Abstract
RhoGDI (Rho GDP-dissociation inhibitor alpha or RhoGDIα) has been identified as a regulator of Rho GTPases, which are essential for tumor progression, but its role in cancer remains controversial and little is known in hepatocellular carcinoma (HCC). Using immunohistochemistry, we analyzed RhoGDI expression in 147 clinicopathologically characterized HCC cases. RhoGDI expression was detected in cytoplasm of HCC tissues. Statistical analysis showed that there was no relationship between RhoGDI expression and clinicopathological features. Importantly, a significant trend was identified between loss of RhoGDI expression in HCC and worsening clinical prognosis. Multivariate survival analysis showed that negative RhoGDI expression was recognized as an independent prognostic factor of patient’s survival. Our results suggest that RhoGDI protein is a valuable marker of prognosis for patients with HCC.
Keywords: Rho GDP-dissociation inhibitor alpha, hepatocellular carcinoma, poor prognosis
Introduction
Hepatocellular carcinoma (HCC) ranks as the fifth most common malignant disorder and the third leading cause of cancer-related deaths worldwide [1]. Although it is especially prevalent in certain areas of Asia and Africa, an increasing incidence in western countries, including the United States, has recently been observed [2]. The prognosis of HCC remains poor despite advances in surgical or locoregional therapies. The search for an effective and efficient therapy for HCC is still ongoing [3]. With the increasing understanding of the tumor biology of HCC, more and more molecular markers with high sensitivity and specificity for HCC have been found and could be helpful for early diagnosis and the development of future targeted HCC therapeutics.
Rho GDIs (GDP-dissociation inhibitors) were identified as key regulators of Rho family GTPases typified by its ability to prevent nucleotide exchange and membrane association. Structural studies on GTPase-GDIs complexes, in combination with biochemical and cell biological results, have provided insight as to how GDIs exert their effects on nucleotide binding, the membrane association-dissociation cycling of the GTPase and how these activities are controlled [4-6]. Despite the initial negative roles attributed to RhoGDI, however, recent evidence has come to suggest that it may also act as a positive regulator necessary for the correct targeting and regulation of Rho activities by conferring cues for spatial restriction, guidance and availability to effectors [7,8]. The expression of RhoGDIs is altered in a variety of cancers and they have been shown to mediate several processes during tumorigenesis and cancer progression [9-12].
In our previous study, we performed comparative proteomic analysis to identify RhoGDI as an up-regulated protein in metastatic CRC [13]. The further data suggested that RhoGDI may promote CRC progressionby stimulating tumor cell growth and migration [14]. A recent research based on small-samples assay revealed that miR-151 is a crucial stimulus for HCC invasion and metastasis by directly targeting RhoGDI [15]. Despite their critical cellular function little is known about its clinical significance in HCC progression.
In the present study, we determined the expression of RhoGDI in primary HCC using immunohistochemistry, and investigated the relationship between its expression and clinicopathological parameters. We also evaluated the potential prognostic value of RhoGDI in the post-resectional survival of HCC patients.
Materials and methods
Tumor tissue sample
All cases of tumor tissue were provided by the Tumor Tissue Bank of Nanfang Hospital. A total of 147 patients were involved in the study. Formalin-fixed tumor tissues from all the patients including 126 tumor tissues and 106 adjacent non-tumors tissues were used for immunohistochemical analysis. In each case, a diagnosis of primary HCC had been made, and the patients had undergone elective surgery for HCC in Nanfang Hospital during 2001 to 2002. The Tumor Tissue Bank of Nanfang Hospital possesses a comprehensive set of clinicopathological data, including age, gender, size of primary tumor, tumor differentiation, serum alpha-fetoprotein (AFP), hepatitis B surface antigen (HBsAg), liver cirrhosis, postsurgical recurrence and metastasis. Complete follow-up, ranging from 0 to 83 months, was available for all patients and the median survival was 42 months. At the time of censoring the data, there had been 89 (60.5%) deaths in the patient group. The pathological diagnosis was performed at the Department of Pathology of Nanfang Hospital, Southern Medical University. The tumor tissues were fixed in 4% formaldehyde, embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE). The study was approved by the Ethics Committee of Southern Medical University and all aspects of the study comply with the Declaration of Helsinki. Ethics Committee of the Southern Medical University specifically approved that not informed consent was required because data were going to be analyzed anonymously. All patient data were de-identified for analysis.
