Abstract
Objective: To study the effect of GnRH-II on the cell proliferation, apoptosis and secreting vascular endothelial growth factor (VEGF) of ectopic, eutopic and normal endometrial stromal cells (ESC) from patients with or without endometriosis (EMs) in vitro. Methods: The ectopic, eutopic and normal ESC were isolated, cultured and identified, then added 0 M, 10-10 M, 10-8 M, 10-6 M GnRH-II. The growth and proliferation of three ESC were measured by MTT assay; the cell apoptosis were detected with the method of Hoechst staining and Flow Cytometry test; ELISA was used to measure the VEGF concentration change by three ESC secretion. Results: GnRH-II inhibited the proliferation of ectopic, eutopic ESC from patients with endometriosis and normal ESC from control patients, in a dose- and time-dependent manner (P<0.05); GnRH-II increased the apoptotic rate of three ESC in a dose-dependent manner (P<0.05); The concentration of VEGF in three ESC was significantly decreased after the treatment of GnRH-II, in a dose-dependent manner (P<0.01); And these above effects were the strongest on the ectopic than on the eutopic or normal, there were statistical significance (P<0.05); and three was no significantly difference between the eutopic and normal (P>0.05). Conclusions: GnRH-II significantly inhibited the cell proliferation, induced cell apoptosis and decreased the VEGF secreting of ectopic, eutopic and normal ESC in EMs in vitro, and these effects were the strongest on ectopic ESC, which suggested that GnRH-II may become a new effective treatment for endometriosis.
Keywords: Endometriosis (EMs), gonadotropin releasing hormone-II (GnRH-II), endometrial stromal cells (ESC), cell proliferation, cell apoptosis, vascular endothelial growth factor (VEGF), in vitro
Introduction
Endometriosis (short for EMs) is a common gynecological disease in reproductive-aged women. Disease associated pelvic pain, infertility and sexual dysfunction has a significant adverse clinical, social and financial impact. Its pathogenesis is unclear [1,2], recurrent rate is high [3], treatment is thorny [4,5], which make EMs become difficult and hot research. Therefore, to study the pathogenesis of EMs and to find effective treatment method becomes an urgent issue. Since Tsutsumi et al. [6] first cloned successfully GnRH-I receptor (gonadotropin releasing hormone-IR, GnRH-IR) in 1992, a lot of researches on receptor structure and function have been done, which promoted greatly the GnRH-Ia and its analogues to research and use widely in the clinic [7-10]. GnRH agonists (that is GnRH-Ia) exerted anti-proliferative and pro-apoptotic effects on cultured endometriotic cells [11,12]. The anti-proliferative effects of GnRH-I and GnRH analogues have also been demonstrated in some cancer cells from reproductive organs [13,14].
GnRH-II (II-type gonadotropin releasing hormone) is a new discovery, may be the earliest evolution of the formation of GnRH. Its distribution is more widely than GnRH-I in the body [15]. GnRH-II is widely distributed in the central nervous system as well as in peripheral tissues of the female reproductive tract, such as the placenta, endometrium and ovarian granulosa cells [16-20]. Therefore, it may have more important and unique physiological functions than GnRH-I [15]. Both GnRH-I and GnRH-II can play roles through integrating with GnRH-I receptors and GnRH-II receptors in marmosets, but they have different degree affinity [15]. The affinity of GnRH-I receptors with GnRH-I is 48 times of that with GnRH-II, while GnRH-II receptors with GnRH-II is 421 times of that with GnRH-I, which indicate that GnRH-II may have much better treatment effect than GnRH-I [21]. Thus, GnRH-II has become the hot topic of study in recent years. Research found that: GnRH-II express in some reproductive system tumors such as ovarian cancer, breast cancer and prostate cancer, and it can inhibit tumor cell growth [22], and its anti-proliferative effect is stronger than GnRH-I on endometrial cancer and ovarian cancer cells [23].
These results indicate that similar to GnRH-I agonists or GnRH-I antagonists, GnRH-II antagonists have anti-tumor effect both in vivo and in vitro [13,14,22-24]. Endometriosis has some malignant tumor biological behaviors although it is a benign hormone-dependent disease, therefore, it is worthy studying whether GnRH-II has direct effects on endometriosis ESC in vitro.
Materials and methods
The source of ectopic, eutopic and normal endometrium
In total, 46 women underwent gynecological laparoscopic surgery were recruited to the study. 30 women had endometriosis (aged 31.2±6.1 years; mean±SD) and 16 women did not have endometriosis but had parovarian cyst (n=5) and mature cystic teratoma of the ovary (n=11) (aged 32.7±9.2 years), there was not statistical difference between two groups’ age (P>0.5). Endometriotic tissues were collected from the walls of endometriomas in patients with ovarian endometriosis, and endometrial tissues were collected by curettage at the same time of surgery. Tissues fixed with formalin were for pathological diagnosis and fresh tissues were for cell culture. All patients with or without endometriosis had regular menstrual cycle. None of the patients received any hormonal therapy within 6 months before surgery. All samples were collected with informed consent from each patient and approval from the local ethics committee of the Second Xiangya Hospital of Central South University in China.
