopsy (n=12) specimens were marked as follows: MUC1 100% (30/30), MUC4 83% (24/29), MUC5AC 83% (25/30), mesothelin 82% (23/28), MUC2 7% (2/30), and CDX2 36% (10/28). Loss of DPC4 expression was seen in 16 of 29 (55%) cases. Reactive mucosa adjacent to PDAC expressed MUC4, MUC5AC and mesothelin in 65% (17/26), 19% (5/27), and 19% (5/26) of cases, respectively. While MUC5AC and mesothelin had high diagnostic accuracy for detection of PDAC, MUC2, CDX2 and DPC4 expression demonstrated negative correlation with PDAC, with absent expression being highly specific for PDAC. Conclusion: Immunohistochemical labeling for PDAC biomarkers may aid the diagnosis of PDAC in duodenal biopsy, especially in situations where diagnosis of a pancreatic mass is challenging.
Keywords: Pancreatic ductal adenocarcinoma, duodenal mucosal invasion, immunohistochemistry
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer deaths in the United States with a 5-year overall survival rate less than 6% [1,2]. The best chance for cure is surgical resection; however, less than 20% of patients are eligible for this procedure due to advanced disease at diagnosis [2]. Even in this select surgical population, the 5-year overall survival is only 18-24% [1,3,4]. These facts underscore the significant medical problem presented by this disease.
The initial workup for obstructive jaundice and/or a pancreatic mass may include endoscopic duodenal biopsy. The pathologic diagnosis of PDAC on such endoscopic biopsies is challenging at best. Common pitfalls include overdiagnoses of reactive epithelial changes, or underdiagnoses of histologically bland but biologically aggressive PDAC colonizing duodenal mucosa. In cases of apparently benign duodenal biopsies on hematoxylin and eosin stain, an immunohistochemical panel identifying duodenal mucosal involvement by PDAC may increase the sensitivity of detection with accurate diagnosis, thus accelerating a potential curative resection.
Many secreted and plasma membrane-bound molecules are over-expressed in PDAC. These include mesothelin, growth factor receptors, and a number of mucins (MUC1, MUC4, and MUC5AC) [5-18]. Mucins are highly complex molecules with extensive O-linked glycosylation of their extracellular domain, and may display specific epitope structures during the development and progression of PDAC [19]. Several monoclonal antibodies (MAbs) have been developed to label specific mucin epitopes that display altered distribution as a consequence of neoplasia [20,21]. Loss of expression of tumor suppressor genes is also common in PDAC. For example, loss of the tumor suppressor gene DPC4/SMAD4 occurs in approximately half of all cases of PDAC, and is associated with aggressive behavior and widespread tumor metastases [22-24]. Additionally, the majority of PDAC specimens do not have an intestinal phenotype, and markers of intestinal differentiation such as MUC2 and CDX2 may help differentiate PDACs from adjacent non-neoplastic duodenal epithelium or primary duodenal adenocarcinoma [25-27].
In this study, we examined 18 consecutive pancreaticoduodenectomy specimens and 12 duodenal biopsies showing duodenal mucosal invasion by PDAC by immunohistochemical labeling to develop a strategic panel of biomarkers for improved detection of PDAC in the duodenal mucosa.
Materials and methods
Tissue specimens
This HIPAA-compliant study was approved by the Institutional Review Board at Vanderbilt University. One hundred fifty patients with PDAC who underwent pancreaticoduodenectomy at Vanderbilt University Medical Center from 01/2005 to 12/2009 were identified, among which 18 (12%) demonstrated duodenal mucosal invasion by PDAC. Formalin-fixed, paraffin-embedded sections containing the duodenal-ampullary-pancreatic junctions from these 18 cases were used to perform immunohistochemical studies. Twelve duodenal biopsies from patients with duodenal mucosal invasion by PDAC were also identified from 01/2009 to 12/2011 and subjected to immunohistochemical studies.
Immunohistochemistry
Immunohistochemical labeling for MUC1, MUC2, MUC4, MUC5AC, MUC6, mesothelin, CDX2, and DPC4 was performed on 5 μm-thick formalin-fixed, paraffin-embedded sections of duodenal-ampullary-pancreatic junctions and duodenal biopsies as described below (MAb clones, their commercial source, and dilution employed for these studies are provided in Table 1). Biomarker expression patterns were independently recorded by three pathologists (SCW, PG, CS) and were compared with tumor grade. All stains were recorded as follows: diffuse, ≥25%; focal, 1%-25%; negative, <1%. The intensity of the stain was graded as strong, moderate or weak.
Table 1.