Immunohistochemistry (IHC)
Immunohistochemistry was performed to investigate the expression of RhoGDI in 147 human HCC tissues. The sections were incubated with primary antibodies against RhoGDI (1:50) for 1 hour at room temperature. Mayer’s hematoxylin was used for nuclear counterstaining. The sections were mounted with a synthetic medium. The slides were reviewed by two or three pathologists blind to the study. To evaluate RhoGDI expression levels, immunostained slides were evaluated using a method described previously [16,17]. The discrepancies (<5%) were resolved by simultaneous re-evaluation.
Statistical analysis
All statistical analyses were carried out using the SPSS 12.0 statistical software package. Mann-Whitney U test was used to analyze the relationship between RhoGDI expression and clinicopathologic characteristics. Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test. The significance of various variables for survival was analyzed by the Cox proportional hazards model in the multivariate analysis. P<0.05 in all cases was considered statistically significant.
Results
RhoGDI is expressed in HCC tissues and non-tumorous tissues
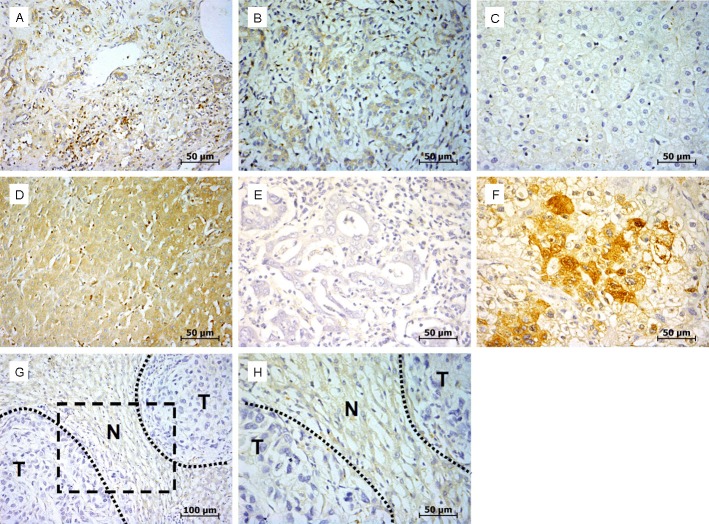
RhoGDI expression was detected in cytoplasm of liver cells, inflammatory cells, and biliary epithelial cells, but not in stromal cells (Figure 1A). Immunohistochemistry clearly allowed to localize RhoGDI expression in 58.5% (62 of 106) of adjacent non-tumorous tissues tested. As compared to these non-tumorous tissues, we observed RhoGDI protein expression in 68.3% (86 of 126) of all HCC samples (P=0.123).
Figure 1.
Expression analysis of RhoGDI in HCC by immunohistochemistry. A, B: Expression of RhoGDI in inflammatory cells and biliary epithelial cells. C: Negative expression of RhoGDI in adjacent non-tumorous tissue. D: Positive expression of RhoGDI in adjacent non-tumorous tissue. E: Negative expression of RhoGDI in HCC. F: Positive expression of RhoGDI in HCC. G: Negative expression of RhoGDI in HCC and positive expression of RhoGDI in adjacent non-tumorous tissue were simultaneously showed in the same fields. H: Corresponding high magnifi cation of picture G. Scale bars were showed in the lower right corner of each picture.
Correlation between RhoGDI expression and the clinicopathological features
To evaluate the clinical relevance of RhoGDI expression, expression of RhoGDI protein was compared to clinicopathological and biological parameters. As shown in Table 1, there was no significant correlation between expression of RhoGDI protein and gender, tumor size, tumor differentiation, hepatitis B surface antigen (HBsAg), liver cirrhosis and recurrence except age of HCC patients.
Table 1.