Main reagents
GnRH-II was obtained from Bachem (Switzerland), DMEM/F12 medium was from GIBCO (USA), IV-type collagenase and progesterone were from Sigma (USA), anti-human monoclonal antibodies against vimentin, keratin, prolactin mouse and fetal bovine serum were from Wuhan Boster (China), vascular endothelial growth factor (VEGF) ELISA Kit was from Peprotechnology Inc. (Rocky Hill, NJ, USA). Hoechst 33258 staining kit was from Shanghai Biyuntian (China). The FACS Calibur flow cytometer was manufactured by Becton Dickinson (USA). MTT kit and trypsin were from Amresco Inc. (USA). Besides stated above, other chemicals were from Wuhan Boster (China).
Isolation, culture, and identification of endometrial stromal cells (ESC)
Surgical operation procedures were carried out in our hospital. Primary endometrial stromal cells were cultured by the following steps described by Morimoto [25] and Liu [26]: ectopic, eutopic and normal endometrial tissues were dissected after rinsing, then digested 2-3 h until the tissues disappeared by adding 0.1% type IV collagenase solution and 0.25% trypsin digestion at pH 7.4 at 37°C, and then isolated cells with 100 μm and 38 μm cell strainers, then centrifuged at 800 rpm for 5 min, removed the supernatant, added DMEM/F12 medium (containing 10% newborn bovine serum), finally, cells growth and morphology changes were observed under inverted microscope. 104/mL cells were seeded into 25 cm2 cell culture plates at 37°C in 5% CO2 incubators, semi-amount medium was replaced every 2-3 d, till cells fusion were grown to 80%, the culture of primary cells were completed. Cells counting and cells growth curve were successfully done. Vimentin, keratin, and prolactin (PRL) were used to identify of cultured ESC. Due to PRL was produced only by ESC in non-pregnant period, and not by glands and fibroblasts, so we used PRL to identify the ESC. Cells were identified directly in 6-well culture plates, passaged the adhesive cells, replaced the medium, added 10-8 mol/L progesterone stimulating 6 d. The ESC were identified according to instruction kit by immunocytochemical ABC method.
ESC intervention
Intervention of the third generation ESC when they grew to 80% confluence, then transferred to serum-free medium for 24 h before treatment with the GnRH-II analogue. 3-well was repeated, and took the average value. Cells were treated with serum-free fresh DMEM/F12 medium of either GnRH-II (10-10 M, 10-8 M, 10-6 M) or 2.5% NBS with the FD 0.5 ml/well (cell growth inhibition with 0.1 ml/well). (1) Cultured 24 h, 48 h, 72 h, respectively, then added MTT 0.02 ml/well, continued to culture 4 h, cell growth inhibition was determined with MTT method (the absorbance A is read directly in the wells at an optimal wavelength of 490 nm), cell growth inhibition rate R=(1-A in experimental group/A in control)×100%; (2) Cultured for 24 h, Hoechst staining and flow cytometry were used to determine apoptosis in three ESC; (3) Cultured 48 h, the medium fluid was aspirated, then stored under -20°C, ELISA method determined the concentration of VEGF (OD values were measured at an optimal wavelength of 450 nm).
Statistical analysis
The data were computerized and analyzed using the SPSS ver. 11.5. The value was expressed as mean±standard deviation (x̅±S). Two-tailed Student’s t-test was used for analyzing for paired data. Multiple comparisons were analyzed by ANOVA followed by Tukey’s multiple comparison test. Significant statistical difference was defined as P<0.05.
Results
ESC morphological changes and identification
Of 30 endometriosis patients, 25 cases cell culture succeeded in eutopic, 20 cases in ectopic; 14 cases succeeded in 16 control patients, the others failed due to bacteria contamination. Success rate was 83.33% in eutopic, 66.67% in ectopic and 87.5% in control ESC.
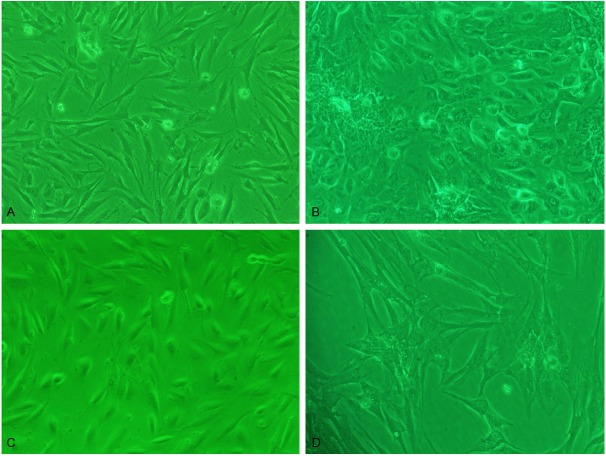
Cell morphology were observed under inverted microscope, normal, eutopic and ectopic ESC adherent to wall after 24 h cultured, the similar morphology of three ESC: cells were bigger, mainly in spindle shape; hammer or round or irregular shape was less. At the beginning of culture, cells grew in polarity, after 3-4 d, cells grew with extending, straight or upright appearance, similar to fibroblasts. But the three ESC had some differences: the primary normal and eutopic stromal cells were more like the fibroblasts and the ectopic was mainly polygonal (Figure 1A, 1B). The cultured ESC were thin and transparent cytoplasm, with nuclears were in the middle. The cells could survive for average 5-8 weeks, passage for 3-6 times. As the increasing of passage times, the shape of normal and eutopic cells gradually were dominated by the spindle, with the polarity disappearing and in vortex state; the volume of ectopic cells increased, grew in flat state (Figure 1C, 1D). These cells were identified by immunocytochemical ABC method: Vimentin was positive, cytoplasm was full of brownish-yellow granule (Figure 2A, 2B); cytokeratin was negative (Figure 2C, 2D); PRL (prolactin) was positive after progestogen effect, cytoplasm was full of brownish-yellow granule, which were proved to be the endometrial stromal cells (Figure 2E, 2F).
