Abstract
The management of burns and injuries using novel treatment strategies involving epidermal stem cells (ESC) requires a better understanding of the biology of these cells, in particular, their isolation and the maintenance of their unique characteristics in culture. The purpose of this study was to describe an improved method for isolating putative ESC from fetal rat skin and to maintain them long term in culture. Single ESC suspensions were obtained from fetal rat skin by enzyme digestion containing 0.5% neutral protease. The target cells were harvested by rapid adherence on type IV collagen plates and were cultured in complex DMEM. After primary isolation, cells were continuously cultured in K-serum free medium. After reaching 70-80% confluence, the cells were digested with 0.25% trypsin at 37°C for 5-10 minutes, and passaged at a ratio of 1:2. The cultured ESC showed good growth, resulting in cell viability of over 98%. Four days later, clones containing 100-200 cells were detected, showing cobblestone-like characteristics. The rapidly adherent cells were positive for keratin 15, 19 and P63. Eighty three percent of cells expressed β1 integrin. The growth-curve showed that the rapidly adherent cells were in the exponential growth phase. The protocol described in this paper provides a simplified and effective method to isolate and maintain long-term culture of epidermal stem cells from fetal rat skin. This method should be valuable for isolating and studying ESC from various transgenic rat lines that are currently available.
Keywords: Epidermal stem cells, stem cell isolation, cell culture, fetal rat skin
Introduction
In skin, the epidermis provides the surface barrier, which is enfolded to form various structures including hair follicles, sebaceous glands, and sweat glands. In terms of stem cell populations, it is clear that the inter-follicular epidermis is capable of maintaining the replacement of surface keratinocytes for very long periods of time, providing no localized injury or cell killing occurs [1]. The inter-follicular epidermis must therefore have a population of resident stem cells that are the source of the steady-state cell replacement in this stable state. Recent data indicate that epidermal stem cells (ESC) exist in the epidermal basal layer (BL) and the hair follicle outer root sheath (ORS) [2]. Like stem cells of other tissues, ESC are important because they not only play a central role in homeostasis and wound repair, but also represent a major target of tumor initiation and gene therapy. A number of pathogenetic and clinical - therapeutic approaches to treat a variety of dermatoses may become possible with knowledge about keratinocyte proliferation, differentiation, and regeneration [3]. Previously, several methods to isolate and culture epidermal stem cells have been described [4-7]. However, the isolation and long-term culture of ESC has remained difficult with complex culture protocols and limited reproducibility of the cell isolation process. In addition, it is difficult to maintain the cells for more than three to five passages in culture. Through continuously improving the isolation and culture method of epidermal stem cells, satisfactory results have been achieved in culturing ESC by our group. Here, for the first time, we describe an improved method to isolate putative epidermal stem cells from fetal rat skin and to maintain them for long-term in culture. This method is further improved and simplified from the previously published methods.
Materials and methods
The isolation and culture of epidermal stem cells
SD pregnant rats were obtained from the Experimental Animal Center of Sun Yat-Sen University and kept under standard conditions according to the ethical committee of Medical Sciences Department. In the study, we used fetal rats (19-21 days gestational age). After sacrificing the rats, the skin from the torso was taken and rinsed twice with D-Hanks buffer, and immersed in D-Hanks buffer containing 200 U/ml penicillin and 200 U/ml streptomycin (Hyclone, Cat. No. SH30010) for 30 min. Under sterile conditions, the skin was washed thoroughly with PBS to remove subcutaneous tissue and trimmed into 0.5 × 0.5 cm pieces. Then it was digested at 4°C overnight in digestion buffer containing 0.5% neutral protease (GIBCO Cat. No. 17105041). The next morning, the skin sample was incubated at 37°C for 30 minutes. After peeling off the epidermis and cutting into the microskin, the skin sample was oscillated and digested with 0.25% trypsin (Hyclone, Cat. No. SH3008742.01) at 37°C for 15 minutes to prepare a single cell suspension. The digestion reaction was stopped by addition of an equal volume of compound DMEM high-glucose medium that contained 20% fetal bovine serum (FBS) (Hyclone, Cat. No. SH30022.01B) relative to the volume of trypsin. The cells were filtered using a 200-mesh filter and then centrifuged at 1,000 rpm for 5 min. After removal of the supernatant, the cells were resuspended in compound DMEM high-glucose medium containing 20% FBS and seeded in a flask coated with type IV collagen (Sigma Cat. No. C8374) at a cell density of 3 × 106/ml. After 15 min at 37°C, the cells were observed under an inverted phase contrast microscope and some were adherent. The cells in suspension were collected. Then, 4 ml DMEM high-glucose medium containing 20% FBS (Hyclone Cat. No. SH30022.01B) was added to the adherent cells, and the cells were cultured with 5% CO2 and saturated humidity at 37°C. The culture medium was changed to K-SFM medium (GIBCO, Cat. No. 17005042) after 24 hours, and the cells continued to be passaged. Half of the medium was replaced every other day, and the complete medium was replaced every 2-3 days. When they reached 70-80% confluence, cells were digested with 0.25% trypsin at 37°C oscillating for 5-10 min, and passaged at a ratio of 1:2.