Correlation between the clinicopathological features and RhoGDI expression
| Characteristics | RhoGDI expression | ||
|---|---|---|---|
|
| |||
| Negative (%) | Positive (%) | P value | |
| Group | |||
| Non-tumor | 44 (41.5) | 62 (58.5) | 0.123 |
| Tumor | 40 (31.7) | 86 (68.3) | |
| Gender | |||
| Male | 36 (32.1) | 76 (67.9) | 0.787 |
| Female | 4 (28.6) | 10 (71.4) | |
| Age | |||
| <50 | 29 (41.4) | 41 (58.6) | 0.009* |
| ≥50 | 11 (19.6) | 45 (80.4) | |
| Tumor size (cm in diameter) | |||
| ≤5 | 14 (32.6) | 29 (67.4) | 0.888 |
| >5 | 26 (31.3) | 57 (68.7) | |
| Tumor differentiation | |||
| Low | 5 (29.4) | 12 (70.6) | 0.888 |
| Middle | 16 (34.0) | 31 (66.0) | |
| High | 19 (30.6) | 43 (69.4) | |
| Liver cirrhosis | |||
| No | 15 (26.8) | 41 (73.2) | 0.285 |
| Yes | 25 (35.7) | 45 (64.3) | |
| HBsAg status | |||
| Negative | 6 (37.5) | 10 (62.5) | 0.597 |
| Positive | 34 (30.9) | 76 (69.1) | |
| Serum AFP | |||
| Negative | 10 (29.4) | 24 (70.6) | 0.732 |
| Positive | 30 (32.6) | 62 (67.4) | |
| Postsurgical recurrence | |||
| Negative | 52 (66.7) | 26 (33.3) | 0.634 |
| Positive | 30 (62.5) | 18 (37.5) | |
| Postsurgical metastasis | |||
| No | 32 (31.1) | 71 (68.9) | 0.729 |
| Yes | 8 (34.8) | 15 (65.2) | |
Statistically significant (P<0.05).
RhoGDI expression is closely related to long-term survival of patients with HCC
To investigate the prognostic value of RhoGDI expression, the association between RhoGDI expression and overall survival was initially evaluated using Kaplan-Meier survival curves with the log-rank test. There was a significant trend toward poorer survival for patients whose primary tumors showed a negative immunoreactivity, compared with those patients whose primary tumors showed positive immunoreactivity (Log rank=7.626, P=0.006; Figure 2).
Figure 2.
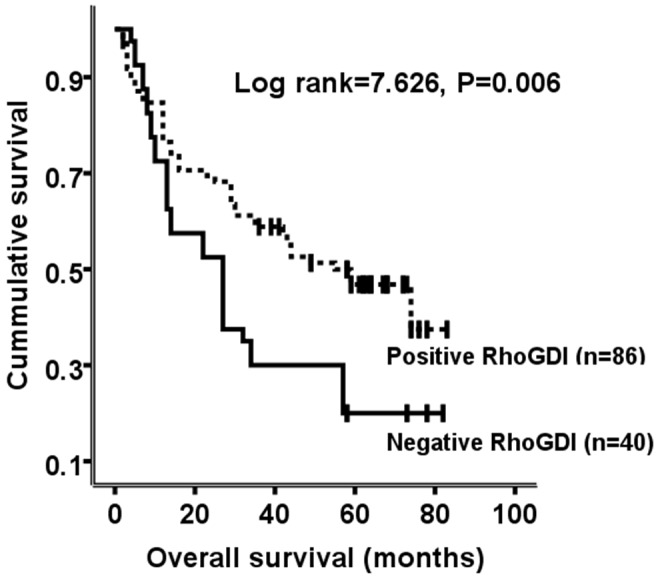
Kaplan-Meier survival analysis of overall survival in patients with HCC according to RhoGDI expression. The log-rank test was used to calculate P values.
Loss of RhoGDI is an independent factor to predict poor prognosis of patients with HCC
In addition, tumor size, tumor differentiation and postsurgical metastasis were also significantly correlated with survival in Kaplan-Meier analysis and log-rank test (Table 2). We did multivariate survival analysis, which included RhoGDI expression, tumor size, tumor differentiation, postsurgical metastasis, to determine if RhoGDI expression is an independent predictor of overall patient survival. In this analysis, RhoGDI expression, tumor differentiation and tumor size were recognized as independent prognostic factors of patient’s survival (Table 2). Thus, our findings indicate that decreased RhoGDI expression has a significant correlation with poor prognosis of patients with HCC.