Figure 1.
ESC morphology was observed under inverted microscope: A. Eutopic ESC of primary culture 6 d. The cell was more like the fibroblasts cell (×100); B. Ectopic ESC of primary culture 6 d. The cell was mainly polygonal cell (×100); C. Eutopic ESC of the passage 3 culture 6 d. Cell was spindle and in vortex state (×100); D. Ectopic ESC of the passage 3 culture 6 d. Cell was large and in flat state (×100).
Figure 2.
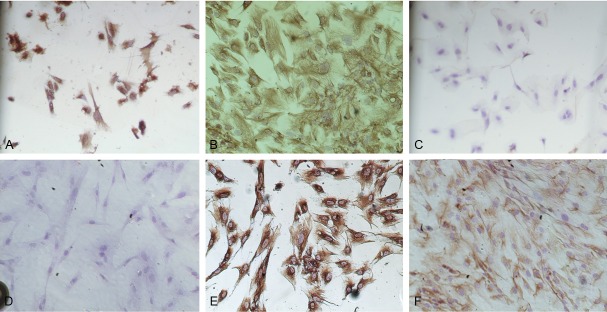
Identification of ESC: A. Positive for vimentin in eutopic ESC. Cytoplasm was with brownish-yellow granules (ABC, ×100); B. Positive for vimentin in ectopic ESC. Cytoplasm was with brownish-yellow granules (ABC, ×100); C. Negative for cytokeratins in eutopic ESC (ABC, ×100); D. Negative for cytokeratins in ectopic ESC (ABC, ×100); E. Positive for PRL in eutopic ESC. Cytoplasm was with brownish-yellow granules (ABC, ×100); F. Positive for PRL in ectopic ESC. Cytoplasm was with brownish-yellow granules (ABC, ×100).
Endometrial stromal cells growth curve
The amount of ESC gradually increased with the prolong of incubation time, they grew more fast in 6-8 d. The normal and eutopic ESC grew faster than the ectopic ESC, but there was no statistical difference (P>0.05) (×105) (Figure 3).
Figure 3.
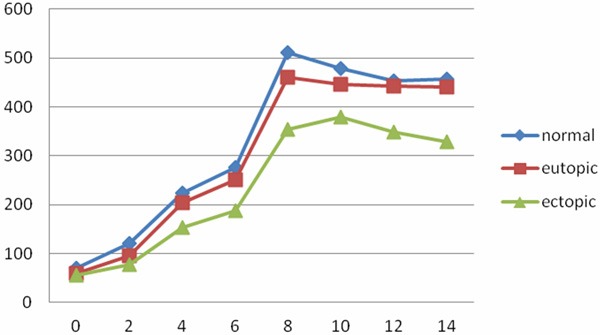
Cell growth curve in cultivate three ESC from 0 to 14 d (×105).
Cell proliferate inhibition rate compare of GnRH-II on cultured three ESC in vitro
Normal, eutopic and ectopic ESC were cultured in vitro, then added different concentrations of GnRH-II (10-10 M, 10-8 M, 10-6 M) for 24 h, 48 h and 72 h. As shown in Table 1. As concentration increased of GnRH-II, or prolonged the time in three ESC, the cell proliferation inhibition rate (%) increased. Which had statistical significant in the difference concentration and time, P<0.05, in a dose- and time-dependent manner; and the effect on ectopic was stronger than on normal or eutopic, the difference was statistical significant, P<0.05; There was no difference between the eutopic and normal ESC, P>0.05.
Table 1.
Cell proliferation inhibition rates compare of GnRH-II on cultured three ESC in vitro (x̅±S)%
| 24 h | 48 h | 72 h | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| 10-10 M | 10-8 M | 10-6 M | 10-10 M | 10-8 M | 10-6 M | 10-10 M | 10-8 M | 10-6 M | |||
| Eutopic | 15.32±1.43∆ | 26.41±2.75*,∆ | 38.06±4.15*,∆ | 30.41±3.50▲,∆ | 41.78±4.49*,▲,∆ | 53.34±5.83*,▲,∆ | 50.01±3.70▲,∆ | 63.29±4.47*,▲,∆ | 81.58±3.44*,▲,∆ | ||
| Ectopic | 38.42±2.67^ | 51.35±3.70*,^ | 62.84±4.13*,^ | 53.41±3.79▲,^ | 64.47±4.78*,▲,^ | 76.39±5.71*,▲,^ | 70.29±2.87▲,^ | 83.25±3.73*,▲,^ | 93.39±4.85*,▲,^ | ||
| Normal | 14.77±1.46 | 27.53±2.88* | 36.93±3.77* | 31.12±3.69▲ | 40.48±4.65*,▲ | 52.47±5.11*,▲ | 49.03±4.82▲ | 62.06±4.44*,▲ | 80.21±4.25*,▲ | ||
Note: Effect of GnRH-II on culture of three ESC in different concentration and different time in vitro. The proliferation inhibition rate had statistically difference, in a dose- and time-dependent;
P<0.05;
P<0.05 in each ESC.