Immunohistochemistry
Cells were fixed with 4% paraformaldehyde for 30 min., washed with PBS three times for 5 min and incubated with 3% peroxide in a humidity box at room temp for 10 min. Following washing with PBS three times X 5 min., cells were blocked with 10% normal goat serum at room temp for 30 min. Incubation with primary antibodies was performed at 4°C overnight. The primary antibodies used were rabbit Anti-CK15 (BIOSS Cat. No. bs-1772R); rabbit Anti-CK19 (BIOSS Cat. No. bs-1028R); rabbit Anti-P63 (BIOSS Cat. No. bs-0723R). Following 3 X 5 min. washes with PBS, cells were incubated with the second antibody at room temp for 30 min., washed 3 X with PBS for 5 min, and incubated with DAB using the ChemMate TM DAKO Envision TM Detection Kit (DAKO, GK500705). Staining was stopped by washing with PBS 3 times. Cells were counter-stained with hematoxylin. Superfluous stain was removed with water. Then cells were dehydrated with 50, 75, 85, 95, and 100% gradient ethanol (once per step) and cleared with xylene twice for 10 min. prior to mounting in neutral resin. An Olympus CX41 microscope was used to observe cells.
Cell proliferation assay
Cells from different groups were digested, dispersed by pipetting, and cell numbers were counted. The cell concentration was adjusted to 1 × 105 cells/ml and distributed in a 96-well microplate (100 μl/well; i.e. 1 × 104 cells/well). After cells adhered, they were collected at different time points (0 h, 24 h, 48 h, 72 h) and MTS was added at a ratio of 1/10 (i.e., 10 μl detection solution was added in 100 μl medium) according to the instructions in the CellTiter96®AQueous One Solution Cell Proliferation Assay (MTS) (Promega, Cat. No. G3582). After 4 hours incubation, MTS levels were read at OD 490 using a microplate reader (Thermo Fisher Scientific, Multiscan MK3).
Marker detection by flow cytometry
Cells were counted and the cell concentration adjusted to 1 × 106 cells/100 μl. One hundred microliters of cells were aliquoted into three tubes. Tube A was the blank control, tube B contained mouse IgG1 K Isotype Control PE, tube C contained the FITC-labeled hamster anti-rat CD29 (BD Pharmingen™, Cat. No. 555005). Antibodies were diluted 1:1, and incubated in a dark chamber at room temp for 20 min. Then cells were washed with 500 μl PBS by centrifugation at 300 g for 10 min. FITC-labeled anti-mouse IgG (Abcam, Cat. No. ab6785) diluted 1:50 was added, cells were incubated for 20 min, and then washed once by centrifugation at 300 g for 10 min. Cells were resuspended in 0.2 ml PBS and the reaction analyzed by flow cytometry using a FACSDiva, version 6.1 flow cytometer.
