Abstract
Maspin, a member of the serpin family of protease inhibitors, has been shown to inhibit tumor growth and suppress metastasis in several malignancies, including lung cancer. Previous studies have reported that p63 and p53 control maspin expression by transactivating the promoter. The present study analyzed immunohistochemical studies to determine the expression and coexpression patterns of maspin, p63 and p53 in non-small cell lung carcinoma, specifically squamous cell carcinoma and adenocarcinoma. The results showed that 83/86 cases (96.5%) of squamous cell carcinoma and 82/161 cases (50.9%) of adenocarcinoma included in this study were positive for maspin. There were 79/86 cases (91.9%) of squamous cell carcinoma and 16/161 cases (9.9%) of adenocarcinoma with positive expression for p63. In addition, 77/86 cases (89.5%) of squamous cell carcinoma and 99/161 cases (61.5%) of adenocarcinoma were positive for p53. Maspin, p63 and p53 expression were each significantly higher in squamous cell carcinoma than adenocarcinoma. Squamous cell carcinomas more highly coexpress maspin and p63, as well as maspin and p53, when compared with adenocarcinomas. The high frequency of coexpression of maspin and p63, as well as maspin and p53, in squamous cell carcinoma, suggests that p63 and p53 may be involved in the pathway to control maspin expression. Therapeutic targeting on maspin, p63 and p53 molecules might be beneficial in the management of patients with squamous cell carcinomas of the lung in the future.
Keywords: Maspin, p63, p53, lung, adenocarcinoma, squamous cell carcinoma
Introduction
Maspin, a member of the serine protease inhibitor (serpin) family, was originally detected in normal breast epithelial cells [1]. Maspin expression was previously shown to be downregulated as breast epithelial cells progressed to a higher grade of breast tumor and lost in metastasis [2]. In contrast, other studies found that maspin expression was upregulated in breast, ovarian, and pancreatic carcinoma cells and only minimally expressed in normal cells [3-6]. More recently, maspin expression and clinical significance have been described in non-small cell lung carcinoma [7-9].
Unlike most of the known protease inhibitors, maspin inhibits cell invasion by blocking cell motility and angiogenesis [10,11]. While maspin is known to function as a tumor suppressor, the underlying mechanisms of maspin at the molecular level are still unclear. Zou et al. suggested that p53 upregulates maspin expression by binding to the maspin promoter after observing a strong induction of maspin expression in breast and prostate cancer cells subsequent to p53 expression [12]. Transcription factors Ets, such as Pdef, and Ap1 have also been reported to transactivate maspin expression [13,14]. Another study found that maspin expression is solely dependent on the presence of p63 [15].
p63, a homologue of p53, does not act like a classical tumor suppressor because it is rarely mutated or deleted in tumors. Instead, it regulates programmed cell death, development and differentiation [16]. The p63 gene encodes multiple proteins with remarkably divergent abilities to transactivate p53 reporter genes and induce apoptosis [17]. It also has been reported that p63 acts as a substitute for p53 in transactivation of maspin [18], particularly in lung tumorigenesis [15]. While p63 expression was not seen in normal lung tissue, it has been observed in normal bronchial tissue with genomic amplification in the development of squamous cell carcinoma of the lung. Unlike squamous cell carcinoma, genomic amplification of p63 was not seen in adenocarcinoma [19].
The expression of maspin, p63 and p53 in non-small cell lung carcinoma has been evaluated separately. There is, however, limited information about the coexpression of maspin and p63 or p53 despite the potential role of p63 and p53 in transactivating maspin expression. The aims of this study are (1) to determine the expression frequency of maspin, p63 and p53 in non-small cell lung carcinoma, specifically squamous cell carcinoma and adenocarcinoma, and (2) to investigate the correlation between maspin and p63 expression, as well as between maspin and p53 expression, in squamous cell carcinoma and adenocarcinoma of the lung.
Method
Case selection
A total of 247 surgically resected squamous cell carcinoma and adenocarcinoma of the lung specimens were collected from the surgical pathology archives at University of Rochester Medical Center, Rochester, New York. These 2547included 161 cases of adenocarcinoma and 86 cases of squamous cell carcinoma.
