Abstract
IgG4-related sclerosing disease is an established disease entity with characteristic clinicopathological features. Recently, the association between IgG4-related sclerosing disease and the risk of malignancies has been suggested. IgG4-related autoimmune pancreatitis with pancreatic cancer has been reported. Further, a few cases of extraocular malignant lymphoma in patients with IgG4-related sclerosing disease have also been documented. Herein, we describe the first documented case of anaplastic large cell lymphoma (ALCL) following IgG4-related autoimmune pancreatitis and cholecystitis and diffuse large B-cell lymphoma (DLBCL). A 61-year-old Japanese male, with a past history of DLBCL, was detected with swelling of the pancreas and tumorous lesions in the gallbladder. Histopathological study of the resected gallbladder specimen revealed diffuse lymphoplasmacytic infiltration with fibrosclerosis in the entire gallbladder wall. Eosinophilic infiltration and obliterative phlebitis were also noted. Immunohistochemically, many IgG4-positive plasma cells had infiltrated into the lesion, and the ratio of IgG4/IgG-positive plasma cells was 71.6%. Accordingly, a diagnosis of IgG4-related cholecystitis was made. Seven months later, he presented with a painful tumor in his left parotid gland. Histopathological study demonstrated diffuse or cohesive sheet-like proliferation of large-sized lymphoid cells with rich slightly eosinophilic cytoplasm and irregular-shaped large nuclei. These lymphoid cells were positive for CD30, CD4, and cytotoxic markers, but negative for CD3 and ALK. Therefore, a diagnosis of ALK-negative ALCL was made. It has been suggested that the incidence of malignant lymphoma may be high in patients with IgG4-related sclerosing disease, therefore, intense medical follow-up is important in patients with this disorder.
Keywords: IgG4-related sclerosing disease, cholecystitis, malignant lymphoma, anaplastic large cell lymphoma
Introduction
IgG4-related sclerosing disease is an established disease entity showing characteristic clinicopathological features [1-3]. This disease concept was first established in sclerosing pancreatitis (autoimmune pancreatitis) in 2001 [1]. Since then, it has been recognized that this disorder can involve various organs, such as liver, bile duct, gallbladder, nasal cavity, salivary gland, lacrimal gland, lung, aorta, kidney, pituitary gland, and retroperitoneum [4-21]. IgG4-related sclerosing disease is a systemic fibroinflammatory disease characterized clinically by the formation of tumor-like lesions and elevated serum IgG4 concentration [1,3], and histopathologically by the presence of fibrosclerosis and dense lymphoplasmacytic infiltration with abundant IgG4-positive plasma cells and high IgG4/IgG-positive plasma cell ratio accompanied by eosinophilic infiltration and obliterative phlebitis [2-5].
Recently, an association between IgG4-related sclerosing disease and the risk of malignancies has been suggested [22]. IgG4-related autoimmune pancreatitis with pancreas cancer has been reported [23-26]. Further, a few cases of extraocular malignant lymphoma in patients with IgG4-related sclerosing disease have also been documented [27-31], although the association between ocular adnexal marginal zone B-cell lymphoma and IgG4-related dacryoadenitis has been recognized [32-36]. Herein, we describe the first documented case of anaplastic large cell lymphoma (ALCL) following IgG4-related autoimmune pancreatitis and cholecystitis and diffuse large B-cell lymphoma (DLBCL).
Case report
A 61-year-old Japanese male was detected with swelling of the pancreas, dilatation of the pancreatic duct, and tumorous lesions in the gallbladder by magnetic resonance imaging (Figure 1A). He had been diagnosed with eosinophilic granuloma of the nasal cavity at the age of 53. At the age of 56, he presented with abdominal pain, and abdominal CT demonstrated multiple lymph nodes swelling in his abdominal cavity. A biopsy from the abdominal lymph node revealed DLBCL (Stage IIIb). Eight cycles of R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisolone) therapy were performed, and he was free from tumor recurrence for 5 years.
Figure 1.