Primary antibodies comprising the immunopanel
| Antibody (clone) | Dilution | Species | Commercial source | Catalog# |
|---|---|---|---|---|
| MUC1 (VU4H5) | 1:100 | mouse | Cell Signaling | 4538 |
| MUC2 (Ccp58) | 1:300 | mouse | Abcam | ab49460 |
| MUC4 (8G7) | 10 μg/mL | mouse | Santa Cruz Biotech | sc-53945 |
| MUC5AC (Ab-1) | 1:300 | mouse | Thermo Scientific | MS-145 |
| MUC6 (N.A.) | 1:200 | mouse | Novacastra | NCL-MUC6 |
| CDX2 | 1:400 | rabbit | Cell Signaling | 3977 |
| DPC4 (SMAD4) | 1:50 | mouse | Santa Cruz Biotech | sc-7966 |
| Mesothelin | 1:200 | rabbit | Abcam | ab93620 |
Unstained slides were deparaffinized by routine methods with antigen retrieval performed by heating the slides in a pH 6.0 citrate buffer at 100°C for 20 minutes followed by a 10-minute cooling to room temperature. All slides were then quenched with 0.03% H2O2 with sodium azide for 5 minutes. Slides were blocked for 20 minutes with serum-free protein block (Dako, Carpentaria, CA), followed by application of primary antibody (Table 1) for 60 min (except for anti-MUC1 MAb which was left on the tissue sections overnight). MAbs were detected by incubation with Envision+HRP-labeled polymer (Dako) for 30 minutes, followed by 5 minute incubation with DAB (Dako).
Statistics
A statistical comparison of biomarker expression was performed. For each biomarker, the labeling results of individual specimens were converted to either negative (0) or positive (1) values for benign duodenal tissue and duodenal mucosa involved by PDAC, with the data analyzed by receiver-operating characteristic curves.
Results
Eighteen of 150 resected PDACs demonstrated duodenal mucosal involvement. These cases were graded as follows: well-differentiated (2/18, 11%), moderately differentiated (10/18, 56%), and poorly differentiated (6/18, 33%). Mucosal involvement manifested as malignant cells colonizing the duodenal epithelium (Figure 1A) and/or malignant glands infiltrating the mucosa (Figure 2A). The biomarker profiles were similar for both types of mucosal involvement (Figures 1B-H and 2B-H), and are summarized in Table 2 for benign duodenal mucosa and duodenal mucosal invasion by PDAC.
Figure 1.

Duodenal mucosal involvement by pancreatic ductal adenocarcinoma (PDAC) in an epithelial colonization fashion (original magnification 200X). A. Hematoxylin and eosin stain showing duodenal epithelial cells replaced by malignant cells (Black arrows: malignant epithelium; Yellow arrows: normal epithelium); B. Diffuse strong MUC1 expression in PDAC; C. Diffuse MUC4 expression in PDAC; D. MUC5AC expression in PDAC; E. Diffuse mesothelin expression in PDAC; F. Lack of MUC2 expression in PDAC (Black arrows: malignant epithelium); G. No CDX2 expression in PDAC (Black arrows: malignant epithelium); H. Lack of DPC4 expression in PDAC (Black arrows: malignant epithelium).
Figure 2.
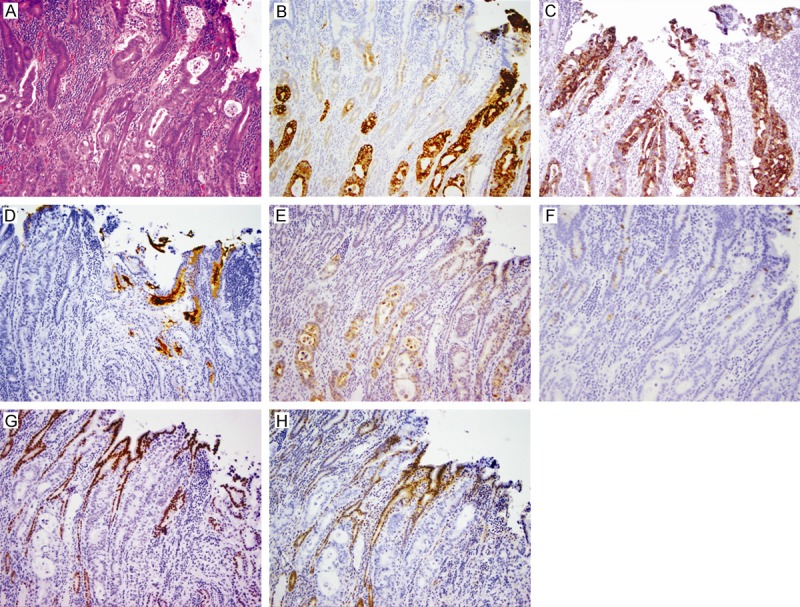
Duodenal mucosal involvement by pancreatic ductal adenocarcinoma in a glandular infiltrating fashion (original magnification 100X). A. Hematoxylin and eosin stain showing malignant glands in the duodenal mucosa; B. Diffuse strong MUC1 expression in malignant glands; C. Diffuse strong expression of MUC4 in malignant glands; D. Focal strong MUC5AC expression in malignant glands (note: MUC5AC-positive mucin in the glands); E. Diffuse mesothelin expression in malignant glands; F. Lack of MUC2 expression in malignant glands; G. No CDX2 expression in malignant glands; H. Loss of DPC4 in malignant glands.