Immunohistochemical analysis
Representative areas of squamous cell carcinoma and adenocarcinoma of the lung from the formalin-fixed, paraffin-embedded specimens were identified and tissue microarrays were constructed with two or more repeated tissue cores (1.0 mm in thickness) from individual cases. Sections of 4-μm in thickness were cut from the tissue microarrays and stained with hematoxylin and eosin to confirm the presence of the expected tissue histology within each tissue core. Additional sections from the tissue microarrays were cut for immunohistochemistry. Immunohistochemical studies were performed on the tissue microarrays. Tissue sections from the tissue microarrays were deparaffinized according to established procedures and rehydrated through graded alcohols. They were then washed in distilled water and placed in phosphate buffered saline. Antigen retrieval was performed by heating sections in a 99°C water bath with pH 6 Tris buffer or pH 9 EDTA buffer for 20 minutes followed by a brief cool down period. Slides were then rinsed with buffered saline for several minutes and placed on a Dako Autostainer. They were covered with fresh buffered saline to prevent drying during staining. All sections were incubated with various primary monoclonal mouse antibodies against maspin (1:500, clone G167-70, BD Biosciences, San Jose, CA), p53 (1:75, clone DO-7, DAKO, Carpentaria, CA) and p63 (1:50, clone BC4A4, Biocare, Concord, CA) at room temperature. The antibodies were detected using the Dako Flex HRP detection kit and counterstained with Dako Flex hematoxylin. A specimen with known maspin, p63 or p53 overexpression served as positive controls. Negative controls were performed by replacing the various antibodies with normal serum. Slides were mounted using a xylene based mounting medium and viewed under a light microscope.
Microscopic evaluation
Manual evaluation of maspin, p63 and p53 expression was performed by BC and HX blinded to all clinical and pathologic information. Discordant cases were reviewed by another pathologist and a final consensus was reached. Maspin, p63 and p53 immunoreactivities were semi-quantitatively assessed by the percentage of stained tumor cells and the intensity of staining. The percentage (0-100%) of positive tumor cells for maspin, p63 and p53 was determined. The intensities of maspin, p63 and p53 staining were graded as 0, 1+ (weak), 2+ (moderate), or 3+ (strong). Maspin, p63 and p53 proteins were considered expressed if more than 5% of tumor cells were stained and if there was at least a weak intensity of staining. Concordance was considered if both maspin and p63 or p53 were expressed in the same tumor.
Statistical analysis
Statistical Analysis was carried out by the SAS system. The Fisher’s exact test and χ2-test were used to compare positivity rate of maspin, p63 and p53 between squamous cell carcinoma and adenocarcinoma of the lung. These tests were also used to assess the concordance of maspin and p63 or p53 positivity in the two types of non-small cell lung carcinoma. A p-value of less than 0.05 was considered statistically significant.
Results
Both nuclear and cytoplasmic maspin expression was observed in squamous cell carcinoma and adenocarcinoma of the lung. p63 and p53, however, had only nuclear staining. The expression of maspin, p63 and p53 were distributed throughout the full thickness of the lesion in squamous cell carcinoma and adenocarcinoma.
Table 1 summarizes the immunohistochemical staining results. Maspin, p63 and p53 were expressed in squamous cell carcinoma of the lung with the majority of cases exhibiting strong and diffuse positivity. The frequencies of their staining were 83/86 (96.5%) for maspin, 79/86 (91.9%) for p63, and 77/86 (89.5%) for p53. There were similar diffuse patterns of staining between maspin, p63 and p53 in squamous cell carcinoma (Figure 1). When the concordance between maspin and p63 expression was compared, the frequency was 78/86 (90.7%; Table 1). When the concordance between maspin and p53 was compared, the frequency was 75/86 (87.2%; Table 1).
Table 1.
Immunohistochemical expression and coexpression of maspin, p63 and p53 in squamous cell carcinoma and adenocarcinoma of the lung
| Squamous Cell Carcinoma | Adenocarcinoma | |||
|---|---|---|---|---|
| n | 86 | 161 | ||
| Markers | ||||
| maspin+ | 83* | (96.5%) | 82 | (50.9%) |
| p63+ | 79* | (91.9%) | 16 | (9.9%) |
| p53+ | 77* | (89.5%) | 99 | (61.5%) |
| maspin+/p63+ | 78* | (90.7%) | 8 | (5.0%) |
| maspin+/p53+ | 75* | (87.2%) | 62 | (38.5%) |
P<0.01 in comparison with adenocarcinoma of the lung.
+, Positive staining.
Figure 1.
