Abstract
Inositol polyphosphate phosphatase-like 1 (INPPL1), also known as SH2-containing inositol 5’-phosphatase 2 (SHIP2), has been suggested to act downstream of the PI3K/AKT pathway and play an important function in tumor development and progression. However, the associations between SHIP2 expression and the clinical features to determine its clinicopathologic significance in hepatocellular carcinoma (HCC) have not been investigated. In the present study, one-step quantitative PCR reverse transcription-polymerase chain reaction (qPCR) and immunohistochemistry (IHC) analysis with HCC tissue microarrays (TMA) were employed to evaluate the expression of SHIP2 in HCC. The results showed that SHIP2 expression in the mRNA and protein levels was significantly higher in HCC tissue than in corresponding non-cancerous tissue (p = 0.0014 and p < 0.001, respectively). The expression of SHIP2 protein in HCC was related to tumor differentiation, α-fetoprotein level, liver cirrhosis, and five-year survival rate (all p < 0.05). Kaplan-Meier method and log-rank test indicated that high expression of SHIP2 (p = 0.017) and tumor differentiation (p = 0.036) showed significant correlations with poor prognosis of HCC patients. The data indicate that SHIP2 expression is correlated with significant characteristics of HCC, and it may be useful as an unfavorable prognostic factor in HCC.
Keywords: INPPL1, SHIP2, HCC, TMA, qPCR, immunohistochemistry
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies all over the world. The incidence of HCC has been increasing in eastern Asia during the past several decades [1,2]. In China, the number of HCC patients accounts for more than half of HCC cases worldwide, and the incidence of HCC in Qidong of Jiangsu Province in China is one of the highest globally [2,3]. HCC is a complex malignant tumor, and its development and progression is influenced by various factors. Two important risk factors for developing HCC are cirrhosis and hepatitis B virus infection [4]. Currently, surgery is the most common treatment for HCC. However, the recurrence rate is high and surgery affects long-term recovery and survival in HCC patients after curative resection [5,6]. For advanced HCC patients, several novel molecular targeted therapies can be applied but with limited therapeutic effects [7-9]. Therefore, studies should focus on biomarker development to distinguish patients with poor prognosis or at high risk of early recurrence and to serve as basis for molecular targeted therapy for HCC.
Inositol polyphosphate phosphatase-like 1 (INPPL1), also known as SH2-containing inositol 5’-phosphatase 2 (SHIP2), has been suggested to act downstream of phosphoinositide-3’ kinase (PI3K), which is a significant component of intracellular signaling mechanisms [10]. PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate to generate phosphatidylinositol 3,4,5-triphospate (PIP3). PIP3 then activates several cellular enzymes, including the protein kinase Akt to regulate cell growth, reproduction and survival [11]. SHIP2 is able to dephosphorylate PIP3 at the 5’ position to generate a different lipid molecule phosphatidylinositol 3,4-bisphosphate [12]. Irregular expression of SHIP2 may disrupt the normal PI3K/AKT pathway, which has an important function in controlling cell proliferation and death, as well as tumor development and progression [13]. Therefore, a potential role for SHIP2 in cancer seems highly plausible, given the oncogenic effect of the PI3K/Akt pathway and the characteristic of SHIP2 [14]. A recent study and our most recent research have both reported that SHIP2 exhibits oncogenic properties and elevated expression of SHIP2 is correlated with poor survival in breast cancer and non-small cell lung cancer (NSCLC) [15,16]. However, the associations between SHIP2 expression and the corresponding clinical features for determining the clinicopathologic significance of SHIP2 in HCC have not been investigated.
In the present study, SHIP2 mRNA and protein expressions were investigated in a number of HCC samples with tumor-adjacent normal tissues. Moreover, the association between SHIP2 expression and clinicopathologic data in a selected group of patients with HCC was analyzed. Finally, the prognostic significance of SHIP2 protein expression in HCC was evaluated.
