Abstract
Objective: Hepatorenal syndrome is one of the serious complications of cirrhosis and closely associated with the increasing intra-abdominal pressure (IAP). The study aims to explore the potential mechanism of intra-abdominal hypertension in the development of hepatorenal syndrome in mouse models. Methods: Eighty male mice were randomly divided into model group (subcutaneous injection of carbon tetrachloride) and control group (subcutaneous injection of olive oil). After 12 weeks, parts of the mice were sacrificed and liver histopathology was detected. Then, albumin (30 g/L) and normal saline were separately injected into the peritoneal cavity of mice to induce the different IAP levels (0, 5, 10 and 20cmH2O). Blood urea nitrogen, serum creatinine and renal histopathology were examined 24 hours later. Results: Blood urea nitrogen and serum creatinine levels were statistically significant high in the group of IAP= 10 and 20cmH2O as compared with the IAP= 0cmH2O. From results of renal histopathology, the constrictive renal tubular lumen and inflammatory infiltration in the interstitial were observed in groups of IAP= 5 and 10cmH2O. Besides, the formed casts and hyperemia in the renal interstitial could be detected in group of IAP= 20cmH2O. The cellular swelling and edema of renal tubular epithelial cells were found in model group simultaneously. Conclusions: Our study suggested that intra-abdominal hypertension was a significant pathological mechanism and a potential independent risk factor of hepatorenal syndrome.
Keywords: Intra-abdominal hypertension, hepatorenal syndrome, abdominal compartment syndrome, cirrhosis
Introduction
Hepatorenal syndrome (HRS) is one of the serious complications in the end-stage liver disease [1-3]. The peripheral vasodilatation is regarded as the classic pathological mechanism in the development of HRS, which is associated with the increasing intra-abdominal pressure (IAP) [4,5]. In clinical practice, IAP could be elevated with the accumulation of ascites in cirrhotic patients. Besides, oliguria would suddenly appear and the increased blood urea nitrogen (BUN) and serum creatinine (SCr) could be detected [6-8]. However, the specific role of IAP in the HRS and its relationship with cirrhosis remains unknown. The aim of this study is to investigate the correlation between intra-abdominal hypertension (IAH) and HRS in mouse models. In addition, we attempt to elaborate the specific effect of IAH in the development of HRS.
Materials and methods
Animals
The study was carried out in strict accordance with the institution’s guidelines for experimental animals. The protocol was approved by the Animal Experimental Ethics Committee of Tongji Hospital, Tongji University. Male ICR mice, weighing 3035 g (n=80), were housed under standard conditions at constant room temperature, humidity and regular 12 h/12 h light/dark cycles. The mice had free access to water and were fed with standard diet.
Animal model of cirrhosis
Eighty male ICR mice were randomly divided into two groups: model group (subcutaneous injection of carbon tetrachloride, 0.3 ml/100 g, 3 times/week) and control group (subcutaneous injection of olive oil, 0.3 ml/100 g, 3 times/week). Parts of the mice in the model group were sacrificed 12 weeks later and the liver histopathology was examined to ensure a cirrhotic model.
Animal model of IAP
The mice in model and control group were separately randomly divided into 4 sub-groups (7 mice per sub-group). Different volume of albumin (30 g/L) and normal saline were injected into the peritoneal cavity of mice to model the different IAP (0, 5, 10 and 20cmH2O). Abdominal circumference and body weight of each mouse were monitored during the modeling. Albumin and normal saline and were added separately to retain the certain IAP as the circumference or weight decreased by 10%. All the mice were sacrificed 24 hours later.
IAP measurement
A midline incision was performed and a percutaneous peripheral intravenous catheter was introduced into the peritoneal cavity. The IAP was recorded continuously with this catheter connected to a pressure transducer from a monitor system (Petas, KMA 275, Ankara, Turkey).
BUN and SCr determination
Blood samples of all the mice were collected and stored at -20 °C until examination. BUN and SCr were measured using an enzymatic method in the Central Laboratory of Clinical Chemistry in Tongji Hospital, Tongji University.
Histopathology
After the sacrifices of each mouse, specimens of liver and kidney were surgically removed and fixed in neutral buffered formalin. The samples were then sectioned in the coronal plane (5 μm slice thickness) and prepared with hematoxylin and eosin staining for routine light microscopy. Histopathological assessment was performed by two experienced pathologists.