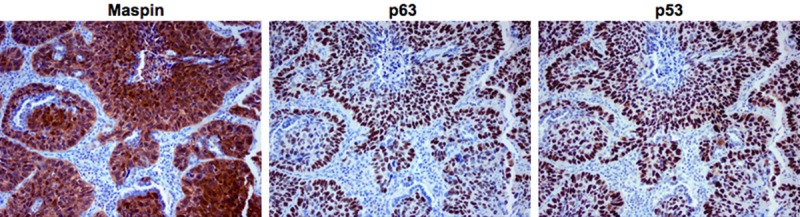
Maspin, p63 and p53 immunohistochemical expression patterns in squamous cell carcinoma of the lung. Squamous cell carcinoma was strongly and diffusely positive for maspin, p63 and p53 (original magnification x 200).
The immunohistochemical staining results of the lung adenocarcinoma were more variable. Adenocarcinoma had a much lower positivity for all of the markers compared with squamous cell carcinoma. The staining frequencies of maspin, p63 and p53 were 82/161 (50.9%), 16/161 (9.9%), and 99/161 (61.5%), respectively (Table 1). The patterns of staining were similar between maspin, p63 and p53 when positive. However, the markers were not consistently coexpressed in the same tumor (Figure 2). The frequency of concordance between maspin and p63 was 8/161 (5.0%; Table 1) and between maspin and p53 was 62/161 (38.5%; Table 1).
Figure 2.
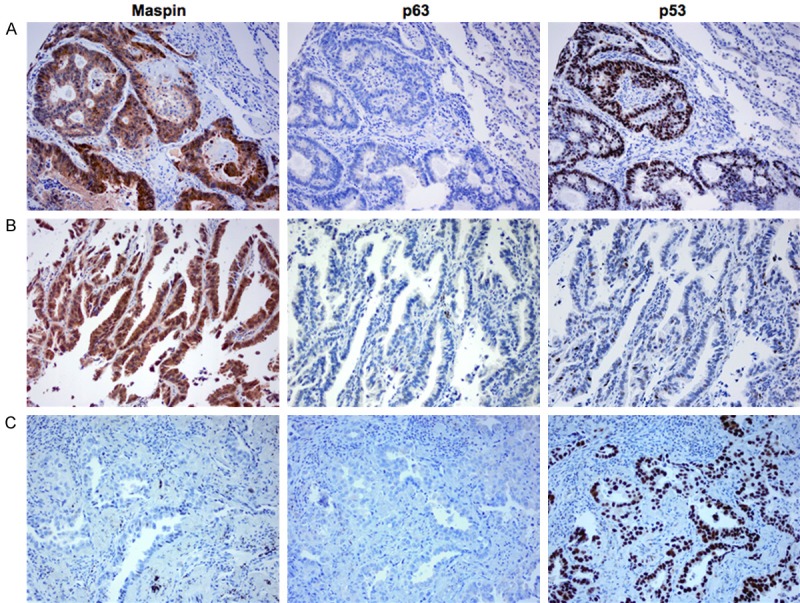
Maspin, p63 and p53 immunohistochemical expression patterns in adenocarcinoma of the lung. A: Adenocarcinoma co-expressing both maspin and p53, and exhibiting p63 negativity. B: Adenocarcinoma exhibiting maspin positivity, and both p63 and p53 negativity. C: Adenocarcinoma exhibiting both maspin and p63 negativity, and p53 positivity (original magnification x 200).
Statistical analyses of the immunohistochemical staining results revealed that the positivity of maspin in squamous cell carcinoma was significantly greater than adenocarcinoma (p<0.01). The differences between squamous cell carcinoma and adenocarcinoma were also statistically significant with p<0.01 for both p63 and p53 expression. When concordance rates were evaluated, the coexpression rate of maspin and p63 in squamous cell carcinoma was significantly higher than the rate in adenocarcinoma (p<0.01). There was also significant difference of p<0.01 between the coexpression rates of maspin and p53 in squamous cell carcinoma and adenocarcinoma.
Discussion
In this study, we reported significantly higher expression of maspin, p63 and p53 in squamous cell carcinoma of the lung when compared with adenocarcinoma. The results extended beyond previous observations of individual expression by examining the coexpression of maspin and p63, as well as of maspin and p53. Squamous cell carcinoma was more likely to coexpress maspin and p63 when compared with adenocarcinoma. Squamous cell carcinoma was also more likely to coexpress maspin and p53 when compared with adenocarcinoma.