Materials and methods
Patients and tissue samples
A total of 111 formalin-fixed, paraffin-embedded HCC tissues and 100 matched tumor-adjacent normal tissues were obtained from the First Affiliated Hospital of Nanjing Medical University between 2002 and 2007. Before surgical therapy, none of the patients had received neoadjuvant chemotherapy, radiation therapy or immunotherapy. Related important clinical information (including gender, age, tumor size, serum level of α-fetoprotein (AFP), and other) was collected from each patient’s medical records, which included a five-year follow-up period after surgery. Clinical staging was performed according to the 2002 American Joint Committee on Cancer/International Union Against Cancer TNM staging system [17]. The average age of the group was 51.92 years (range 26-75 years). A panel of 20 fresh HCC tissues and corresponding adjacent non-cancerous tissues, obtained from the tissue bank of The First People’s Hospital of Kunshan Affiliated with Jiangsu University (Suzhou, China) were also enrolled in this study. Written informed consent was accomplished from the patients for publication of this study and any accompanying images. Study protocol was approved by the Ethics Committee of local hospital.
One-step quantitative real-time reverse transcription-polymerase chain reaction (qPCR)
Total RNA was extracted from a subset of the frozen tissues mentioned above using the Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s guidelines. One-step qPCR analysis was performed with a SensiMixTM One-Step Kit (Quantace, London, UK) using a Real Time PCR system (Bio-Rad IQ5, Hercules, CA, USA) according to the standard protocol. The primers for SHIP2 were as follows: forward primer 5’-TCG TCA CCA GCG ACC ATT C-3’ and reverse primer 5’-AGC CCT TTC TTG GAG ATG AAC TG-3’. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA level was used to standardize the measurements of the target gene and the primers for GAPDH were as follows: forward primer 5’-TGC ACC ACC AAC TGC TTA GC-3’ and reverse primer 3’-GGC ATG GAC TGT GGT CAT GAG-5’. Amplification conditions consisted of 30 minutes at 42°C for reverse transcription and 2 minutes at 94°C for Taq activation, followed by 35 cycles of 95°C for 20 seconds, 56°C for 20 seconds, and elongation at 72°C for 30 seconds. Each measurement was performed in triplicate.
Tissue microarrays (TMA) construction and immunohistochemistry (IHC)
TMA was produced by Alenabio Biotech (Xi’an, China). Core tissue biopsies (2 mm in diameter) were collected from individual paraffin-embedded HCC and arranged in the recipient paraffin blocks. The TMA was cut into 4 μm sections and placed on SuperfrostTM charged glass microscope slides. IHC analysis was performed as previously described [18]. TMA sections (4 μm) were deparaffinized in 100% xylene and re-hydrated in graded ethanol solutions. Tissue sections were incubated with a primary anti-SHIP2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in TBS containing 1% bovine serum albumin for 1 hour. After washing, sections were then washed with TBS and incubated with horseradish peroxidase-conjugated anti-goat antibody (Dako Cytomation, Carpentaria, CA). For the negative control reactions, phosphate-buffered saline was used in place of the primary anti-SHIP2 antibody. Four fields in each slide were randomly chosen, and the percentage of positive staining was evaluated using immunohistochemistry score (IHS). Results of IHC were analyzed according to a previously described method [19,20]. Specifically, the IHS was determined by evaluating of both staining density and intensity. The staining intensity was scored as follows: 0 (negative), 1 (weakly positive), 2 (moderately positive), and 3 (strongly positive). The percentage of SHIP2-positive cells was also scored according to the following four categories: 1 represents 0% to 10%, 2 represents 11% to 50%, 3 represents 51% to 80%, and 4 represents 81% to 100%. Sum of the intensity and the percentage scores led to the final IHS: samples with a sum score below 3 (IHS ≤ 3) were considered as low SHIP2 expression while those with a sum score above 4 (IHS ≥ 4) as high SHIP2 expression.
Statistical analysis
Statistical analyses were carried out by using STATA Version 12.0 (Stata Corporation, College Station, TX). The expression of SHIP2 mRNA in fresh frozen HCC tissues and in the corresponding non-cancerous tissues normalized to GAPDH was analyzed with the Wilcoxon non-parametric signed-rank test. The relationship between SHIP2 expression and clinicopathological factors was analyzed by chi-square test. Survival rate was estimated by Kaplan-Meier method. Univariate and multivariate analysis was carried out using Cox’s proportional hazards regression models. For all tests, the significance level for statistical analysis was set at p < 0.05.