And the ectopic was the strongest;
P<0.05.
There was no difference between eutopic and normal ESC;
P>0.05.
Apoptosis effect of GnRH-II on cultured normal, eutopic and ectopic ESC in vitro
After three ESC in logarithmic growing phase were treated with different concentration GnRH-II (0, 10-10 M, 10-8 M and 10-6 M), Hoechst staining was used for analysis of apoptotic cells by morphology. Results demonstrated that the morphological changes of apoptosis of three ESC were shown as karyorrhexis, karyolysis, karyopyknosis, or even the formation of apoptotic bodies, which confirmed that GnRH-II can induce the apoptosis of three ESC in morphologic characters in vitro. The apoptosis rates (%) of three ESC were seen in Table 2. The ectopic was higher than the eutopic, there was statistical significance differences (P<0.05), while the eutopic and normal had no differences (P>0.05), which meant that GnRH-II had stronger apoptosis-inducing effect on ectopic cells than that on eutopic or normal ESC; Furthermore, with the increase of GnRH-II concentration (0, 10-10 M, 10-8 M and 10-6 M), the apoptosis rate increased in each group, showing a dose-dependent manner (P<0.05).
Table 2.
Apoptosis rates compare of GnRH-II on three ESC by Hoechst staining (x̅±S)%
| Different concentrations of GnRH-II | ||||
|---|---|---|---|---|
|
| ||||
| 0 | 10-10 M | 10-8 M | 10-6 M | |
| Eutopic cells | 5.78±0.53∆ | 18.62±2.59*,∆ | 47.41±3.57*,∆ | 66.16±6.46*,∆ |
| Ectopic cells | 5.79±0.66 | 31.30±2.93*,▲ | 59.28±4.25*,▲ | 79.43±6.42*,▲ |
| Normal cells | 5.83±0.56 | 19.17±4.37* | 46.77±3.48* | 65.89±5.99* |
Note: With the increase of GnRH-II concentration, the apoptosis rates increased in stroma cells, in a dose-dependent manner;
P<0.05.
And GnRH-II had a stronger apoptosis-inducing effect on ectopic ESC than on eutopic or normal ESC;
P<0.05.
There was no difference between eutopic and normal stroma cells;
P>0.05.
Meanwhile, the apoptosis were detected in three ESC by flow cytometry. And the result of flow cytometry is the same as the Hoechst staining. As shown in Table 3. The apoptosis rates (%) of ectopic ESC were significance higher than normal or eutopic (P<0.05), while the eutopic and normal groups had no differences (P>0.05), which meant that GnRH-II had stronger apoptosis-inducing effect on ectopic cells than that on eutopic or normal ESC; Furthermore, with the increasing of GnRH-II concentration (0, 10-10 M, 10-8 M and 10-6 M), the apoptosis rate increased in each group, showing a dose-dependent manner (P<0.05).
Table 3.
Apoptosis rates compare of GnRH-II on three ESC by flow cytometry (x̅±S)%
| Different concentrations of GnRH-II | ||||
|---|---|---|---|---|
|
| ||||
| 0 M | 10-10 M | 10-8 M | 10-6 M | |
| Eutopic cells | 5.87±0.59∆ | 19.30±3.28*,∆ | 46.30±5.32*,∆ | 69.70±8.77*,∆ |
| Ectopic cells | 5.99±0.78 | 33.40±3.97*,▲ | 62.41±6.74*,▲ | 83.30±9.42*,▲ |
| Normal cells | 5.76±0.64 | 18.97±3.79* | 45.66±4.62* | 68.89±6.11* |
Notes: With the increase of GnRH-II concentration, the apoptosis rates of three ESC were increased, in a dose-dependent manner;
P<0.05.
And GnRH-II had the stronger apoptosis-inducing effect on ectopic ESC than that on eutopic or normal ESC;
P<0.05.
There was no difference between eutopic and normal ESC;
P>0.05.
Effect of GnRH-II on VEGF secreted by cultured three ESC in vitro
VEGF were determined by ELISA after three ESC in logarithmic growing phase were treated with or without GnRH-II for 24 h in vitro. When GnRH-II was 0 M, the level of VEGF in normal, eutopic and ectopic ESC were (733.51±183.44), (726.05±166.35) and (695.12±203.11) pg/ml, respectively, there were no statistical difference among three groups (P>0.05). Then, we added different concentration GnRH-II (10-10 M, 10-8 M, 10-6 M) to culture 48 h, determined VEGF concentration, calculated inhibition rate (inhibition rate=(VEGF level without adding GnRH-II-VEGF level with adding GnRH-II)/VEGF level without GnRH-II). As shown in Table 4: GnRH-II increased significantly VEGF secretion inhibition rates in three ESC, there was statistical difference, P<0.01, and in dose-dependent manner; And the ectopic was stronger than the eutopic or the normal, P<0.01, there was statistical difference; There was no difference between the eutopic and the normal, P>0.05.
Table 4.