Maspin, a member of the serpin family of protease inhibitors, was first shown to inhibit tumor growth and suppress metastasis in breast cancer [1]. To date, several studies have investigated the role of maspin in lung tumorigenesis. These studies have generally focused on comparing the levels of maspin expression in normal lung tissues and various lung carcinomas. They also examined the relationship between maspin expression and prognosis. Normal epithelial cells in the proximal airway were found to express maspin [20]. In lung carcinomas, some studies reported a significant increase in maspin expression in squamous cell carcinoma that was higher than in other histological types, such as adenocarcinoma. These studies also found maspin as an independent factor in predicting a favorable prognosis in patients with squamous cell carcinoma of the lung [7-9]. In fact, Akyildiz et al. found the sensitivity and specificity of maspin positivity to detect adenocarcinoma of the lung to be only 59% and 73%, respectively [21]. However, others found maspin expression to have favorable prognostic significance in adenocarcinoma [22-24]. Yet another study found that overexpression of maspin in both adenocarcinoma and squamous cell carcinoma is an unfavorable prognostic factor [25]. The results from this study were consistent with previous studies that found maspin expression in squamous cell carcinoma to be significantly higher than adenocarcinoma. This suggests that maspin plays a more functional role in the tumorigenesis of squamous cell carcinoma than in adenocarcinoma.
The precise mechanisms of maspin as a tumor suppressor gene are still unclear. Different pathways have been suggested for maspin expression. Both p53 and p63, a homolog of p53, are potential regulators of the maspin gene. One of the earlier studies found p53 binding to the maspin gene promoter and upregulating the maspin expression [12]. Other studies reported both p63 and p53 transactivate the maspin promoter [15,18]. In the present study, the high concordance rate of maspin and p63, as well as of maspin and p53, in squamous cell carcinoma of the lung supported the potential role of p63 and p53 as transactivators of maspin expression. However, maspin may not be involved in the regulation of p63 and p53 expression in adenocarcinoma.
In conclusion, maspin, p63 and p53 are highly expressed in squamous cell carcinoma of the lung and expression concordance between maspin and p63 or p53 is much higher in squamous cell carcinoma, but not in adenocarcinoma. These findings indicate that both p63 and p53 may control maspin expression in squamous cell carcinoma more likely than in adenocarcinoma of the lung.
Disclosure of conflict of interest
None.
References
- 1.Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 2.Maass N, Hojo T, Rosel F, Ikeda T, Jonat W, Nagasaki K. Down regulation of the tumor suppressor gene maspin in breast carcinoma is associated with a higher risk of distant metastasis. Clin Biochem. 2001;34:303–307. doi: 10.1016/s0009-9120(01)00220-x. [DOI] [PubMed] [Google Scholar]
- 3.Maass N, Hojo T, Ueding M, Luttges J, Kloppel G, Jonat W, Nagasaki K. Expression of the tumor suppressor gene Maspin in human pancreatic cancers. Clin Cancer Res. 2001;7:812–817. [PubMed] [Google Scholar]
- 4.Bieche I, Girault I, Sabourin JC, Tozlu S, Driouch K, Vidaud M, Lidereau R. Prognostic value of maspin mRNA expression in ER alpha-positive postmenopausal breast carcinomas. Br J Cancer. 2003;88:863–870. doi: 10.1038/sj.bjc.6600812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umekita Y, Ohi Y, Sagara Y, Yoshida H. Expression of maspin predicts poor prognosis in breast-cancer patients. Int J Cancer. 2002;100:452–455. doi: 10.1002/ijc.10500. [DOI] [PubMed] [Google Scholar]
- 6.Sood AK, Fletcher MS, Gruman LM, Coffin JE, Jabbari S, Khalkhali-Ellis Z, Arbour N, Seftor EA, Hendrix MJ. The paradoxical expression of maspin in ovarian carcinoma. Clin Cancer Res. 2002;8:2924–2932. [PubMed] [Google Scholar]
- 7.Nakagawa M, Katakura H, Adachi M, Takenaka K, Yanagihara K, Otake Y, Wada H, Tanaka F. Maspin expression and its clinical significance in non-small cell lung cancer. Ann Surg Oncol. 2006;13:1517–1523. doi: 10.1245/s10434-006-9030-z. [DOI] [PubMed] [Google Scholar]