Table 2.
Univariate and multivariate analyses of individual parameters for correlations with overall survival rate: Cox proportional hazards model
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | CI (95%) | P value | HR | CI (95%) | P value | ||
| Gender | 0.816 | 0.409-1.627 | 0.563 | ||||
| Age | 0.909 | 0.595-1.389 | 0.660 | ||||
| Tumor size | 1.660 | 1.064-2.592 | 0.026* | 1.709 | 1.040-2.809 | 0.034* | |
| Tumor differentiation | 1.619 | 1.176-2.228 | 0.003* | 1.739 | 1.217-2.486 | 0.002* | |
| Liver cirrhosis | 1.288 | 0.841-1.974 | 0.245 | ||||
| HBsAg status | 1.797 | 0.901-3.582 | 0.096 | ||||
| Serum AFP level | 1.288 | 0.849-1.952 | 0.234 | ||||
| Postsurgical metastasis | 1.757 | 1.054-2.929 | 0.031* | 1.638 | 0.937-2.863 | 0.083 | |
| Postsurgical recurrence | 1.021 | 0.667-1.562 | 0.924 | ||||
| RhoGDI expression | 0.538 | 0.341-0.847 | 0.007* | 0.457 | 0.287-0.729 | 0.001* | |
Abbreviations: HR, Hazard radio; CI, Confidence interval.
Statistically significant (P<0.05).
Discussion
Rho family GTPases play important roles in a variety of cellular functions. RhoA, Rac1 and Cdc42, the defining members of this family, were initially linked to changes in the filamentous actin system involving the formation of stress fibres, membrane ruffles/lamellipodia and filopodia respectively [18]. Now it is widely accepted that their roles extend beyond these initial observations and cover many aspects of cellular regulation including morphology and migration, gene transcription, cell cycle progression and cytokinesis, phagocytosis and vesicular traffic, as well as regulation of a range of enzymatic functions, e.g. NADPH oxidase [19].
Rho GDIs (GDP-dissociation inhibitors) were identified as key regulators of Rho family GTPases typified by its ability to prevent nucleotide exchange and membrane association. These function by extracting Rho family GTPases from membranes and solubilizing them in the cytosol. Moreover, both in vitro and in vivo they interact only with prenylated Rho proteins [20,21]. They also inhibit nucleotide exchange and GTP hydrolyzing activities on Rho proteins by interacting with their switch regions and probably restricting accessibility to GEFs and GAPs.
Three human Rho GDIs have been identified: the ubiquitously expressed RhoGDI (or GDIa/GDI1) [22,23], the hematopoietic cell-selective Ly/D4GDI (or GDIβ/GDI2) [24,25] and RhoGDIγ (or GDI3), specifically expressed in lung, brain and testis [26,27]. Both RhoGDI and D4GDI (hereafter referred to collectively as GDIs) are cytosolic and form 1:1 complexes with Rho family GTPases. Structural studies on GTPase-GDIs complexes, in combination with biochemical and cell biological results, have provided insight as to how GDIs exert their effects on nucleotide binding, the membrane association-dissociation cycling of the GTPase and how these activities are controlled [4-6]. The expression levels of GDIs have been reported to be up- or down-regulated in certain cancers [9-11] and in other pathological conditions (e.g. [12]).
Despite their critical cellular function little is known about the expression of GDIs in HCC. To address this question, we used immunohistochemical assay to analyze the expression of RhoGDI and its clinical significance in HCC. The statistical evaluation, however, indicated no statistically significant relationship parameters. Nevertheless, a trend was identified between negative RhoGDI expression in HCC and worsening clinical prognosis. Similarly, Ding et al. showed that RhoGDI functions as a metastasis suppressor and can block miR-151-induced HCC cell migration and invasion, suggesting its role in HCC progression [15]. Further, we have shown in univariate and multivariate analysis that negative expression of RhoGDI is a significant predictor of poor prognosis for HCC patients. As RhoGDI expression might be served as a new and independent predictor of overall patient survival, it may function as a new and independent predictor of prognosis for HCC patients as well. In combination with other biomarker of HCC, RhoGDI expression status may be useful to stratify patients for novel therapeutic strategies, such as adjuvant chemotherapy, radiosensitization or the establishment of rational treatment selection criteria for patients.