Statistical analysis
Categorical variables were presented as frequencies and percentages, with continuous variables as mean ± SD. The difference between the control and model group were analyzed by Student’s t test. All the data were calculated by SPSS14.0 (SPSS, Inc., Chicago, IL, USA). P< 0.05 was considered as statistical significance.
Results
Cirrhotic mouse models
Compared with the normal in control group, the shape of liver in model group was rough and small nodules could be seen. According to pathological examination, hepatocytes arranged disorderly and the regenerated nodules were detected (Figure 1).
Figure 1.
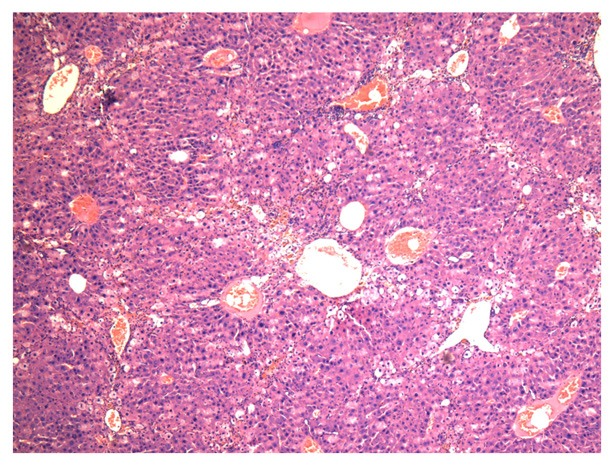
Liver pathologic changes of model group (hematoxylin-eosin stain), ×40.
BUN and SCr levels
BUN levels in control group of IAP= 0cmH2O was 9.1±0.8 mmol/L. It was significant elevated in group of IAP= 10cmH2O (11.6±1.0 mmol/L, P< 0.05) and IAP= 20cmH2O (13.5±2.0 mmol/L, P< 0.01). In model group, the baseline of BUN levels was 9.2±0.6 mmol/L. A significant increase could also be observed in the IAP= 10cmH2O (11.3±1.6 mmol/L, P< 0.01) and IAP= 20cmH2O (14.8±1.9 mmol/L, P< 0.01) (Table 1).
Table 1.
BUN and SCr levels with different IAP
| Control Group | Model Group | |||||||
|---|---|---|---|---|---|---|---|---|
| IAP (cmH2O) | 0 | 5 | 10 | 20 | 0 | 5 | 10 | 20 |
| BUN (mmol/L) | 9.1±0.8 | 9.3±0.9 | 11.6±1.0* | 13.5±2.0** | 9.2±0.6 | 9.6±0.9 | 11.3±1.6Δ | 14.8±1.9Δ |
| SCr (μmol/L) | 94.4±6.9 | 96.9±7.8 | 114.4±8.4** | 130.5±9.8** | 94.3±6.6 | 95.4±6.9 | 128.3±10.9Δ | 140.5±13.9Δ |
Compared with the IAP= 0cmH2O in control group, P< 0.05;
compared with the IAP= 0cmH2O in control group, P< 0.01;
compared with the IAP= 0cmH2O in model group, P< 0.01.
BUN, blood urea nitrogen; SCr, serum creatinine; IAP, intra-abdominal pressure.
The similar change of SCr levels was detected. The baseline levels were 94.4±6.9 μmol/L in control and 94.3±6.6 μmol/L in model group, respectively. It increased greatly in control group of IAP= 10cmH2O (114.4±8.4 μmol/L, P< 0.01) and IAP= 20cmH2O (130.5±9.8, P< 0.01). For the model group, it significant elevated to the IAP= 10cmH2O (128.3±10.9 μmol/L, P< 0.01) and IAP= 20cmH2O (140.5±13.9, P< 0.01) (Table 1). Interestingly, there were no significant differences of BUN and SCr levels in the groups of the same IAP.