Results
Evaluation of SHIP2 mRNA expression by qPCR
Total RNA was extracted from the HCC tissues and subjected to one-step qPCR to determine the expression of SHIP2 mRNA. The mRNA expression of samples from the matched tumor adjacent tissues was used for comparing the expression of the mRNA with that of non-cancerous tissue. When normalized to GAPDH, the means of SHIP2 mRNA expression in HCC and the corresponding non-cancerous tissue were 4.27 ± 1.3275 and 1.77 ± 0.4344 respectively (p < 0.001). SHIP2 expression averaged 2.41-fold higher in HCC samples than in non-cancerous tissues (Figure 1).
Figure 1.
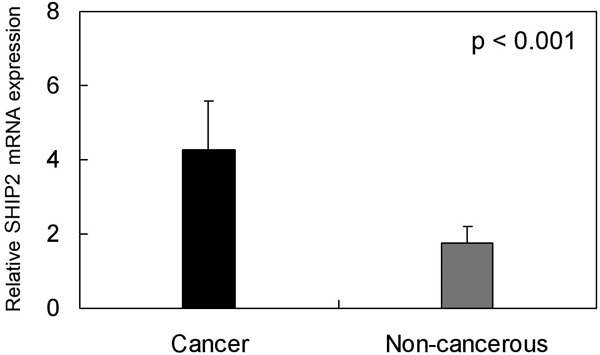
Expression of SHIP2 mRNA in hepatocellular carcinoma (HCC) and tumor adjacent tissues. One-step quantitative real-time polymerase chain reaction (qPCR) was employed to evaluate SHIP2 mRNA expression levels in HCC (cancer) compared with tumor adjacent tissue (non-cancerous). Expression of SHIP2 mRNA in HCC tissues (4.27 ± 1.3275) was higher than in matched tumor adjacent non-cancerous tissues (1.77 ± 0.4344) (p < 0.001), when normalized to the GAPDH internal control.
Association between SHIP2 expression with clinicopathological variables
The relationship between high SHIP2 expression with clinicopathological variables of 111 HCC patients was shown in Table 1. High SHIP2 expression was significantly related to tumor differentiation (p = 0.001), α-fetoprotein level (p = 0.017), liver cirrhosis (p = 0.035), and five-year survival rate (p = 0.008). In contrast, no significant association was discovered between SHIP2 expression and other clinical parameters, such as gender, age, tumor diameter, hepatitis B virus infection, portal vein invasion, lymph node metastasis and TNM stage (Table 1).
Table 1.
Correlation of high SHIP2 expression with clinicopathological characteristics in HCC
| Groups | No. | SHIP2 | X2 | p value | |
|---|---|---|---|---|---|
|
| |||||
| + | % | ||||
| Gender | |||||
| Male | 90 | 48 | 53.33 | 0.7480 | 0.387 |
| Female | 21 | 9 | 42.86 | ||
| Age (years) | |||||
| < 40 | 16 | 10 | 62.50 | 2.3760 | 0.305 |
| 40-60 | 68 | 31 | 45.59 | ||
| > 60 | 27 | 16 | 59.26 | ||
| Tumor diameter (cm) | |||||
| ≥ 5 | 74 | 41 | 55.41 | 1.4605 | 0.227 |
| < 5 | 37 | 16 | 43.24 | ||
| Tumor differentiation | |||||
| Well | 14 | 2 | 14.29 | 22.9127 | 0.001* |
| Moderate | 37 | 12 | 32.43 | ||
| Poor | 60 | 43 | 71.67 | ||
| α-fetoprotein (μg/L) | |||||
| < 13.2 | 20 | 7 | 35.00 | 8.1488 | 0.017* |
| 13.2-1210 | 72 | 35 | 48.61 | ||
| > 1210 | 19 | 15 | 78.95 | ||
| Liver cirrhosis | |||||
| Yes | 91 | 51 | 56.04 | 4.4518 | 0.035* |
| No | 20 | 6 | 30.00 | ||
| Hepatitis B virus infection | |||||
| Yes | 93 | 51 | 54.84 | 2.7919 | 0.095 |
| No | 18 | 6 | 33.33 | ||
| Portal vein invasion | |||||
| Yes | 75 | 41 | 54.67 | 1.0174 | 0.313 |
| No | 36 | 16 | 44.44 | ||
| Lymph node metastasis | |||||
| Yes | 33 | 20 | 60.61 | 1.6101 | 0.204 |
| No | 78 | 37 | 47.44 | ||
| TNM stage | |||||
| Stage I, II | 64 | 31 | 48.44 | 0.5137 | 0.474 |
| Stage III, IV | 47 | 26 | 55.32 | ||
| Five years’ survival | |||||
| Yes | 77 | 46 | 59.74 | 7.0815 | 0.008* |
| No | 34 | 11 | 32.35 | ||
p < 0.05.