Table 2.
Expression of biomarkers in the duodenal mucosa and DMI-PDAC
| Resection | Biopsy | Overall | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Duodenum | PDAC | Duodenum | PDAC | Duodenum | PDAC | |
| MUC1 | 17/18 (94%) | 18/18 (100%) | 9/9 (100%) | 12/12 (100%) | 26/27 (96%) | 30/30 (100%) |
| MUC4 | 8/17 (47%) | 14/17 (82%) | 9/9 (100%) | 10/12 (83%) | 17/26 (65%) | 24/29 (83%) |
| MUC5AC | 3/18 (17%) | 17/18 (94%) | 2/9 (22%) | 8/12 (67%) | 5/27 (19%) | 25/30 (83%) |
| MUC6 | 17/18 (94%) | 7/18 (39%) | - | - | 17/18 (94%) | 7/18 (39%) |
| Mesothelin | 5/17 (29%) | 15/16 (94%) | 0/9 (0%) | 8/12 (67%) | 5/26 (19%) | 23/28 (82%) |
| MUC2a | 0/18 (0%) | 17/18 (94%) | 0/9 (0%) | 11/12 (92%) | 0/27 (0%) | 28/30 (93%) |
| CDX2a | 0/16 (0%) | 11/16 (69%) | 0/9 (0%) | 7/12 (58%) | 0/25 (0%) | 18/28 (64%) |
| DPC4a | 0/17 (0%) | 8/17 (47%) | 0/12 (0%) | 8/12 (67%) | 0/29 (0%) | 16/29 (55%) |
We first compared MUC1 expression in PDACs in the duodenal mucosa to that in benign duodenal mucosa using an antibody reactive with the VNTR (VU4H5). This MAb labeled background duodenal crypts and Brunner’s glands, but rarely duodenal villi. Labeling for MUC1 in the duodenal mucosa progressed from positive in the deep crypts to negative in the villi. Eighteen of 18 (100%) PDACs in the duodenal mucosa labeled for MUC1, with 15 of them (83%) showing strong, diffuse MUC1 expression. MUC1 labeling abruptly transitioned from positive in PDACs to negative in benign duodenal villi, which made it recognizable from background duodenal epithelium (Figure 1B). In addition, compared to background duodenal crypts, PDAC was frequently more intensely labeled.
Immunohistochemical labeling for MUC4 demonstrated that 82% (14/17) of PDACs expressed at least focal MUC4 (Table 2), 4 with focal expression and 10 with moderate to strong, diffuse expression (Figures 1C and 2C). However, scattered MUC4 positive cells were also present in the reactive mucosa adjacent to PDAC in 8 of 17 (47%) cases studied.
The expression of MUC5AC in PDAC was also examined. All but one PDACs (17/18, 94%) were labeled with anti-MUC5AC antibody (Table 2, Figures 1D and 2D). MUC5AC was not expressed by normal duodenal mucosa, but was expressed by gastric metaplasia. Duodenal gastric metaplasia is not uncommon in settings of chronic injury/inflammation. MUC5AC expression is, therefore, not entirely specific for PDAC. However, compared to its weak expression in the metaplastic mucosa, MUC5AC expression in PDAC was very strong in most positive cases, and thick MUC5AC positive mucin was frequently observed within cancerous glands.
MUC6 was frequently expressed by duodenal mucosa (94%), especially Brunner’s glands, whereas only 39% (7/18) of PDACs expressed MUC6. Therefore, MUC6 appeared to be a poor marker for differentiation of PDACs from the duodenal mucosa.
Mesothelin is a biomarker that is highly expressed in PDAC [9,10,13]. Nearly all PDACs (94%) expressed this marker (Figures 1E and 2E). While the labeling was mostly strong, and especially prominent in the luminal surface membranes of the tumor cells, four of them only had focal expression of mesothelin. In addition, the expression of mesothelin was also observed in reactive duodenal mucosa in some (29%) cases.
The expressions of two intestinal biomarkers, MUC2 and CDX2, in PDAC were also explored and compared to the benign mucosa. Background duodenal villi and crypts showed MUC2 labeling in goblet cells (100%), while 17/18 (94%) PDACs lacked the expression of this mucin (Figures 1F and 2F). All duodenal epithelial cells expressed nuclear CDX2. In PDACs, 69% of cases did not express CDX2 (Figures 1G and 2G). Therefore, lack of CDX2 and MUC2 expression may be useful as confirming evidence for detection/diagnosis of PDAC.