Renal histopathology
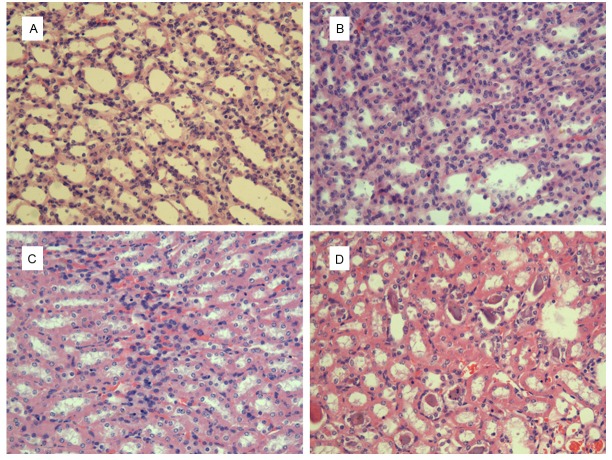
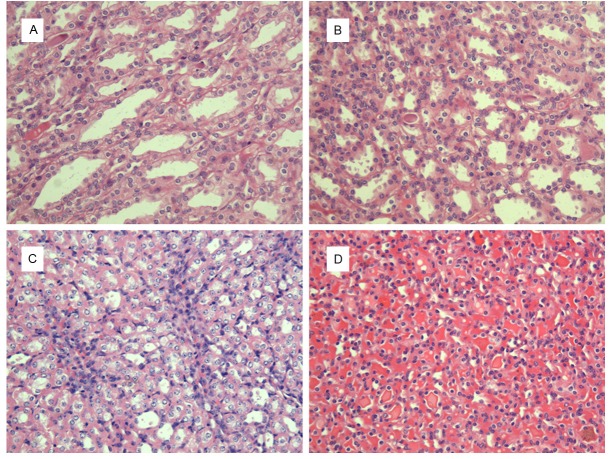
According to renal specimen (hematoxylin-eosin stain, ×200), the renal tubular epithelial cells and lumen were normal in both group of IAP= 0cmH2O (Figures 2A and 3A). The constrictive renal tubular lumen and a few inflammatory infiltration in the interstitial could be observed in the group of IAP= 5cmH2O (Figures 2B and 3B). As for the IAP= 10cmH2O, significant constrictive renal tubular lumen with a lot inflammatory infiltration in the renal interstitial in both group, as well as cellular swelling in model group, were detected (Figures 2C and 3C). Besides, the formed casts and hyperemia in the renal interstitial in two groups and the edema of renal tubular epithelial cells in model group were the characteristics of IAP= 20cmH2O (Figures 2D and 3D).
Figure 2.
Renal pathologic changes of control group, (hematoxylin-eosin stain), ×200. A: IAP= 0cmH2O, the normal renal tubular epithelial cells and lumen; B: IAP= 5cmH2O, the constrictive renal tubular lumen with a few inflammatory infiltration in the renal interstitial; C: IAP= 10cmH2O, the constrictive renal tubular lumen with a lot inflammatory infiltration in the renal interstitial; D: IAP= 20cmH2O, the constrictive renal tubular lumen, the formed casts and hyperemia in the interstitial.
Figure 3.
Renal pathologic changes of model group, (hematoxylin-eosin stain), ×200. A: IAP= 0cmH2O, the normal renal tubular epithelial cells and lumen; B: IAP= 5cmH2O, the constrictive renal tubular lumen with a few inflammatory infiltration in the renal interstitial; C: IAP= 10cmH2O, the renal cellular swelling, significant constrictive renal tubular lumen with a lot inflammatory infiltration in the renal interstitial; D: IAP= 20cmH2O, the edema of renal tubular epithelial cells, the constrictive renal tubular lumen, the formed casts and hyperemia in the interstitial.
Discussion
HRS is one of the most advanced stages of complications in patients with cirrhosis. The hallmark of HRS is intense renal vasoconstriction at the early time point [1-3]. It could be deteriorating with the aggravation of liver disease [2]. The definite mechanism of HRS is still unknown now. Potential pathways involved in the pathology of HRS are as following: 1. the vasodilatation of peripheral arterial with hyperdynamic circulation and subsequent renal vasoconstriction; 2. the activation of the renal sympathetic nervous system; 3. the cardiac dysfunction leading to the renal hypoperfusion; 4. the action of cytokines and vasoactive mediators on the renal and other vascular beds [3-8].