Results
Isolation of primary cells
In this study, cells showed the typical ESC morphology. Microscopy revealed that above 98% of cells were vital. Based on the highly adhesive interaction between the epidermal stem cells and type IV collagen, the adherent cells were small and round with an even distribution after 15 minutes, and some of the cells stretched slightly (Figure 1A). After 48 hours of culture, the cells had stretched completely in the shape of round or small polygons, and there were no mixed fibrocytes (Figure 1B). After 4 days of culture, big clones consisting of 100-200 cells were observed. The cells had big round nuclei with little cytoplasm, which met the general characteristics of stem cells. These epidermal stem cells fused into flakes and covered the bottom of the flask, with a pebble-like appearance (Figure 1C).
Figure 1.
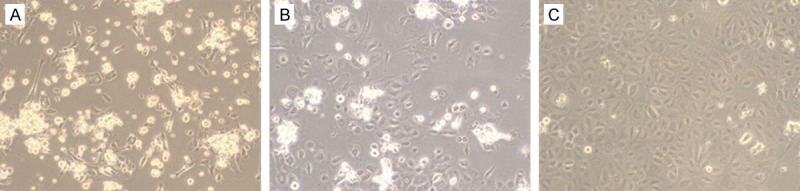
Micrographs of ESC on the first day (A), the second day (B), and the fourth day (C), after isolation and culture (200 X).
Identification of surface markers by immunohistochemistry
Immunohistochemical staining of slices of adherent cells with antibodies against K15 or K19 revealed cytoplasmic staining, with cells expressing both K15 (Figure 2A and 2B) and K19 (Figure 2C and 2D). The nuclei expressed P63 (Figure 2E and 2F), while no staining was observed in the control cells.
Figure 2.
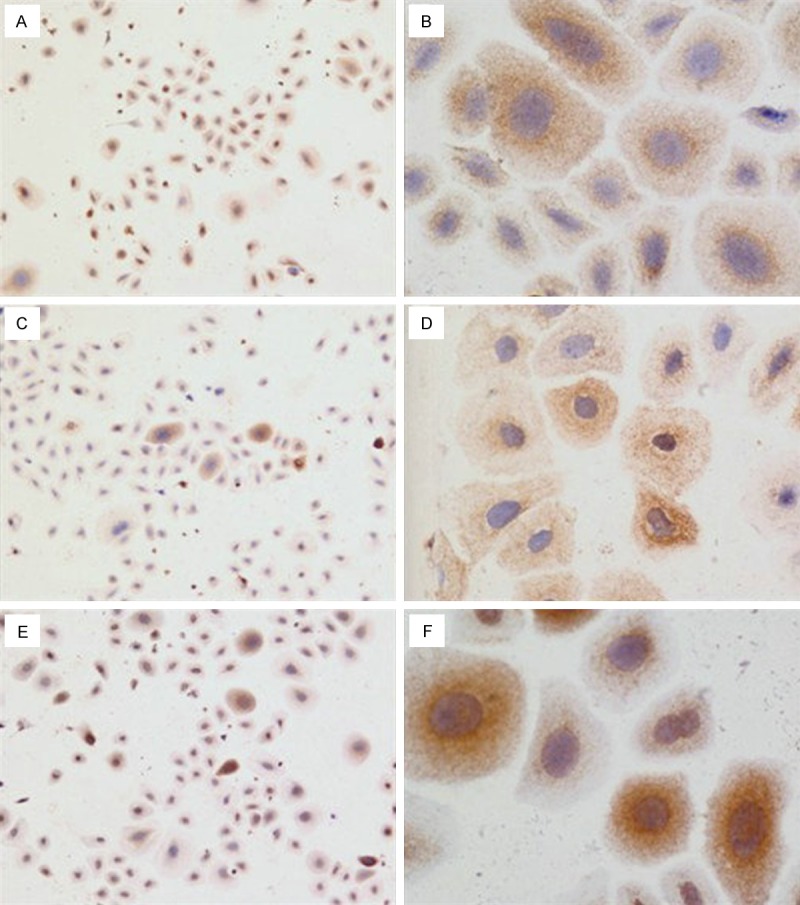
Immunohistochemistry on ESC cells after 5 days in culture stained with (A and B) rabbit anti-CK15, 40 X and 200 X, respectively; C and D: rabbit anti-CK19, 40 X and 200 X, respectively; E and F: rabbit anti-P63, 40 X and 200 X, respectively.