- 8.Takanami I, Abiko T, Koizumi S. Expression of maspin in non-small-cell lung cancer: correlation with clinical features. Clin Lung Cancer. 2008;9:361–366. doi: 10.3816/CLC.2008.n.052. [DOI] [PubMed] [Google Scholar]
- 9.Katakura H, Takenaka K, Nakagawa M, Sonobe M, Adachi M, Ito S, Wada H, Tanaka F. Maspin gene expression is a significant prognostic factor in resected non-small cell lung cancer (NSCLC). Maspin in NSCLC. Lung Cancer. 2006;51:323–328. doi: 10.1016/j.lungcan.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Sheng S, Carey J, Seftor EA, Dias L, Hendrix MJ, Sager R. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci U S A. 1996;93:11669–11674. doi: 10.1073/pnas.93.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 12.Zou Z, Gao C, Nagaich AK, Connell T, Saito S, Moul JW, Seth P, Appella E, Srivastava S. p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem. 2000;275:6051–6054. doi: 10.1074/jbc.275.9.6051. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Maass N, Magit D, Sager R. Transactivation through Ets and Ap1 transcription sites determines the expression of the tumor-suppressing gene maspin. Cell Growth Differ. 1997;8:179–186. [PubMed] [Google Scholar]
- 14.Feldman RJ, Sementchenko VI, Gayed M, Fraig MM, Watson DK. Pdef expression in human breast cancer is correlated with invasive potential and altered gene expression. Cancer Res. 2003;63:4626–4631. [PubMed] [Google Scholar]
- 15.Kim S, Han J, Kim J, Park C. Maspin expression is transactivated by p63 and is critical for the modulation of lung cancer progression. Cancer Res. 2004;64:6900–6905. doi: 10.1158/0008-5472.CAN-04-1657. [DOI] [PubMed] [Google Scholar]
- 16.Strano S, Rossi M, Fontemaggi G, Munarriz E, Soddu S, Sacchi A, Blandino G. From p63 to p53 across p73. FEBS Lett. 2001;490:163–170. doi: 10.1016/s0014-5793(01)02119-6. [DOI] [PubMed] [Google Scholar]
- 17.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 18.Spiesbach K, Tannapfel A, Mossner J, Engeland K. TAp63gamma can substitute for p53 in inducing expression of the maspin tumor suppressor. Int J Cancer. 2005;114:555–562. doi: 10.1002/ijc.20766. [DOI] [PubMed] [Google Scholar]
- 19.Massion PP, Taflan PM, Rahman SM, Yildiz P, Shyr Y, Carbone DP, Gonzalez AL. Role of p63 amplification and overexpression in lung cancer development. Chest. 2004;125:102S. [PubMed] [Google Scholar]
- 20.Yatabe Y, Mitsudomi T, Takahashi T. Maspin expression in normal lung and non-small-cell lung cancers: cellular property-associated expression under the control of promoter DNA methylation. Oncogene. 2004;23:4041–4049. doi: 10.1038/sj.onc.1207557. [DOI] [PubMed] [Google Scholar]
- 21.Akyildiz EU, Oz B, Sehitoglu I, Demir H. The diagnostic utility of maspin in the distinction between malignant mesothelioma and pulmonary adenocarcinoma. J Int Med Res. 2010;38:1070–1076. doi: 10.1177/147323001003800334. [DOI] [PubMed] [Google Scholar]
- 22.Nakashima M, Ohike N, Nagasaki K, Adachi M, Morohoshi T. Prognostic significance of the maspin tumor suppressor gene in pulmonary adenocarcinoma. J Cancer Res Clin Oncol. 2004;130:475–479. doi: 10.1007/s00432-004-0571-x. [DOI] [PubMed] [Google Scholar]
- 23.Zheng HC, Saito H, Masuda S, Wang ZG, Takano Y. Cytoplasmic and nuclear maspin expression in lung carcinomas: an immunohistochemical study using tissue microarrays. Appl Immunohistochem Mol Morphol. 2008;16:459–565. doi: 10.1097/PAI.0b013e3181640bb1. [DOI] [PubMed] [Google Scholar]
- 24.Frey A, Soubani AO, Adam AK, Sheng S, Pass HI, Lonardo F. Nuclear, compared with combined nuclear and cytoplasmic expression of maspin, is linked in lung adenocarcinoma to reduced VEGF-A levels and in Stage I, improved survival. Histopathology. 2009;54:590–597. doi: 10.1111/j.1365-2559.2009.03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirai K, Koizumi K, Haraguchi S, Hirata T, Mikami I, Fukushima M, Yamagishi S, Kawashima T, Okada D, Shimizu K, Kawamoto M. Prognostic significance of the tumor suppressor gene maspin in non-small cell lung cancer. Ann Thorac Surg. 2005;79:248–253. doi: 10.1016/j.athoracsur.2004.06.118. [DOI] [PubMed] [Google Scholar]