Loss of DPC expression is frequently seen in PDAC. Nuclear expression of DPC4 was present in normal duodenal mucosa, stromal and inflammatory cells. However, 47% PDACs did not express DPC4 (Table 2, Figures 1H and 2H). For that reason, complete loss of DPC4 expression is highly specific for PDAC when compared to background benign mucosa; however, the sensitivity is low.
Since some of the biomarkers studied were only focally expressed in the resection specimens, their expression was also examined in duodenal biopsies from patients with known PDAC to determine the feasibility of using these biomarkers in biopsy specimens. Twelve patients were identified, including 1 with well differentiated, 6 moderately differentiated, 2 poorly differentiated, and 3 undifferentiated carcinomas. MUC1 was diffusely and focally expressed in 8 (66%) and 4 (34%) of the 12 cases, respectively. Similar to the findings from the resection specimens, the well to moderately differentiated carcinomas (Figure 3A) had diffuse, much stronger MUC1 labeling than background duodenal mucosa (Figure 3B). Therefore, although MUC1 expression is not specific, the intensity of MUC1 labeling might be helpful in distinguishing PDAC from benign duodenal mucosa.
Figure 3.
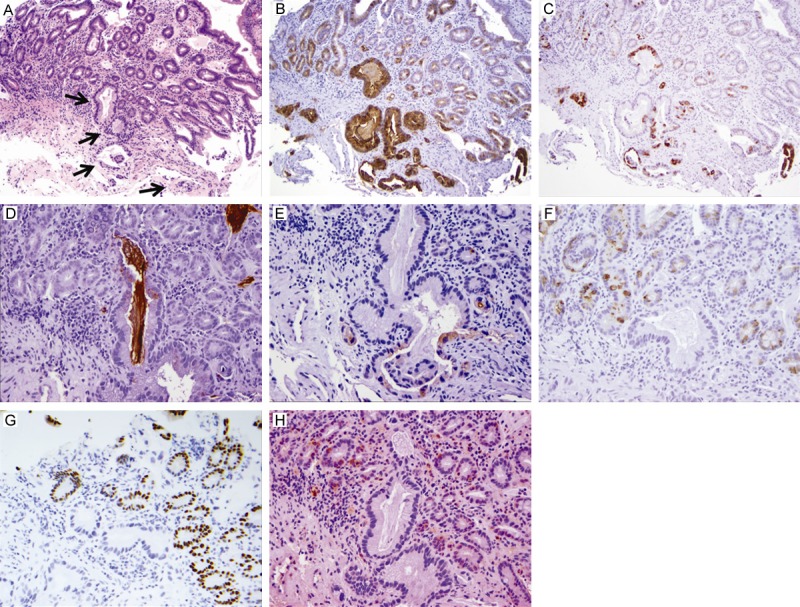
Duodenal mucosal involvement by pancreatic ductal adenocarcinoma (PDAC) in one duodenal biopsy. A. Hematoxylin and eosin stain showing malignant glands in the duodenal biopsy (original magnification 100X; black arrows: malignant glands); B. Diffuse strong MUC1 expression in malignant glands (100X); C. MUC4 expression in malignant glands (100X; note: scattered MUC4 expression in background duodenal mucosa); D. MUC5AC expression in malignant glands (200X; note: MUC5AC positive mucin in the glands); E. Focal mesothelin expression by malignant glands (200X); F. Lack of MUC2 expression in one large malignant glands (200X); G. No CDX2 expression in malignant glands (200X); H. Loss of DPC4 expression in malignant glands (200X).
Ten of the 12 (83%) cases expressed at least focal MUC4 in the biopsies, including 5 (42%) with diffuse, strong expression (Figure 3C). However, the adjacent duodenal mucosa also showed scattered positive cells. Thus MUC4 may not be suitable for differentiating PDAC from duodenal mucosa. MUC5AC was expressed in 67% (8/12) of the cases, and frequently was present within the malignant glands (Figure 3D). Due to the fact that MUC5AC is expressed by the mucosa with gastric metaplasia, the expression of MUC5AC was also not specific for PDAC. However, the intensity and the pattern (thick mucin gel within the glandular structures) of the stain may aid in distinguishing between PDAC and the adjacent mucosa.
MUC1, MUC4, and MUC5AC were expressed by all well to moderately differentiated PDACs in the duodenal biopsies. The expression profiles of each mucin appeared to correlate with grade of tumor differentiation, although expression of MUC4 has been reported to correlate with poor differentiation in PDAC [5,28]. Mesothelin expression was seen in 67% of cases, but unlike the resection specimens, the biopsies did not show mesothelin expression in background duodenal mucosa (Figure 3E). Similar to the mucin markers, mesothelin expression was not detected in the 3 undifferentiated carcinomas.