Expression of CD29 (β1 integrin) and cell cycle detection by flow cytometry
Flow cytometry showed that 83.94% of the adherent cells expressed CD29 (β1 integrin) (Figure 3).
Figure 3.
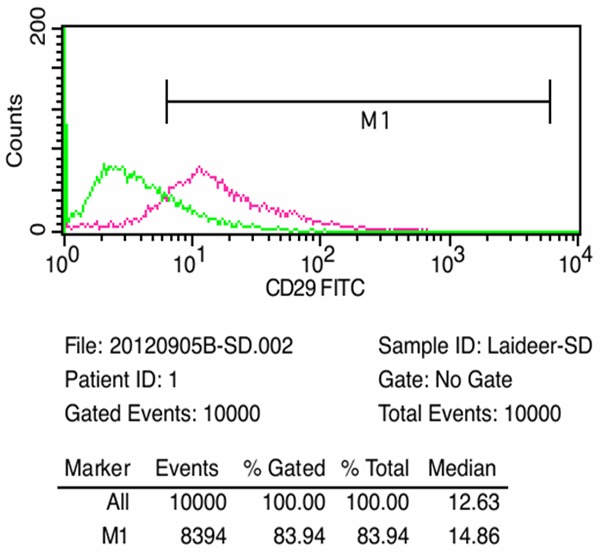
FACS analysis of CD29 markers present on ESC.
Cell proliferation assay
The growth-curve showed that the rapidly adherent cells presented exponential growth (Table 1).
Table 1.
Detection of cell proliferation
| Time | Duplicate 1 | Duplicate 2 | Duplicate 3 | Mean ± S.D. |
|---|---|---|---|---|
| Day 1 | 0.768 | 0.7375 | 0.7515 | 0.75 ± 0.02 |
| Day 2 | 1.28 | 1.287 | 1.31 | 1.29 ± 0.02 |
| Day 3 | 1.551 | 1.567 | 1.602 | 1.57 ± 0.03 |
| Day 4 | 1.826 | 1.755 | 1.862 | 1.81 ± 0.05 |
| Day 5 | 1.928 | 1.977 | 2.019 | 1.97 ± 0.05 |
Discussion
The implementation and improvement of novel treatment strategies using ESC requires a better understanding of the biology of these cells, specifically improvements are needed in the isolation of these cells and the maintenance of their unique characteristics in culture. The purpose of this study was the isolation, culture, and identification of rat ESC in vitro from fetal rat skin.
Different methods for isolating epidermal stem cells have been proposed, including: (1) α 6-bright/CD71-dim human keratinocytes; (2) rapid adhesion of cells to collagen type IV; (3) DNA label-retaining cells representing slow-cycling cells; and (4) side population cells that efflux Hoechst 33342 fluorescent dye [8-11]. In the present study, the adherence of epidermal cells to type IV collagen for 15 min was used to obtain ESC. The non-adherent cells were rinsed off after 15 min to separate the rapidly attaching stem cells from the slower adhering more differentiated keratinocytes. This method is reliable, rapid, simple, convenient and reproducible. However, to address limitations, we made a few significant improvements to the method. One of the advantages of the described protocol, compared to previous cell culture methods, is that fetal rat skin was used. The ESC culture adopted in previous methods used newborn rat skin [12], which is prone to contamination. In addition, the yield and quality of the stem cells is not as good as that from rat embryos. Using fetal rat skin leads to easier ESC isolation. Furthermore, we used KSFM medium in our protocol, which simplifies the preparation of medium, leading to better results. It is more difficult to trypsin digest the cells from the culture flask when passaging rat ESC, if traditional medium is used. Over-digestion may cause damage to the stems cells. our method provides a solution to this issue.