In conclusion, our study evaluated the prognostic significance of RhoGDI expression in a large number of HCC clinical tissue specimens at protein level by immunohistochemical analysis. The most valuable finding of this study is that the overall survival of our study cohort was significantly poorer in negative RhoGDI expression cases that in positive RhoGDI expression cases. In indicates that loss RhoGDI expression is a new and independent predictor for HCC patients. Therefore, its clinical value lies in that closer monitoring and more aggressive treatment should be indicated for the HCC patients whose poor tumor differentiation or whose tumor size exceeds 5 cm or whose RhoGDI expression is absent. This may be a useful way to reduce mortality and prolong the survival time as much as possible. Our finding also provides evidence for molecular target therapy of tumor.
To achieve a better understanding of the mechanism of carcinogenesis and tumor progression in patients with HCC, further molecular, cellular and animal model studies are necessary. Moreover, it will be essential to analyze quantitatively the predictive power of absent RhoGDI expression for HCC and other tumor types in further studies.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 30901792, 81272762, 81201635), Research Foundation of Guangzhou Medical College (2010C33), Key Project of Affiliated Tumor Hospital of Guangzhou Medical College (2011-yz-05), Guangdong Natural Science Funds for Distinguished Young Scholar (S20120011334), Guangdong Natural Science Foundation (S2012040006418, S2013010014254).
Disclosure of conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32:225–237. doi: 10.1016/s0168-8278(00)80428-6. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Chiriva-Internati M, Grizzi F, Wachtel MS, Jenkins M, Ferrari R, Cobos E, Frezza EE. Biological treatment for liver tumor and new potential biomarkers. Dig Dis Sci. 2008;53:836–843. doi: 10.1007/s10620-007-9909-y. [DOI] [PubMed] [Google Scholar]
- 4.Golovanov AP, Chuang TH, DerMardirossian C, Barsukov I, Hawkins D, Badii R, Bokoch GM, Lian LY, Roberts GC. Structure-activity relationships in flexible protein domains: regulation of rho GTPases by RhoGDI and D4 GDI. J Mol Biol. 2001;305:121–135. doi: 10.1006/jmbi.2000.4262. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman GR, Nassar N, Cerione RA. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell. 2000;100:345–356. doi: 10.1016/s0092-8674(00)80670-4. [DOI] [PubMed] [Google Scholar]
- 6.Grizot S, Faure J, Fieschi F, Vignais PV, Dagher MC, Pebay-Peyroula E. Crystal structure of the Rac1-RhoGDI complex involved in nadph oxidase activation. Biochemistry. 2001;40:10007–10013. doi: 10.1021/bi010288k. [DOI] [PubMed] [Google Scholar]
- 7.Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11:545–554. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 8.Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gildea JJ, Seraj MJ, Oxford G, Harding MA, Hampton GM, Moskaluk CA, Frierson HF, Conaway MR, Theodorescu D. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 2002;62:6418–6423. [PubMed] [Google Scholar]
- 10.Jiang WG, Watkins G, Lane J, Cunnick GH, Douglas-Jones A, Mokbel K, Mansel RE. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin Cancer Res. 2003;9:6432–6440. [PubMed] [Google Scholar]
- 11.Jones MB, Krutzsch H, Shu H, Zhao Y, Liotta LA, Kohn EC, Petricoin EF 3rd. Proteomic analysis and identification of new biomarkers and therapeutic targets for invasive ovarian cancer. Proteomics. 2002;2:76–84. [PubMed] [Google Scholar]