MUC2 expression was seen in only one biopsy with features of mucinous (colloid) adenocarcinoma. Background duodenal mucosa demonstrated scattered MUC2 expressing goblet cells, but some cross sections of small duodenal crypts could be lack of goblet cells. Therefore, absent MUC2 expression in small glands may not correlate with invasive tumor glands. However, lack of MUC2 expression in large glandular structures may be suggestive of malignancy (Figure 3F). CDX2 (Figure 3G) and DPC4 (Figure 3H) were not expressed in 58% and 67% of the cases, respectively.
A statistical comparison of biomarker expression is presented in Table 3, sorted by diagnostic accuracy (highest to lowest). MUC2 as a single biomarker provided the highest diagnostic accuracy, but has a negative correlation with PDAC. In addition, as mentioned above, small duodenal crypts might be lack of goblet cells. Therefore, it is unlikely that this biomarker would prove useful as a stand-alone agent, but rather as a means to provide confirmation while another biomarker provides positive reaction with PDAC. Of those biomarkers with positive correlation to PDAC, MUC5AC and mesothelin were not statistically different (P>0.05).
Table 3.
Diagnostic performance of individual biomarkers sorted by diagnostic accuracy
| Sensitivity (%) | Specificity (%) | Diagnostic Accuracy (%) | |
|---|---|---|---|
| MUC2a | 93 | 100 | 97 |
| MUC5AC | 83 | 81 | 82 |
| CDX2a | 64 | 100 | 81 |
| Mesothelin | 82 | 81 | 81 |
| DPC4a | 55 | 100 | 78 |
| MUC4 | 83 | 35 | 60 |
| MUC1 | 100 | 4 | 54 |
| MUC6 | 39 | 6 | 44 |
These biomarkers have a negative correlation with PDAC.
Discussion
A biomarker panel comprised of well-known, reliable immunohistochemical markers (MUC1, MUC5AC, MUC2, mesothelin, and CDX2), and those with potential prognostic and/or theranostic significance (MUC1, MUC4, MUC5AC, and DPC4) was chosen for evaluation of PDAC [5,14,17,28,29]. Some of these biomarkers (MUC1, mesothelin) represent promising targets of immunotherapy through the use of radiolabeled antibodies or vaccines [30-39], while modulation of MUC4 expression lends increased sensitivity to front-line chemotherapy [40], and the expression of MUC1, MUC5AC and mesothelin provide insight into patterns of tumor development, spread, and aggression [7,14,41,42]. Therefore, this immunohistochemical panel may offer a sensitive method of detection of PDAC with an immediate, multifaceted assessment of tumor behavior and susceptibilities. In addition, this panel may have potential to maximize the information derived from biopsies and better direct therapy in the initial workup of pancreatic cancer patients.
Five of the 8 biomarkers analyzed in this study are mucins. Two subfamilies of mucin species have been described: secreted and plasma membrane-bound. Membrane-bound mucins have a structure composed of an integral transmembrane domain, a short cytoplasmic tail, and an extracellular, juxtamembrane domain with homology to the epidermal-growth-factor (EGF) family [43]. A paracrine signaling pathway is implicated, with binding of EGF-like domains to EGF receptors, to regulate growth, motility, differentiation, inflammation, or other functions. One of the membrane-bound mucins, MUC1, is expressed early in the pancreatic ductal dysplasia-carcinoma sequence and is found in approximately 90% of PDAC cases [12,15]. The expression of specific MUC1 structures/epitopes is associated with tumor progression and decreased cellular adhesion through cytoplasmic tail interaction with the β-catenin and MAPK pathways [35,43]. MUC1 is also a receptor for myelin-associated glycoprotein on Schwann cells and oligodendroglia, which contributes to the extensive perineural invasion seen in the majority of PDAC specimens [44]. Additionally, the extracellular juxtamembrane domain interacts with the ErbB family [45], and activates ERK and Akt, promoting cellular proliferation [35]. In vitro studies have shown decreased proliferation and migration through decreased ERK phosphorylation with anti-MUC1 antibody treatment [35], which may represent a novel future immunotherapy.