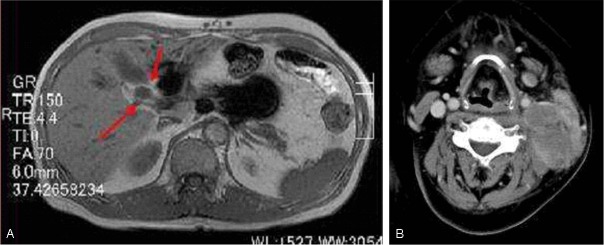
A: Magnetic resonance imaging showing tumorous lesions in the gallbladder (arrows). B: Computed tomography demonstrating a left parotid gland tumor.
Laboratory tests revealed elevated eosinophils and serum IgG and IgG4 concentrations.
Laparoscopic cholecystectomy was performed. Subsequently, he was administered prednisolone (35 mg/day) under a diagnosis of IgG4-related autoimmune pancreatitis and cholecystitis. Three months later, computed tomography showed a tumorous lesion in his right nasal cavity and maxillary sinus, and a biopsy specimen revealed IgG4-related sclerosing lesion of the nasal cavity. Seven months after the cholecystectomy, he presented with a painful tumor in his left cervical region, and computed tomography demonstrated a large tumorous lesion in his left parotid gland (Figure 1B). A biopsy from the left parotid gland tumor was performed. After a diagnosis of ALCL, he received four cycles of DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin) therapy, and subsequently, autologous peripheral blood stem cell transplantation was performed. However, he succumbed to respiratory failure and was suspected with malignant lymphoma invasion to the brain 16 months after the diagnosis of ALCL.
Abdominal lymph node
Diffuse proliferation of large-sized lymphoid cells and destruction of normal architecture of the lymph node were observed (Figure 2). These lymphoid cells had large cleaved nuclei with conspicuous nucleoli, and mitotic figures and apoptotic bodies were scattered.
Figure 2.
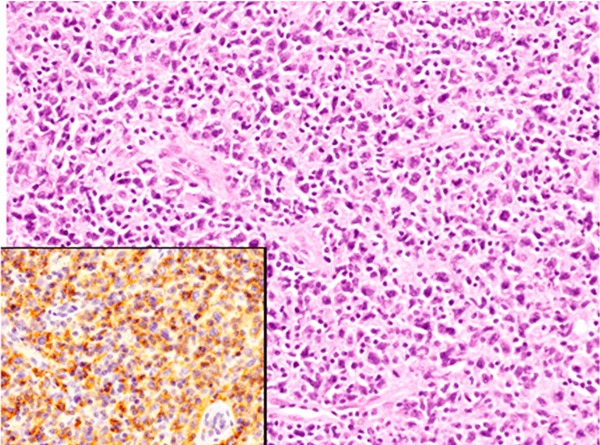
Histopathological and immunohistochemical features of the abdominal lymph node. A: Diffuse proliferation of large-sized lymphoid cells with large cleaved nuclei, HE, x 200. CD20 is expressed in these lymphoid cells (inset), x 200.
Immunohistochemical and in situ hybridization studies were performed using an autostainer (Ventana) by the same method as previously reported [37-41]. The large-sized lymphoid cells were diffusely positive for CD20 (Figure 2, inset) and bcl-2, but negative for CD3, CD10, CD15, CD30, bcl-6, cyclin D1, and anaplastic lymphoma kinase (ALK). Moreover, in situ hybridization revealed no positive signal for Epstein-Barr virus-encoded small RNA (EBER).
Accordingly, a diagnosis of DLBCL was made.