VEGF secreting inhibition rates compare of GnRH-II on three ESC (x̅±S)%
| Different concentration of GnRH-II | |||
|---|---|---|---|
|
| |||
| 10-10 M | 10-8 M | 10-6 M | |
| Eutopic ESC | 0.38±0.13∆ | 0.57±0.07*,∆ | 0.74±0.12*,∆ |
| Ectopic ESC | 0.55±0.14▲ | 0.75±0.11*,▲ | 0.94±0.13*,▲ |
| Normal ESC | 0.36±0.15 | 0.55±0.09* | 0.73±0.10* |
Note: With the increase of GnRH-II concentration, VEGF secreting inhibition rates of three ESC were increased, in a dose-dependent manner;
P<0.05.
And ectopic was stronger than eutopic or normal;
P<0.01.
There was no difference between eutopic and normal;
P>0.05.
Discussion
Culture significance of ESC in endometriosis in vitro
Because of ethical issues, it is difficult to control study EMs in the human body, especially it was impossible to do traumatic check and early drug test. Therefore, in order to study the pathogenesis of EMs, the mechanism of drugs action and treatment effects, it is necessary to build different disease models in vitro. Cell culture, started at the beginning of this century, is widely applied in biology, medicine and other fields, which becomes one of the important contents of basic science, and has the great superiority. Cell culture is a good research method of living cell and tissue. Our experiment used cell culture improvement method, chose normal, eutopic/ectopic endometrial tissue to separate and culture stromal cells, then identified with vimentin, keratin protein (cytokeratin) and prolactin (PRL), respectively. The culture of ESC was successful. The cultured ESC of logarithmic growth phase in vitro were treated with different concentration GnRH-II (0, 10-10 M, 10-8 M, 10-6 M). The effect of GnRH-II were studied on three ESC proliferation, apoptosis, and the influence of VEGF secretion in vitro, which will provide experimental and theoretical basis to explore pathogenesis and drug treatment mechanism of endometriosis.
Effect of GnRH-II on cultured three ESC proliferations in vitro
Imai and Borroni found: GnRHa treat endometriosis by suppress the hypothalamic pituitary ovarian axis, not only reduce estrogen level, make the ectopic endometrial atrophy [27], but also have a direct effect on anti-proliferation and promote apoptosis in cultured endometriosis cells in vitro [11,12,28]. Another studies found [13,14,22-24,29]: GnRH-I, GnRH-II agonists and antagonists can inhibit the growth of tumor cells in some tumor researches of the reproductive system such as ovarian cancer, breast cancer and prostate cancer, and GnRH-II had the strongest cell proliferation-inhibiting effect on these tumor cells than GnRH-I. In addition, GnRH-II displayed an inhibitory effect on the proliferation of SK-OV-3 (an endometrial carcinoma cell line with positive GnRH-II receptors and negative GnRH-I receptors), while GnRH-I agonist (Triptorelin) had no such an effect [23]. Groundkeeper C et al. [30] reported GnRH-II antagonists seem to be suitable drugs for an efficacious and less-toxic endocrine therapy for breast cancers, including triple-negative breast cancers. Montagnani Marelli M et al. [31] reported that GnRH-II exerts a specific and significant antiproliferative action on prostate cancer cells, this anti-tumor effect was mediated by the activation of type I GnRH-R (but not type II GnRH-R) and by its coupled cAMP intracellular signaling pathway.
GnRH-II receptor system function has been the hotspot of research in recent years. The function of GnRH-II has been confirmed to inhibit M current and regulate sex function. In addition, the study found [32]: GnRH-II receptor system can regulate hormone secretion, have autocrine/paracrine function and inhibit tumor cell proliferation; Another study about endometrium found that [23,33]: the immune response of GnRH-II expressed through the entire menstrual cycle in the stromal cells and epithelial cells; Morimoto C et al. also found that [25] GnRH-II mRNA expression was lower in the eutopic endometrial in women with endometriosis than those of normal endometrium, and exogenous GnRH-II can reduce interleukin 8 (IL-8) and the secretion of cyclooxygenase 2 (cox-2), the later may be associated with important immune response of the disease. Therefore, GnRH-II may be an important factor leading to the endometriosis, GnRH-II have anti-inflammatory effect on ESC.
We added different concentration of GnRH-II into the cultured three ESC in vitro to detect the cell proliferation inhibition by MTT, the results found: the cell proliferation inhibition rate (%) increased as GnRH-II concentration increased, or prolonged the exposure time in three ESC, in a dose- and time-dependent manner, P<0.05; And the effect on ectopic is stronger than on normal or eutopic, the difference had statistical significance, P<0.05; There was no difference between eutopic and normal stroma cells, P>0.05. These results suggested GnRH-II had anti-proliferative effect, especially to the ectopic ESC which was consistent with the above reported [22-25]. Klemmt PA et al. [34] also found that ectopic endometrium survival ability is poor with EMs patients, may also become targeted treatment on ectopic endometrium. These results suggest that GnRH-II may become one of treatment methods in endometriosis.
Apoptosis effect of GnRH-II on three ESC in vitro
It’s well known, GnRHa (GnRH-Ia), because its effective apoptosis-inducing and proliferation-inhibiting effects on endometrial stroma cells, has become the ideal drug in treatment of EMs. GnRHa could obviously inhibit proliferation of eutopic and ectopic endometrial stroma cells in patient with endometriosis; promote cells apoptosis; and decrease the expression of VEGF to achieve the aim of EMs treatment [25,28,35]. Results obtained from in vitro culture proved that GnRHa Leuprorelin could inhibit the eutopic ESC in patients with EMs and promote cells apoptosis, the mechanism might be associated with its up-regulation of Bax and Fas-L expressions and down-regulation of Bcl-2 expression [36]. GnRH-I (100 ng/ml) could promote the apoptosis of endometrial stroma cells in both EMs group and the normal group while antide (10-7 M) could cancel out such an effect [37]. And another study found that GnRHa had the strongest pro-apoptosis effect on endometrial stroma cells by adding GnRHa, LING21US (Levonorgestrel-releasing intrauterine system) and MPA (medroxyprogesterone acetate) with transmission electron microscopy (TEM) [38].