The increasing resistance of blood flow through the cirrhotic liver and the vasodilation of the spleen circulation lead to the blood accumulation in splenic blood pooling. Vasodilator factors are further induced due to the decreased circulating volume [4,5]. Then, the low effective circulating volume unloads the pressure baroreceptors in the carotid body and aortic arch with subsequent compensatory activation of sympathetic nervous system, rennin angiotensin aldosterone system and non-osmotic release of vasopressin. It results in a hyperdynamic circulation with increased cardiac output, decreased systemic vascular resistance, hypotension, and renal vasoconstriction. Further splanchnic vasodilation will occur with the progress of cirrhosis [1-3]. Although this hypothesis provides a rational explanation of the hemodynamic changes in cirrhosis and HRS, it has not been validated in human studies. Besides, factors, such as the vasoactive agents affecting both the systemic and renal circulation, are operative in HRS and involve in the regulation of intrarenal hemodynamics and glomerular filtration rate [6-8].
In clinical practice, IAP could be raised due to the increasing ascites in patients with cirrhosis. To a certain extent, oliguria would suddenly appear and the elevated BUN and SCr could be detected. In our study, BUN and SCr levels were raised both in the control and model group of IAP= 10 and 20cmH2O with statistical significance. However, there were no significant differences of BUN and SCr with the same IAP in both groups. Accordingly, we concluded that IAH was the significant pathological mechanism and an independent risk factor in the occurrence and development of HRS.
IAH occurs over a wide range of persistent IAP with large variability in the manner of clinical manifestation, the lack of standardized definitions has hampered efforts to study this problem. In 2006, the world society for Abdominal Compartment Syndrome (ACS) published consensus definitions of IAH/ACS based on the current evidence and expert opinions. Accordingly, IAH was defined as sustained or repeated pathologic elevation of IAP≥ 12mmHg. Sustained elevation of IAP> 20mmHg accompanying new organ dysfunction, such as cardiovascular, respiratory and renal systems, was defined as ACS [9-12].
In recent years, both IAH and ACS are increasingly associated with morbidity and mortality in critical patients [13,14]. Therefore, elevated IAP plays a more significant role in the development of multiple system organ failure and is regarded as an independent predictor of mortality [15,16]. Several mechanisms were proposed as the etiologies of renal dysfunction or failure induced by IAH/ACS. Renal blood flow was demonstrated to preferentially diminish in comparison to that of celiac or superior mesenteric artery when IAP persistently increases [17]. Moreover, renal vein pressure and renal vascular resistance are significantly elevated. All of these changes shunt blood away from the renal cortex and functional glomeruli, which leads to impaired glomerular and tubular function and significant urinary output reduction [18,19]. Recent studies suggested that the compression of renal vein was vital in the development of renal dysfunction [19,20]. Parenchymal compression and “renal compartment syndrome” may result in renal ischemia and subsequent failure [21,22]. In addition, factors, such as reduction of cardiac output and elevated levels of catecholamines, renin, angiotensin and inflammatory cytokines, may lead to further renal dysfunction as IAP approaches the range of ACS [23]. Diuretics, albumin or other treatments are often insensitive or ineffective to the recovery of renal function in the cirrhotic patients with ascites. However, it could be improved after the extraction of ascites. Early abdominal decompression, rather than late, is gaining more popularity with better outcomes. From the results of our study, we can also conclude that IAH is a significant pathological mechanism and an independent risk factor of HRS.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81070343); National Natural Science Foundation of China (81370559); Shanghai Science and Technology Innovation Action Plan (12431901002).
Disclosure of conflict of interest
None.