Another member of the membrane-bound mucin subfamily, MUC4, is aberrantly expressed in precancerous pancreatic intraepithelial neoplasias (PanINs) and PDACs, and is not expressed in normal pancreas. Expression correlates with PanIN dysplasia [17], and with tumor grade [8,28]. MUC4 contains an extracellular juxtamembrane domain EGF-like motif which interacts with HER2/neu (ErbB2) and shows sequence homology to heregulin [43]. Signaling is postulated to occur through p27 and MAPK pathways, and is associated with tumor growth, proliferation, resistance to apoptosis, survival, and tumor invasion/metastasis [8]. In a recent multivariate analysis, MUC4 was the only significant factor associated with a poor prognosis [8]. In vitro studies have shown that MUC4 downregulation improves gemcitabine response [40]. Our study showed no association of immunohistochemical expression with poor tumor grade, which may be reflective of a small sample size. Scattered MUC4 positive duodenal epithelial cells were seen and especially prominent in the area closely adjacent to PDAC. Although MUC4 is not specific for PDAC, identification of its expression may help choose a better treatment strategy for patients with PDAC in the future.
MUC5AC, a member of the secreted subfamily, is a gastric-type mucin expressed by normal gastric epithelium but not normal pancreas. Like MUC1, MUC5AC is expressed early in the pancreatic-ductal dysplasia sequence [7,20]; however, unlike MUC1, it is expressed less frequently and shows loss of expression in high grade tumors [7,14]. Some studies have demonstrated that expression is associated with a more favorable prognosis [7,14], while others have shown an association with a poor prognosis [18,41]. Loss of MUC5AC expression is associated with lymphovascular invasion and lymph node metastasis [14], while xenograft studies have shown that MUC5AC expression may allow pancreatic cancer cells to evade immunosurveillance [19]. Recent studies show that MUC5AC expression is associated with decreased E-cadherin-dependent cell-cell adhesion and the β-catenin signaling pathway [41]. The association of MUC5AC staining intensity with tumor grade was observed in the present study, with undifferentiated carcinoma showing little to no expression.
Expression of MUC5AC was observed in the majority of PDAC. However, this biomarker was also expressed in gastric mucin cell metaplasia of the duodenal mucosa, a phenomenon following chronic stimulation due to inflammatory processes or malignant involvement. We observed that expression of the biomarker was weak in gastric mucin cell metaplasia, whereas the expression was often strong in PDAC. In addition, gastric mucin cell metaplasia is easily recognized on histologic ground and usually located at the surface of the mucosa. Therefore, immunohistochemical labeling for MUC5AC can still be a powerful tool to diagnose PDAC.
Mesothelin, a GPI-anchored protein whose function is unknown, but may mediate cell-cell adhesion, is a recently identified biomarker for PDAC and is not expressed in normal pancreas, although its expression in duodenal mucosa has been observed in some cases. Its expression is minimal in PanINs but is found in nearly all cases of PDAC [13,46]. Studies of pancreatic fine needle aspirate biopsies demonstrate a high specificity for pancreatic cancer as compared with normal pancreas or reactive atypia [10,16]. Due to its relative specificity for a small number of tumors (PDAC, ovarian carcinoma, and mesothelioma), it is a new target for immunotherapies, with some success [30-32,34,36]. Patients with PDAC may be chosen for mesothelin-targeting therapy based on IHC detection of its expression using duodenal biopsy, should PDAC be present.
Well to moderately differentiated PDAC may invade the duodenum, where tumor glands/cells can closely mimic reactive duodenal epithelium. Here we demonstrated that MUC5AC and mesothelin, having a positive correlation with the presence of PDAC, may be used to discriminate PDAC from duodenal epithelium. Confirmation may be demonstrated by use of a biomarker with negative correlation, that is, high expression within the background, histologically normal duodenal tissue, such as MUC2. In addition, the intensity and pattern of MUC1 may be useful to distinguish PDAC from background duodenal mucosa. However, morphology remains the most important part of diagnosis, and immunohistochemical results should be evaluated in context of morphology and the patient’s clinical history.
In summary, detection of PDAC in biopsy specimens is a diagnostic challenge. When hematoxylin and eosin observations show morphologically suspicious glands/cells, immunohistochemical stains for these biomarkers, may be helpful in the diagnostic workup and provide timely information that can provide evidence of the tumor’s biologic behavior for efficient therapeutic decision-making.
Acknowledgements
This project is funded by NIH grant P50CA095103 to CS and KW.
Disclosure of conflict of interest
None.
References
- 1.Surveillance Epidemiology and End Results. SEER Fact Sheets. 2012.