Our study revealed that GnRH-II induced apoptosis of three ESC, shown as karyorrhexis, karyolysis, karyopyknosis, and even apoptotic body formation. Hoechst staining morphologically confirmed that GnRH-II induced ESC apoptosis in a dose-dependent manner (P<0.05); exhibited significant differences among three groups (P<0.05), and the ectopic was the strongest (P<0.05). In addition, the flow cytometry found the similar results. Which further confirmed the reliability of the results and the conclusions we had drawn: GnRH-II had the effect of promoting apoptosis, especially on the ectopic ESC. Leuprorelin (GnRH-Ia) could promote the apoptosis of eutopic ESC in patients with EMs, and such an effect could be canceled out by GnRH-I antagonist antide 10-7 M [25]. The similar results had been proved between in our study and other studies [25,28]. Cell apoptosis is actually the process of caspase irreversible finite cascade amplification reaction process of hydrolysis of the substrate. Fister et al. [39] confirmed that GnRH-II antagonist resulted in cell apoptosis of human endometrial cancer and ovarian cancer by activation of caspases-3 in dose-dependent manner. Based on our results, together with other related studies [25,39], it is reasonable that GnRH-II can serve as a new drug in treatment of EMs someday, and its action mechanism may be realized partly through its apoptosis-promoting effect on endometrial stroma cells.
The effect of GnRH-II on VEGF secretion of ESC in vitro
Although the pathogenesis of EMs are varied, the ectopic endometrium to grow successfully must have new blood vessels to provide blood in the abdominal cavity. Angiogenesis plays an important role in the pathogenesis of EMs [40]. VEGF is currently recognized as the most key one in numerous promote angiogenesis factors, and it is a kind of multifunctional cytokine, especially to promote vascular endothelial cell hyperplasia, form new blood vessels. which provide blood supply for the success planting of the ectopic endometrium in the abdominal cavity, and these help to develop EMs [41]. Our study found three ESC can secrete VEGF, and the level of VEGF had no difference (P>0.05). GnRH-II increased significantly VEGF secretion inhibition rate in three ESC, in a dose-dependent manner, P<0.01; And the ectopic was stronger than the eutopic or normal, P<0.01; There was no difference between the eutopic and the normal, P>0.05. Which provided a experimental reference for GnRH-II treatment endometriosis.
In summary, GnRH-II can affect directly on ESC by inhibited ESC proliferation; induced ESC apoptosis; reduce the release of VEGF, especially to the ectopic ESC of EMs in vitro which provided the experimental and theoretical basis for GnRH-II treatment of endometriosis.
Acknowledgements
The subject was supported by the grants from The Health Department of Hunan Province (2010-016) and Hunan Province Natural Science Foundation Project (12jj3100).
Disclosure of conflict of interest
None.
References
- 1.Hey-Cunningham AJ, Peters KM, Barrera-Villa Zevallos H, Berbic M, Markham R, Fraser IS. Angiogenesis, lymphangiogenesis and neurogenesis in endometriosis. Front Biosci (Elite Ed) 2013;1:1033–1056. doi: 10.2741/e682. [DOI] [PubMed] [Google Scholar]
- 2.Polak G, Wertel I, Kwaśniewski W, Derewianka-Polak M, Kotarski J. The role of iro metabolism and oxidative stress in the pathogenesis of endometriosis. Ginekol Pol. 2013;84:62–64. doi: 10.17772/gp/1542. [DOI] [PubMed] [Google Scholar]
- 3.Paka C, Miller J, Nezhat C. Predictive factors and treatment of recurrence of endometriosis. Minerva Ginecol. 2013;65:105–111. [PubMed] [Google Scholar]
- 4.Taylor RN, Hummelshoj L, Stratton P, Vercellini P. Pain and endometriosis: Etiology, impact, and therapeutics. Middle East Fertil Soc J. 2012;17:221–225. doi: 10.1016/j.mefs.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheaves C. Advances in endometriosis treatment. Nurse Pract. 2013;38:42–47. doi: 10.1097/01.NPR.0000425826.90435.a8. [DOI] [PubMed] [Google Scholar]
- 6.Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6:1163–1169. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- 7.Schrager S, Falleroni J, Edgoose J. Evaluation and treatment of endometriosis. Am Fam Physician. 2013;87:107–113. [PubMed] [Google Scholar]
- 8.Ge CX, Zhu XH, Tang XQ. Efficacy of conservative laparoscopic surgery combined with Goserelin in treatment of 206 patients with severe ovarian endometriosis at short-term and long-term follow-up. Zhonghua Fu Chan Ke Za Zhi. 2012 Aug;47:603–7. [PubMed] [Google Scholar]