- 12.Kasper B, Tidow N, Grothues D, Welte K. Differential expression and regulation of GTPases (RhoA and Rac2) and GDIs (LyGDI and RhoGDI) in neutrophils from patients with severe congenital neutropenia. Blood. 2000;95:2947–2953. [PubMed] [Google Scholar]
- 13.Zhao L, Wang H, Sun X, Ding Y. Comparative proteomic analysis identifies proteins associated with the development and progression of colorectal carcinoma. FEBS J. 2010;277:4195–4204. doi: 10.1111/j.1742-4658.2010.07808.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Wang H, Li J, Liu Y, Ding Y. Overexpression of Rho GDP-dissociation inhibitor alpha is associated with tumor progression and poor prognosis of colorectal cancer. J Proteome Res. 2008;7:3994–4003. doi: 10.1021/pr800271b. [DOI] [PubMed] [Google Scholar]
- 15.Ding J, Huang S, Wu S, Zhao Y, Liang L, Yan M, Ge C, Yao J, Chen T, Wan D, Wang H, Gu J, Yao M, Li J, Tu H, He X. Gain of miR-151 on chromosome 8q24.3 facilitates tumour cell migration and spreading through downregulating RhoGDIA. Nat Cell Biol. 2010;12:390–399. doi: 10.1038/ncb2039. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Wang H, Liu C, Liu Y, Wang X, Wang S, Sun X, Li J, Deng Y, Jiang Y, Ding Y. Promotion of colorectal cancer growth and metastasis by the LIM and SH3 domain protein 1. Gut. 2010;59:1226–1235. doi: 10.1136/gut.2009.202739. [DOI] [PubMed] [Google Scholar]
- 17.Coppola D, Szabo M, Boulware D, Muraca P, Alsarraj M, Chambers AF, Yeatman TJ. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10:184–190. doi: 10.1158/1078-0432.ccr-1405-2. [DOI] [PubMed] [Google Scholar]
- 18.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 19.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 20.Faure J, Dagher MC. Interactions between Rho GTPases and Rho GDP dissociation inhibitor (Rho-GDI) Biochimie. 2001;83:409–414. doi: 10.1016/s0300-9084(01)01263-9. [DOI] [PubMed] [Google Scholar]
- 21.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Ueda T, Kikuchi A, Ohga N, Yamamoto J, Takai Y. Purification and characterization from bovine brain cytosol of a novel regulatory protein inhibiting the dissociation of GDP from and the subsequent binding of GTP to rhoB p20, a ras p21-like GTP-binding protein. J Biol Chem. 1990;265:9373–9380. [PubMed] [Google Scholar]
- 23.Fukumoto Y, Kaibuchi K, Hori Y, Fujioka H, Araki S, Ueda T, Kikuchi A, Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990;5:1321–1328. [PubMed] [Google Scholar]
- 24.Lelias JM, Adra CN, Wulf GM, Guillemot JC, Khagad M, Caput D, Lim B. cDNA cloning of a human mRNA preferentially expressed in hematopoietic cells and with homology to a GDP-dissociation inhibitor for the rho GTP-binding proteins. Proc Natl Acad Sci U S A. 1993;90:1479–1483. doi: 10.1073/pnas.90.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scherle P, Behrens T, Staudt LM. Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding protein, is expressed preferentially in lymphocytes. Proc Natl Acad Sci U S A. 1993;90:7568–7572. doi: 10.1073/pnas.90.16.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adra CN, Manor D, Ko JL, Zhu S, Horiuchi T, Van Aelst L, Cerione RA, Lim B. RhoGDIgamma: a GDP-dissociation inhibitor for Rho proteins with preferential expression in brain and pancreas. Proc Natl Acad Sci U S A. 1997;94:4279–4284. doi: 10.1073/pnas.94.9.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zalcman G, Closson V, Camonis J, Honore N, Rousseau-Merck MF, Tavitian A, Olofsson B. RhoGDI-3 is a new GDP dissociation inhibitor (GDI). Identification of a non-cytosolic GDI protein interacting with the small GTP-binding proteins RhoB and RhoG. J Biol Chem. 1996;271:30366–30374. doi: 10.1074/jbc.271.48.30366. [DOI] [PubMed] [Google Scholar]