Expression of SHIP2 in HCC by IHC
IHC analysis was performed to confirm the elevated expression of SHIP2 in HCC. Elevated SHIP2 expression was detected in 57 (51.55%) out of 111 HCC tissues and in 21 (21.00%) out of 100 matched tumor-adjacent non-cancerous tissues. The results showed statistical significance (p < 0.01) and were consistent with the SHIP2 mRNA levels in the HCC samples. Positive staining was mainly localized in the cytoplasmic membrane and cytoplasm of HCC cells. Typical IHC staining patterns for SHIP2 in HCC are shown in Figure 2.
Figure 2.
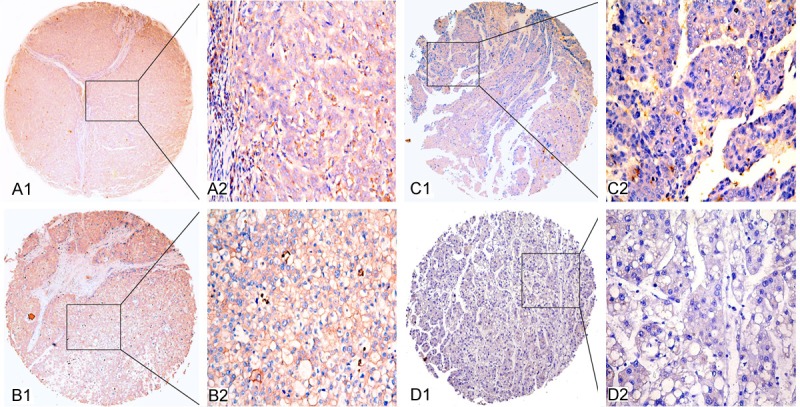
Representative figures of SHIP2 protein expression in hepatocellular carcinoma (HCC) and adjacent non-cancerous tissues. A1 and A2: Strong immunohistochemical (IHC) staining of SHIP2 in poorly differentiated HCC tissue sample. B1 and B2: Strong IHC staining of SHIP2 in well differentiated HCC tissue sample. C1 and C2: Weak IHC staining of SHIP2 in HCC tissue sample. D1 and D2: Negative IHC staining of SHIP2 in tumor adjacent non-cancerous tissue sample. Original magnification × 40 in A1, B1, C1 and D1; × 400 in A2, B2, C2 and D2.
Survival analysis
As expected, the overexpression of SHIP2 protein showed a significant correlation with the decreased five-year survival rate of 111 HCC patients (p = 0.001). In addition, certain HCC clinical prognostic factors, such as differentiation (p = 0.005), lymph node metastasis (p = 0.040), and advanced TNM stage classification (p = 0.014), also showed a statistically significant correlation with the decreased five-year survival rate based on Cox regression univariate analysis (Table 2). All of the above significant factors were enrolled in a multivariate analysis. The results indicated that high SHIP2 expression (p = 0.017) and tumor differentiation (p = 0.036) are two independent prognostic factors for HCC (Table 2). Kaplan-Meier survival curves indicated that HCC patients with high SHIP2 expression and poor tumor differentiation had a significantly shorter survival time (Figure 3).
Table 2.
Univariate and multivariate analysis of prognostic factors in HCC for five-year survival
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | p >|z| | 95% CI | HR | p >|z| | 95% CI | ||
| SHIP2 expression | |||||||
| High versus Low | 2.169 | 0.001* | 1.358-3.465 | 1.822 | 0.017* | 1.113-2.981 | |
| Gender | |||||||
| Male versus Female | 1.176 | 0.574 | 0.668-2.071 | ||||
| Age (years) | |||||||
| < 40 and 40-60 versus > 60 | 0.956 | 0.870 | 0.555-1.645 | ||||
| Tumour diameter (cm) | |||||||
| ≥ 5 versus < 5 | 1.221 | 0.416 | 0.756-1.974 | ||||
| Tumor differentiation | |||||||
| Well-Moderate versus poor | 1.956 | 0.005* | 1.223-3.129 | 1.696 | 0.036* | 1.034-2.783 | |
| α-fetoprotein (μg/L) | |||||||
| < 13.2 versus 13.2-1210 and > 1210 | 0.704 | 0.325 | 0.349-1.418 | ||||
| Liver cirrhosis | |||||||
| Yes versus No | 1.665 | 0.135 | 0.853-3.249 | ||||
| Hepatitis B virus infection | |||||||
| Yes versus No | 1.027 | 0.929 | 0.565-1.871 | ||||
| Portal vein invasion | |||||||
| Yes versus No | 1.440 | 0.158 | 0.868-2.389 | ||||
| Lymph node metastasis | |||||||
| Yes versus No | 1.629 | 0.040* | 1.023-2.592 | 0.987 | 0.976 | 0.426-2.289 | |
| TNM stage | |||||||
| Stage I, II versus Stage III, IV | 0.561 | 0.014* | 0.353-0.890 | 0.552 | 0.167 | 0.238-1.281 | |
p < 0.05.