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 4.Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, Zahurak ML, Dooley WC, Coleman J, Sauter PK, Pitt HA, Lillemoe KD, Cameron JL. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225:621–633. doi: 10.1097/00000658-199705000-00018. discussion 633-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westgaard A, Schjolberg AR, Cvancarova M, Eide TJ, Clausen OP, Gladhaug IP. Differentiation markers in pancreatic head adenocarcinomas: MUC1 and MUC4 expression indicates poor prognosis in pancreatobiliary differentiated tumours. Histopathology. 2009;54:337–347. doi: 10.1111/j.1365-2559.2009.03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamasaki H, Ikeda S, Okajima M, Miura Y, Asahara T, Kohno N, Shimamoto F. Expression and localization of MUC1, MUC2, MUC5AC and small intestinal mucin antigen in pancreatic tumors. Int J Oncol. 2004;24:107–113. [PubMed] [Google Scholar]
- 7.Yonezawa S, Horinouchi M, Osako M, Kubo M, Takao S, Arimura Y, Nagata K, Tanaka S, Sakoda K, Aikou T, Sato E. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999;49:45–54. doi: 10.1046/j.1440-1827.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Zhang JJ, Zhu R, Zhu Y, Liang WB, Gao WT, Yu JB, Xu ZK, Miao Y. The increase in the expression and hypomethylation of MUC4 gene with the progression of pancreatic ductal adenocarcinoma. Med Oncol. 2011;28(Suppl 1):S175–S184. doi: 10.1007/s12032-010-9683-0. [DOI] [PubMed] [Google Scholar]
- 9.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 10.Baruch AC, Wang H, Staerkel GA, Evans DB, Hwang RF, Krishnamurthy S. Immunocytochemical study of the expression of mesothelin in fine-needle aspiration biopsy specimens of pancreatic adenocarcinoma. Diagn Cytopathol. 2007;35:143–147. doi: 10.1002/dc.20594. [DOI] [PubMed] [Google Scholar]
- 11.Bhardwaj A, Marsh WL Jr, Nash JW, Barbacioru CC, Jones S, Frankel WL. Double immunohistochemical staining with MUC4/p53 is useful in the distinction of pancreatic adenocarcinoma from chronic pancreatitis: a tissue microarray-based study. Arch Pathol Lab Med. 2007;131:556–562. doi: 10.5858/2007-131-556-DISWPI. [DOI] [PubMed] [Google Scholar]
- 12.Chu PG, Schwarz RE, Lau SK, Yen Y, Weiss LM. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol. 2005;29:359–367. doi: 10.1097/01.pas.0000149708.12335.6a. [DOI] [PubMed] [Google Scholar]
- 13.Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–845. [PubMed] [Google Scholar]
- 14.Jinfeng M, Kimura W, Hirai I, Sakurai F, Moriya T, Mizutani M. Expression of MUC5AC and MUC6 in invasive ductal carcinoma of the pancreas and relationship with prognosis. Int J Gastrointest Cancer. 2003;34:9–18. doi: 10.1385/IJGC:34:1:09. [DOI] [PubMed] [Google Scholar]
- 15.Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004;122:61–69. doi: 10.1309/9R66-73QE-C06D-86Y4. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy DM, Maitra A, Argani P, Rader AE, Faigel DO, Van Heek NT, Hruban RH, Wilentz RE. Novel markers of pancreatic adenocarcinoma in fine-needle aspiration: mesothelin and prostate stem cell antigen labeling increases accuracy in cytologically borderline cases. Appl Immunohistochem Mol Morphol. 2003;11:238–243. doi: 10.1097/00129039-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, Wilentz RE, Hruban RH, Argani P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 18.Takikita M, Altekruse S, Lynch CF, Goodman MT, Hernandez BY, Green M, Cozen W, Cockburn M, Sibug Saber M, Topor M, Zeruto C, Abedi-Ardekani B, Reichman ME, Hewitt SM. Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res. 2009;69:2950–2955. doi: 10.1158/0008-5472.CAN-08-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshi H, Sawada T, Uchida M, Saito H, Iijima H, Toda-Agetsuma M, Wada T, Yamazoe S, Tanaka H, Kimura K, Kakehashi A, Wei M, Hirakawa K, Wanibuchi H. Tumor-associated MUC5AC stimulates in vivo tumorigenicity of human pancreatic cancer. Int J Oncol. 2011;38:619–627. doi: 10.3892/ijo.2011.911. [DOI] [PubMed] [Google Scholar]
- 20.Gold DV, Karanjawala Z, Modrak DE, Goldenberg DM, Hruban RH. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin Cancer Res. 2007;13:7380–7387. doi: 10.1158/1078-0432.CCR-07-1488. [DOI] [PubMed] [Google Scholar]
- 21.Gold DV, Lew K, Maliniak R, Hernandez M, Cardillo T. Characterization of monoclonal antibody PAM4 reactive with a pancreatic cancer mucin. Int J Cancer. 1994;57:204–210. doi: 10.1002/ijc.2910570213. [DOI] [PubMed] [Google Scholar]