Abbreviations
- ACS
abdominal compartment syndrome
- BUN
blood urea nitrogen
- HRS
hepatorenal syndrome
- IAH
intra-abdominal hypertension
- IAP
intra-abdominal pressure
- SCr
serum creatinine
References
- 1.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 2.Platt JF, Illis JH, Rubin JM, Merion RM, Lucey MR. Renal duplex Doppler ultrasonography: A noninvasive predictor of kidney dysfunction and hepatorenal failure in liver disease. Hepatology. 1994;20:362–369. [PubMed] [Google Scholar]
- 3.Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1:1066–1079. doi: 10.2215/CJN.01340406. [DOI] [PubMed] [Google Scholar]
- 4.Flint A. Clinical report on hydro-peritoneum, based on analysis of forty-six cases. Am J Med Sci. 1963;45:306–339. [Google Scholar]
- 5.Hecker R, Sherlock S. Electrolyte and circulatory changes in terminal liver failure. Lancet. 1956;271:1121–1125. doi: 10.1016/s0140-6736(56)90149-0. [DOI] [PubMed] [Google Scholar]
- 6.Ring-Larsen H. Renal blood flow in cirrhosis: Relaion to systemic and portal haemodynamics and liver function. Scand J Clin Lab Invest. 1977;37:635–642. doi: 10.3109/00365517709100657. [DOI] [PubMed] [Google Scholar]
- 7.Dagher L, Moore K. The hepatorenal syndrome. Gut. 2001;49:739–737. doi: 10.1136/gut.49.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore K. Endothelin and vascular function in liver disease. Gut. 2004;53:159–161. doi: 10.1136/gut.2003.024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, D’Amours S, Wendon J, Hillman K, Johansson K, Kolkman K, Wilmer A. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–1732. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 10.Burch JM, Moore EE, Moore FA, Franciose R. The abdominal compartment syndrome. Surg Clin N Amer. 1996;76:833–842. doi: 10.1016/s0039-6109(05)70483-7. [DOI] [PubMed] [Google Scholar]
- 11.Nathens AB, Brenneman FD, Boulanger BR. The abdominal compartment syndrome. Can J Surg. 1997;40:254–258. [PMC free article] [PubMed] [Google Scholar]
- 12.Latenser BA, Kowal-Vern A, Kimball D, Chakrin A, Dujovny N. A pilot study comparing percutaneous decompression with decompressive laparotomy for acute abdominal compartment syndrome in thermal injury. J Burn Care Rehabil. 2002;23:190–195. doi: 10.1097/00004630-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Ball CG, Kirkpatrick AW, Mcbeth P. The secondary abdominal compartment syndrome: it is time. Can J Surg. 2008;51:399–405. [PMC free article] [PubMed] [Google Scholar]
- 14.Daugherty EL, Hongyan L, Taichman D, Hansen-Flaschen J, Fuchs BD. Abdominal compartment syndrome is common in medical intensive care unit patients receiving large-volume resuscitation. J Intensive Care Med. 2007;22:294–299. doi: 10.1177/0885066607305247. [DOI] [PubMed] [Google Scholar]
- 15.Ivatury RR, Porter JM, Simon RJ, Islam S, John R, Stahl WM. Intra-abdominal hypertension after life-threatening penetrating abdominal trauma: prophylaxis, incidence, and clinical relevance to gastric mucosal pH and abdominal compartment syndrome. J Trauma. 1998;44:1016–1021. doi: 10.1097/00005373-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Schein M, Ivatury R. Intra-abdominal hypertension and the abdominal compartment syndrome. Br J Surg. 1998;85:1027–1028. doi: 10.1046/j.1365-2168.1998.00831.x. [DOI] [PubMed] [Google Scholar]
- 17.Barnes GE, Laine GA, Giam PY, Smith EE, Granger HJ. Cardiovascular responses to elevation of intra-abdominal hydrostatic pressure. Am J Physiol. 1985;248:R208–R213. doi: 10.1152/ajpregu.1985.248.2.R208. [DOI] [PubMed] [Google Scholar]
- 18.Harman PK, Kron IL, Mclachlan HD, Freedlender AE, Nolan SP. Elevated intra-abdominal pressure and renal function. Ann Surg. 1982;196:594–597. doi: 10.1097/00000658-198211000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloomfield GL, Blocher CR, Fakhry IF, Sica DA, Sugerman HJ. Elevated intra-abdominal pressure increases plasma rennin activity and aldosterone levels. J Trauma. 1997;42:997–1004. doi: 10.1097/00005373-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Richards WO, Scovill W, Shin B, Reed W. Acute renal failure associated with increased intra-abdominal pressure. Ann Surg. 1983;197:183–187. doi: 10.1097/00000658-198302000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson RA, Howdieshell TR. Abdominal compartment syndrome. South Med J. 1988;91:326–332. doi: 10.1097/00007611-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Stone HH, Fulenwider JT. Renal decapsulation in the prevention of post-ischemic oliguria. Ann Surg. 1977;186:343–355. doi: 10.1097/00000658-197709000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikami O, Fujise K, Matsumoto S, Shingu K, Ashida M, Matsuda T. High intra-abdominal pressure increases plasma catecholamine concentrations during pneumoperitoneum for laparoscopic procedures. Arch Surg. 1998;133:39–43. doi: 10.1001/archsurg.133.1.39. [DOI] [PubMed] [Google Scholar]