Figure 3.
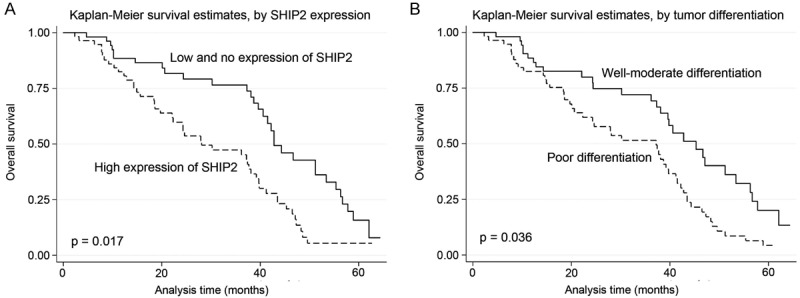
Survival analysis of 111 hepatocellular carcinoma (HCC) patients by Kaplan-Meier method. A: Overall survival rate in patients with high SHIP2 expression (dashed line) was significantly lower than that in patients with no or low SHIP2 expression (solid line). B: Overall survival rate in patients with poor differentiation of tumor (dashed line) was significantly lower than that in patients with well-moderate differentiation of tumor (solid line).
Discussion
A growing number of novel treatment strategies have been developed for HCC, including molecular targeted therapy [21], gene therapy [22], and immunotherapy [23]. However, satisfactory therapeutic outcomes have not been achieved, and the survival rate of HCC is still low [24]. Considering that several studies have suggested substantial molecular markers for HCC [4,6], further identification of new prognostic markers remains important for the prevention and treatment of HCC.
Among the signaling pathways known to control cell proliferation and apoptosis, aberrant regulation of the PI3K/AKT/mTOR pathway plays an important role in tumor development and progression [25]. SHIP2 and phosphatase and tensin homologue on chromosome 10 (PTEN) are two significant lipid phosphatases involved in the downstream action of the PI3K/Akt pathway [10]. Although the exact function of SHIP2 remains unclear, several studies have suggested that SHIP2 displays important function in signaling pathways, specifically in growth factor and integrin signaling, as well as in receptor endocytosis [26,27]. A recent study reported that SHIP2 silencing in breast cancer cells reduces cell proliferation in vitro and cancer growth in vivo and inhibits tumor metastases [28]. In summary, the above findings suggest that SHIP2 exerts an oncogenic effect in human cancer.
In the present study, the mRNA and protein levels of SHIP2 in HCC and tumor-adjacent non-cancerous tissues were evaluated using qPCR and IHC. Both analyses showed that SHIP2 was highly expressed in HCC compared with the corresponding non-cancerous tissues. The obtained data were consistent with the previous report, which showed overexpression of SHIP2 in breast cancer cells compared with non-tumor cells [15]. Moreover, high expression of SHIP2 in HCC was found to be correlated with certain clinical pathologic characteristics, including tumor differentiation, α-fetoprotein level, liver cirrhosis, and five-year survival rate.
Univariate analysis indicated that in addition to SHIP2 expression, tumor differentiation, lymph node metastasis, and TNM stage were also correlated with the life span of HCC patients. Multivariate analysis further demonstrated that high expression of SHIP2 could be considered as an independent factor for poor prognosis of HCC patients, as well as tumor differentiation. The obtained data agree with results from our latest research, which showed that SHIP2 could be identified as a harmful prognostic factor for NSCLC [16].