- 9.Grigoriadis C, Papaconstantinou E, Mellou A, Hassiakos D, Liapis A, Kondi-Pafiti A. Clinicopathological changes of uterine leiomyomas after GnRH agonist therapy. Clin Exp Obstet Gynecol. 2012;39:191–194. [PubMed] [Google Scholar]
- 10.Demeestere I, Brice P, Peccatori FA, Kentos A, Gaillard I, Zachee P, Casasnovas RO, Van Den Neste E, Dechene J, De Maertelaer V, Bron D, Englert Y. Gonadotropin-releasing hormone agonist for the prevention of chemotherapy-induced ovarian failure in patients with lymphoma: 1-year follow-up of a prospective randomized trial. J. Clin. Oncol. 2013;31:903–909. doi: 10.1200/JCO.2012.42.8185. [DOI] [PubMed] [Google Scholar]
- 11.Borroni R, Di Blasio AM, Gaffuri B, Santorsola R, Busacca M, Vigano P, Vignali M. Expression of GnRH receptor gene in human ectopic endometrial cells and inhibition of their proliferation by leuprolide acetate. Mol Cell Endocrinol. 2000;159:37–43. doi: 10.1016/s0303-7207(99)00199-9. [DOI] [PubMed] [Google Scholar]
- 12.Imai A, Takagi A, Tamaya T. Gonadotropin-releasing hormone analog repairs reduced endometrial cell apoptosis in endometriosis in vitro. Am J Obstet Gynecol. 2000;182:1142–1146. doi: 10.1067/mob.2000.104804. [DOI] [PubMed] [Google Scholar]
- 13.Tang X, Yano T, Osuga Y, Matsumi H, Yano N, Xu J, Wada O, Koga K, Kugu K, Tsutsumi O, Schally AV, Taketani Y. Cellular mechanisms of growth inhibition of human epithelial ovarian cancer cell line by LH-releasing hormone antagonist Cetrorelix. J Clin Endocrinol Metab. 2002;87:3721–3727. doi: 10.1210/jcem.87.8.8726. [DOI] [PubMed] [Google Scholar]
- 14.Limonta P, Moretti RM, Marelli MM, Motta M. The biology of gonadotropin hormone-eleasing hormone: role in the control of tumor growth and progression in humans. Front Neuroendocrinol. 2003;24:279–295. doi: 10.1016/j.yfrne.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Millar RP. GnRH-II and type-II GnRH receptors. Trends Endocrinol Metab. 2003;14:35–43. doi: 10.1016/s1043-2760(02)00016-4. [DOI] [PubMed] [Google Scholar]
- 16.Siler-Khodr TM, Grayson M. Action of chicken II GnRH on the human placenta. J Clin Endocrinol Metab. 2001;86:804–810. doi: 10.1210/jcem.86.2.7210. [DOI] [PubMed] [Google Scholar]
- 17.Cheon KW, Lee HS, Parhar IS, Kang IS. Expression of the second isoform of gonadotrophin-releasing hormone (GnRH-II) in human endometrium throughout the menstrual cycle. Mol Hum Reprod. 2001;7:447–452. doi: 10.1093/molehr/7.5.447. [DOI] [PubMed] [Google Scholar]
- 18.Choi KC, Auersperg N, Leung PC. Expression and antiproliferative effect of a second form of gonadotropin-releasing hormone in normal and neoplastic ovarian surface epithelial cells. J Clin Endocrinol Metab. 2001;86:5075–5078. doi: 10.1210/jcem.86.10.8100. [DOI] [PubMed] [Google Scholar]
- 19.Kang SK, Tai CJ, Nathwani PS, Leung PC. Differential regulation of two forms of gonadotropin-releasing hormone messenger ribonucleic acid in human granulosa-luteal cells. Endocrinology. 2001;142:182–192. doi: 10.1210/endo.142.1.7895. [DOI] [PubMed] [Google Scholar]
- 20.Khosravi S, Leung PC. Differential regulation of gonadotropin-releasing hormone (GnRH)I and GnRH-II messenger ribonucleic acid by gonadal steroids in human granulosa luteal cells. J Clin Endocrinol Metab. 2003;88:663–672. doi: 10.1210/jc.2002-020866. [DOI] [PubMed] [Google Scholar]
- 21.Harrison GS, Wierman ME, Nett TM, Glode LM. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr Relat Cancer. 2004;11:725–748. doi: 10.1677/erc.1.00777. [DOI] [PubMed] [Google Scholar]
- 22.Enomoto M, Endo D, Kawashima S, Park MK. Human type-II GnRH receptor mediates effects of GnRH on cell proliferation. Zool Sci. 2004;21:763–770. doi: 10.2108/zsj.21.763. [DOI] [PubMed] [Google Scholar]
- 23.Grundker C, Gunthert AR, Millar RP, Emons G. Expression of gonadotropin-releasing hormone-II (GnRH-II) receptor in human endometrial and ovarian cancer cells and effects of GnRH-II on tumor cell proliferation. J Clin Endocrinol Metab. 2002;87:1427–1430. doi: 10.1210/jcem.87.3.8437. [DOI] [PubMed] [Google Scholar]
- 24.Schubert A, Hawighorst T, Emons G, Gründker C. Agonists and antagonists of GnRH-I and -II reduce metastasis formation by triple-negative human breast cancer cells in vivo. Breast Cancer Res Treat. 2011;130:783–790. doi: 10.1007/s10549-011-1358-9. [DOI] [PubMed] [Google Scholar]