Many attempts have been made to identify putative markers of ESC. Several molecules such as β1 integrin, α6 integrin, P63, K15, K19, and β-catenin have been reported as putative ESC markers [13-16]. In this study, additional approaches were used to identify ESC to compensate for the lack of specific markers; the cells showed the typical epidermal stem cell morphology. Moreover, the adhesive cells were positive for epidermal stem cell markers, including K15, K19 and P63. In addition, the results of flow cytometry showed that 83.94% of cells were CD29 positive. The cell growth curve illustrated that the cells were dividing exponentially. After long-term culture and about 10 passages, no significant change was observed in cell morphology and the ability to form clones or the proliferation ability of the cells. These features met the characteristics of epidermal stem cells.
The protocol described in this paper provides a simplified and effective method to isolate and maintain long-term culture of epidermal stem cells from fetal rat skin. This method should be valuable for isolating and studying ESC from various transgenic rat lines that are currently available.
Acknowledgements
Sources of Support: The project was supported by National Natural Science Foundation of China; Grant # 81071557, supplemental support from the Department of Burns Surgery at First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, China.
Disclosure of conflict of interest
The authors do not have conflicts of interest to report.
References
- 1.Potten CS, Booth C. Keratinocyte stem cells: a commentary. J Invest Dermatol. 2002;119:888–99. doi: 10.1046/j.1523-1747.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 2.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rzepka K, Schaarschmidt G, Nagler M, Wohlrab J. Epidermal stem cells. J Dtsch Dermatol Ges. 2005;3:962–973. doi: 10.1111/j.1610-0387.2005.05071.x. [DOI] [PubMed] [Google Scholar]
- 4.Dunnwald M, Tomanek-Chalkley A, Alexandrunas D, Fishbaugh J, Bickenbach JR. Isolating a pure population of epidermal stem cells for use in tissue engineering. Exp Dermatol. 2001;10:45–54. doi: 10.1034/j.1600-0625.2001.100106.x. [DOI] [PubMed] [Google Scholar]
- 5.Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A. 2000;97:10960–10965. doi: 10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DS, Cho HJ, Choi HR, Kwon SB, Park KC. Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell Mol Life Sci. 2004;61:2774. doi: 10.1007/s00018-004-4288-4. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Miao C, Guo W, Jia L, Zhou J, Ma B, Peng S, Liu S, Cao Y, Duan E. Enrichment of putative human epidermal stem cells based on cell size and collagen type IV adhesiveness. Cell Res. 2008;18:360–371. doi: 10.1038/cr.2007.103. [DOI] [PubMed] [Google Scholar]
- 8.Terunuma A, Kapoor V, Yee C, Telford WG, Udey MC, Vogel JC. Stem cell activity of human side population and α6 integrin-bright keratinocytes defined by a quantitative in vivo assay. Stem Cells. 2007;25:664–669. doi: 10.1634/stemcells.2006-0434. [DOI] [PubMed] [Google Scholar]
- 9.Bickenback JR, Chism E. Selection and extended growth of murine epidermal stem cell in culture. Exp Cell Res. 1998;244:184–195. doi: 10.1006/excr.1998.4163. [DOI] [PubMed] [Google Scholar]
- 10.Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triel C, Vestergaard ME, Bolund L, Jensen TG, Jensen UB. Side population cells in human and mouse epidermis lack stem cell characteristics. Exp Cell Res. 2004;295:79–90. doi: 10.1016/j.yexcr.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Reiisi S, Esmaeili F, Shirazi A. Isolation, culture and identification of epidermal stem cells from newborn mouse skin. In Vitro Cell Dev Biol Anim. 2010;46:54–9. doi: 10.1007/s11626-009-9245-y. [DOI] [PubMed] [Google Scholar]
- 13.Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109:1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- 14.Zhu AJ, Haase I, Watt FM. Signaling via β1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci U S A. 1999;96:6728–6733. doi: 10.1073/pnas.96.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellegrini G, Dellamhra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li A, Pouliot N, Redvers R, Kaur P. Extensive tissue-regenerative capacity of neonatal human keratinocyte stem cells and their progeny. J Clin Invest. 2004;13:390–400. doi: 10.1172/JCI19140. [DOI] [PMC free article] [PubMed] [Google Scholar]