Interestingly, a recent study which collected 20 HCC tissue samples reported decreased SHIP2 expression levels in HCC tissues compared with non-cancerous tissues which are contradictory to the results of the present study. No solid evidence was discovered to demonstrate the association between SHIP2 expression and HCC prognosis [29]. The conflicting results may be due to the small sample sizes, differences in the pathological samples, and the experimental methods or evaluation system.
In summary, to the best of our knowledge, the present study is the first to report the differential expression of SHIP2 in HCC and the possible use of SHIP2 as a novel prognostic marker in HCC. The present findings demonstrate high expression of SHIP2 in HCC tissue, which is associated with a poor prognosis in HCC patients. Further studies are needed to confirm the prognostic and therapeutic value of SHIP2 for HCC and to elucidate the mechanisms of action of SHIP2 in HCC.
Disclosure of conflict of interest
None.
References
- 1.Palaiologou M, Koskinas J, Karanikolas M, Fatourou E, Tiniakos DG. E2F-1 is overexpressed and pro-apoptotic in human hepatocellular carcinoma. Virchows Arch. 2012;460:439–46. doi: 10.1007/s00428-012-1220-4. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Guo X, Jin Y, Qian G, Tu H. Sequential accumulation of the mutations in core promoter of hepatitis B virus is associated with the development of hepatocellular carcinoma in Qidong, China. J Hepatol. 2008;49:718–25. doi: 10.1016/j.jhep.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Xia QY, Liu S, Li FQ, Huang WB, Shi LN, Zhou XJ. Sperm protein 17, MAGE-C1 and NY-ESO-1 in hepatocellular carcinoma: expression frequency and their correlation with clinical parameters. Int J Clin Exp Pathol. 2013;6:1610–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 6.Tang Q, Liu YF, Zhu XJ, Li YH, Zhu J, Zhang JP, Feng ZQ, Guan XH. Expression and prognostic significance of the αB-crystallin gene in human hepatocellular carcinoma. Hum Pathol. 2009;40:300–5. doi: 10.1016/j.humpath.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Faivre S, Bouattour M, Raymond E. Novel molecular therapies in hepatocellular carcinoma. Liver Int. 2011;31:151–60. doi: 10.1111/j.1478-3231.2010.02395.x. [DOI] [PubMed] [Google Scholar]
- 8.Alves RC, Alves D, Guz B, Matos C, Viana M, Harriz M, Terrabuio D, Kondo M, Gampel O, Polletti P. Advanced hepatocellular carcinoma. Review of targeted molecular drugs. Ann Hepatol. 2011;10:21–27. [PubMed] [Google Scholar]
- 9.Berz D, Wanebo H. Targeting the growth factors and angiogenesis pathways: small molecules in solid tumors. J Surg Oncol. 2011;103:574–86. doi: 10.1002/jso.21776. [DOI] [PubMed] [Google Scholar]
- 10.Blunt MD, Ward SG. Targeting PI3K isoforms and SHIP in the immune system: new therapeutics for inflammation and leukemia. Curr Opin Pharmacol. 2012;12:444–51. doi: 10.1016/j.coph.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 12.Rohrschneider LR, Fuller JF, Wolf I, Liu Y, Lucas DM. Structure, function, and biology of SHIP proteins. Genes Dev. 2000;14:505–20. [PubMed] [Google Scholar]
- 13.Ichihara Y, Wada T, Soeda Y, Ishii Y, Sasahara M, Tsuneki H, Sasaoka T. SH2-containing inositol 5’-phosphatase 2 (SHIP2) selectively impaired hypothalamic insulin signalling and regulation of food intake in mice. J Neuroendocrinol. 2013;25:372–82. doi: 10.1111/jne.12014. [DOI] [PubMed] [Google Scholar]
- 14.Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells lose it. Biochem J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad NK, Tandon M, Handa A, Moore GE, Babbs CF, Snyder PW, Bose S. High Expression of Obesity-Linked Phosphatase SHIP2 in Invasive Breast Cancer Correlates with Reduced Disease-Free Survival. Tumour Biol. 2008;29:330–41. doi: 10.1159/000172970. [DOI] [PubMed] [Google Scholar]