- 25.Morimoto C, Osuga Y, Yano T, Takemura Y, Harada M, Hirata T, Hirota Y, Yoshino O, Koga K, Kugu K, Taketani Y. GnRH-II as a possible cytostatic regulator in the development of endometriosis. Hum Reprod. 2005;20:3212–3218. doi: 10.1093/humrep/dei192. [DOI] [PubMed] [Google Scholar]
- 26.Liu QH, Huang FY, Wang HP, Zou Y. GnRH-II in vitro culture of uterine in patients with endometriosis Endometrial stromal cells secrete VEGF influence. Journal of Central South University (Medical Edition) 2009;9:926–932. [PubMed] [Google Scholar]
- 27.Imai A, Sugiyama M, Furui T, Tamaya T. Treatment of perimenopausal women with uterine myoma: successful use of a depot GnRH agonist leading to a natural menopause. J Obstet Gynaecol. 2003;23:518–520. doi: 10.1080/0144361031000153765. [DOI] [PubMed] [Google Scholar]
- 28.Huang FY, Wang HP, Liu QH, Zou Y. Effect of GnRHa on cell proliferation and VEGF’s secreting of ectopic and eutopic stromal cells from patients with endometriosis in vitro. China Journal of Modern Medicine. 2011;21:79–83. 86. [Google Scholar]
- 29.Kim KY, Choi KC, Park SH, Auersperg N, Leung PC. Extracellular signal-regulated protein kinase, but not c-Jun N-terminal kinase, is activated by type II gonadotropin-releasing hormone involved in the inhibition of ovarian cancer cell proliferation. J Clin Endocrinol Metab. 2005;90:1670–1677. doi: 10.1210/jc.2004-1636. [DOI] [PubMed] [Google Scholar]
- 30.Gründker C, Föst C, Fister S, Nolte N, Günthert AR, Emons G. Gonadotropin-releasing hormone type-II antagonist induces apoptosis in MCF-7 and triple-negative MDA-MB-231 human breast cancer cells in vitro and in vivo. Breast Cancer Res. 2010;12:R49. doi: 10.1186/bcr2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montagnani Marelli M, Moretti RM, Mai S, Januszkiewicz-Caulier J, Motta M, Limonta P. Type I gonadotropin-releasing hormone receptor mediates the antiproliferative effects of GnRH-II on prostate cancer cells. J Clin Endocrinol Metab. 2009;94:1761–1767. doi: 10.1210/jc.2008-1741. [DOI] [PubMed] [Google Scholar]
- 32.Chi XL, Zhou WX, Zhang YX, Chen JP. Type-II GnRH research progress and its receptor system. Proceedings of The Military Academy of Medical Sciences. 2006;30:169–173. [Google Scholar]
- 33.Zou Y, Huang FY. Expression of GnRH-II protein in patient with endometriosis and its Significance. International Journal of Pathology and Clinical Medicine. 2010;30:27–32. [Google Scholar]
- 34.Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. The Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;30:564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han LW, Jiang WG. The effect of GnRHa on endometrial stromal cell proliferation and Angiogenesis in endometriosis. Progress in Obstetrics and Gynecology. 2008;17:737–739. [Google Scholar]
- 36.Bilotas M, Baraño RI, Buquet R, Sueldo C, Tesone M, Meresman G. Effect of GnRH analogues on apoptosis and expression of Bcl-2, Bax, Fas and FasL proteins in endometrial epithelial cell cultures from patients with endometriosis and controls. Hum Reprod. 2007;22:644–653. doi: 10.1093/humrep/del423. [DOI] [PubMed] [Google Scholar]
- 37.Meresman GF, Bilotas MA, Lombardi E, Tesone M, Sueldo C, Barañao RI. Effect of GnRH analogues on apoptosis and release of interleukin-1beta and vascular endothelial growth factor in endometrial cell cultures from patients with endometriosis. Hum Reprod. 2003;18:1767–1771. doi: 10.1093/humrep/deg356. [DOI] [PubMed] [Google Scholar]
- 38.Deng S, Lang JH, Leng JH, Liu ZF, Sun DW, Zhu L, Tan XJ. Effects of medical treatment on apoptosis in eutopic endometrium of patients with endometriosis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007;29:252–6. [PubMed] [Google Scholar]
- 39.Fister S, Günthert AR, Emons G, Gründker C. Gonadotropin-releasing hormone type-II antagonists induce apoptotic cell death in human endometrial and ovarian cancer cells in vitro and in vivo. Cancer Res. 2007;67:1750–1756. doi: 10.1158/0008-5472.CAN-06-3222. [DOI] [PubMed] [Google Scholar]
- 40.Zhao ZZ, Nyholt DR, Thomas S, Treloar SA, Montgomery GW. Polymorphisms in the vascular endothelial growth factor gene and the risk of familial endometriosis. Mol Hum Reprod. 2008;14:531–538. doi: 10.1093/molehr/gan043. [DOI] [PubMed] [Google Scholar]
- 41.Gilabert-Estellés J, Ramón LA, España F, Gilabert J, Vila V, Réganon E, Castelló R, Chirivella M, Estellés A. Expression of angiogenic factors in endometriosis: relationship to fibrinolytic and metalloproteinase systems. Hum Reprod. 2007;22:2120–2127. doi: 10.1093/humrep/dem149. [DOI] [PubMed] [Google Scholar]