- 22.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P, Herman JM, Cameron JL, Yeo CJ, Halushka MK, Eshleman JR, Raben M, Klein AP, Hruban RH, Hidalgo M, Laheru D. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J. Clin. Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iacobuzio-Donahue CA, Wilentz RE, Argani P, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Dpc4 protein in mucinous cystic neoplasms of the pancreas: frequent loss of expression in invasive carcinomas suggests a role in genetic progression. Am J Surg Pathol. 2000;24:1544–1548. doi: 10.1097/00000478-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Hruban RH, Goggins M, Kern SE. Molecular genetics and related developments in pancreatic cancer. Curr Opin Gastroenterol. 1999;15:404–409. doi: 10.1097/00001574-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Shi J, Anandan V, Wang HL, Diehl D, Blansfield J, Gerhard G, Lin F. Reevaluation and identification of the best immunohistochemical panel (pVHL, Maspin, S100P, IMP-3) for ductal adenocarcinoma of the pancreas. Arch Pathol Lab Med. 2012;136:601–609. doi: 10.5858/arpa.2011-0326-OA. [DOI] [PubMed] [Google Scholar]
- 26.Park SY, Kim BH, Kim JH, Lee S, Kang GH. Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch Pathol Lab Med. 2007;131:1561–1567. doi: 10.5858/2007-131-1561-POIMHD. [DOI] [PubMed] [Google Scholar]
- 27.Yeh TS, Ho YP, Chiu CT, Chen TC, Jan YY, Chen MF. Aberrant expression of cdx2 homeobox gene in intraductal papillary-mucinous neoplasm of the pancreas but not in pancreatic ductal adenocarcinoma. Pancreas. 2005;30:233–238. doi: 10.1097/01.mpa.0000153615.55761.00. [DOI] [PubMed] [Google Scholar]
- 28.Saitou M, Goto M, Horinouchi M, Tamada S, Nagata K, Hamada T, Osako M, Takao S, Batra SK, Aikou T, Imai K, Yonezawa S. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, Adsay V, Abrams RA, Cameron JL, Kern SE, Yeo CJ, Hruban RH, Goggins M. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–4121. [PubMed] [Google Scholar]
- 30.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 31.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, Pastan I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I. V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 32.Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, Schweizer C, Weil S, Laheru D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16:6132–6138. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan R, Viner JL, Wang QC, Margulies I, Kreitman RJ, Pastan I. Anti-tumor activity of K1-LysPE38QQR, an immunotoxin targeting mesothelin, a cell-surface antigen overexpressed in ovarian cancer and malignant mesothelioma. J Immunother. 2000;23:473–479. doi: 10.1097/00002371-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Hisatsune A, Nakayama H, Kawasaki M, Horie I, Miyata T, Isohama Y, Kim KC, Katsuki H. Anti-MUC1 antibody inhibits EGF receptor signaling in cancer cells. Biochem Biophys Res Commun. 2011;405:377–381. doi: 10.1016/j.bbrc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Bharadwaj U, Zhang R, Zhang S, Mu H, Fisher WE, Brunicardi FC, Chen C, Yao Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7:286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karacay H, Sharkey RM, Gold DV, Ragland DR, McBride WJ, Rossi EA, Chang CH, Goldenberg DM. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10-90Y-IMP-288 alone and combined with gemcitabine. J Nucl Med. 2009;50:2008–2016. doi: 10.2967/jnumed.109.067686. [DOI] [PubMed] [Google Scholar]
- 38.Gold DV, Cardillo T, Vardi Y, Blumenthal R. Radioimmunotherapy of experimental pancreatic cancer with 131I-labeled monoclonal antibody PAM4. Int J Cancer. 1997;71:660–667. doi: 10.1002/(sici)1097-0215(19970516)71:4<660::aid-ijc24>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 39.Gold DV, Schutsky K, Modrak D, Cardillo TM. Low-dose radioimmunotherapy ((90)Y-PAM4) combined with gemcitabine for the treatment of experimental pancreatic cancer. Clin Cancer Res. 2003;9:3929S–3937S. [PubMed] [Google Scholar]
- 40.Mimeault M, Johansson SL, Senapati S, Momi N, Chakraborty S, Batra SK. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010;295:69–84. doi: 10.1016/j.canlet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inaguma S, Kasai K, Ikeda H. GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin. Oncogene. 2011;30:714–723. doi: 10.1038/onc.2010.459. [DOI] [PubMed] [Google Scholar]
- 42.Maitra A, Adsay NV, Argani P, Iacobuzio-Donahue C, De Marzo A, Cameron JL, Yeo CJ, Hruban RH. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–912. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- 43.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 44.Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67:10222–10229. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 45.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Argani P, Shaukat A, Kaushal M, Wilentz RE, Su GH, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Differing rates of loss of DPC4 expression and of p53 overexpression among carcinomas of the proximal and distal bile ducts. Cancer. 2001;91:1332–1341. [PubMed] [Google Scholar]