- 16.Fu MY, Fan WF, Pu XL, Ni HH, Zhang W, Chang F, Gong Li, Xiong L, Wang J, Gu XF. Elevated expression of SHIP2 correlates with poor prognosis in non-small cell lung cancer. Int J Clin Exp Pathol. 2013 [Epub ahead of print] [PMC free article] [PubMed] [Google Scholar]
- 17.Varotti G, Ramacciato G, Ercolani G, Grazi GL, Vetrone G, Cescon M, Del Gaudio M, Ravaioli M, Ziparo V, Lauro A, Pinna A. Comparison between the fifth and sixth editions of the AJCC/UICC TNM staging systems for hepatocellular carcinoma: multicentric study on 393 cirrhotic resected patients. Eur J Surg Oncol. 2005;31:760–7. doi: 10.1016/j.ejso.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Chen R, Lu M, Wang J, Zhang D, Lin H, Zhu H, Zhang W, Xiong L, Ma J, Mao Y, Zhu J, Xu J. Increased expression of Trop2 correlates with poor survival in extranodal NK/T cell lymphoma, nasal type. Virchows Arch. 2013 doi: 10.1007/s00428-013-1475-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Mao Y, Zhang DW, Zhu H, Lin H, Xiong L, Cao Q, Liu Y, Li QD, Xu JR, Xu LF, Chen RJ. LMP1 and LMP2A are potential prognostic markers of extranodal NK/T-cell lymphoma, nasal type (ENKTL) Diagn Pathol. 2012;7:178. doi: 10.1186/1746-1596-7-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao Y, Zhang DW, Lin H, Xiong L, Liu Y, Li QD, Ma J, Cao Q, Chen RJ, Zhu J, Feng ZQ. Alpha B-crystallin is a new prognostic marker for laryngeal squamous cell carcinoma. J Exp Clin Cancer Res. 2012;31:101. doi: 10.1186/1756-9966-31-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tazi el M, Essadi I, M’rabti H, Touyar A, Errihani PH. Systemic treatment and targeted therapy in patients with advanced hepatocellular carcinoma. N Am J Med Sci. 2011;3:167–75. doi: 10.4297/najms.2011.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu L, Wang Y, Gong L, Zhu J, Gong R, Si J. Suicide gene therapy for hepatocellular carcinoma cells by survivin promoter-driven expression of the herpes simplex virus thymidine kinase gene. Oncol Rep. 2013;29:1435–40. doi: 10.3892/or.2013.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tada F, Abe M, Hirooka M, Ikeda Y, Hiasa Y, Lee Y, Jung NC, Lee WB, Lee HS, Bae YS, Onji M. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol. 2012;41:1601–9. doi: 10.3892/ijo.2012.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie B, Zhou J, Yuan L, Ren F, Liu DC, Li Q, Shu G. Epigenetic silencing of Klotho expression correlates with poor prognosis of human hepatocellular carcinoma. Hum Pathol. 2013;44:795–801. doi: 10.1016/j.humpath.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Prasad NK, Decker SJ. SH2-containing 5’-inositol phosphatase, SHIP2, regulates cytoskeleton organization and ligand-dependent down-regulation of the epidermal growth factor receptor. J Biol Chem. 2005;280:13129–36. doi: 10.1074/jbc.M410289200. [DOI] [PubMed] [Google Scholar]
- 27.Koch A, Mancini A, El Bounkari O, Tamura T. The SH2-domian-containing inositol 5-phosphatase (SHIP)-2 binds to c-Met directlyvia tyrosine residue 1356 and involves hepatocyte growth factor (HGF)-induced lamellipodium formation, cell scattering and cell spreading. Oncogene. 2005;24:3436–47. doi: 10.1038/sj.onc.1208558. [DOI] [PubMed] [Google Scholar]
- 28.Prasad NK, Tandon M, Badve S, Snyder PW, Nakshatri H. Phosphoinositol phosphatase SHIP2 promotes cancer development and metastasis coupled with alterations in EGF receptor turnover. Carcinogenesis. 2008;29:25–34. doi: 10.1093/carcin/bgm213. [DOI] [PubMed] [Google Scholar]
- 29.Sumie S, Kawaguchi T, Komuta M, Kuromatsu R, Itano S, Okuda K, Taniguchi E, Ando E, Takata A, Fukushima N, Koga H, Torimura T, Kojiro M, Sata M. Significance of glucose intolerance and SHIP2 expression in hepatocellular carcinoma patients with HCV infection. Oncol Rep. 2007;18:545–52. [PubMed] [Google Scholar]