Gallbladder
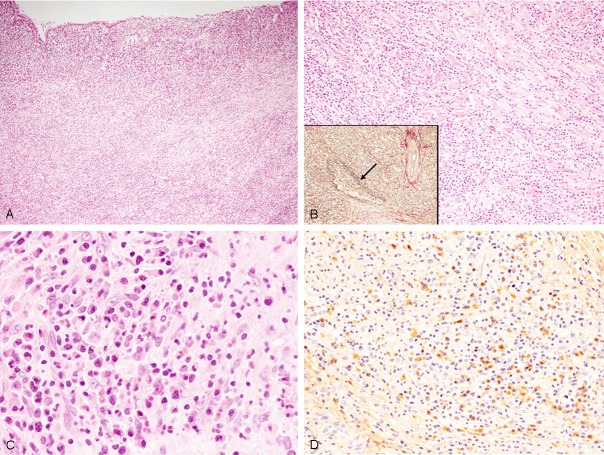
The wall of the gallbladder was thickened, and diffuse lymphoplasmacytic infiltration with fibrosclerosis was observed in the entire gallbladder wall (Figure 3A, 3B). Infiltrating lymphocytes were small in size and had small round nuclei, and plasma cells were also bland in appearance (Figure 3B, 3C). Eosinophilic infiltration was also observed (Figure 3C), but no neutrophils were seen. Obliterative phlebitis was also noted by elastica van Gieson staining (Figure 3B, inset).
Figure 3.
Histopathological and immunohistochemical features of the gallbladder. A: Dense lymphoplasmacytic infiltration is observed in the entire gallbladder wall, HE, x 40. B: Dense lymphoplasmacytic infiltration with fibrosclerotic change is noted, HE, x 100. Obliterative phlebitis (arrow), elastica van Gieson staining x 100. C: Lymphocytes and plasma cells appear mature and are without atypia. Eosinophils are also observed, HE, x 400. D: Many IgG4-positive plasma cells have infiltrated into the lesion, x 200.
Immunohistochemical study revealed that many IgG4-positive plasma cells had infiltrated into the lesion (73/high-power field) (Figure 3D), and the ratio of IgG4/IgG-positive plasmas was 71.6%. Mild CD3-positive T-lymphocytic infiltration was observed, and only a few CD20-positive B-lymphocytes had infiltrated into the lesion. Kappa- and lambda chain-positive plasma cells were evenly distributed as determined by in situ hybridization.
According to these results, a diagnosis of IgG4-positive cholecystitis was made. Although a biopsy from the pancreas was not performed, swelling of the pancreas was suspected to be due to autoimmune pancreatitis because the swelling subsided after administration of prednisolone.
Nasal cavity
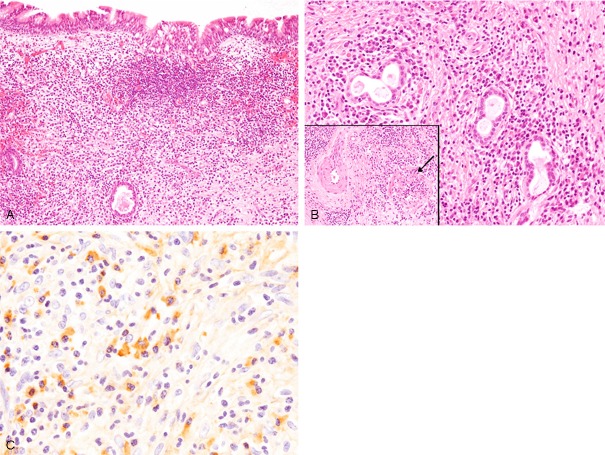
Dense lymphoplasmacytic infiltration with mild fibrosis was observed under the ciliated epithelium and around the nasal glands (Figure 4A, 4B). Lymphocytes and plasma cells appeared mature and without atypia (Figure 4B). Obliterative phlebitis was noted (Figure 4B, inset).
Figure 4.
Histopathological and immunohistochemical features of the nasal tumor. A: Dense lymphoplasmacytic infiltration is observed under the ciliated epithelium, HE, x 100. B: Dense lymphoplasmacytic infiltration is also observed around the nasal glands. Lymphocytes and plasma cells are without atypia. Obliterative phlebitis (arrow, inset), HE, x 200. C: Abundant IgG4-positive plasma cells have infiltrated into the lesion, x 400.
Immunohistochemical study revealed that many IgG4-positive plasma cells had infiltrated into the lesion (68/high-power field) (Figure 4C), and the ratio of IgG4/IgG-positive plasma cells was 79.1%. Kappa- and lambda chain-positive plasma cells were evenly distributed as assessed by in situ hybridization.
Parotid gland
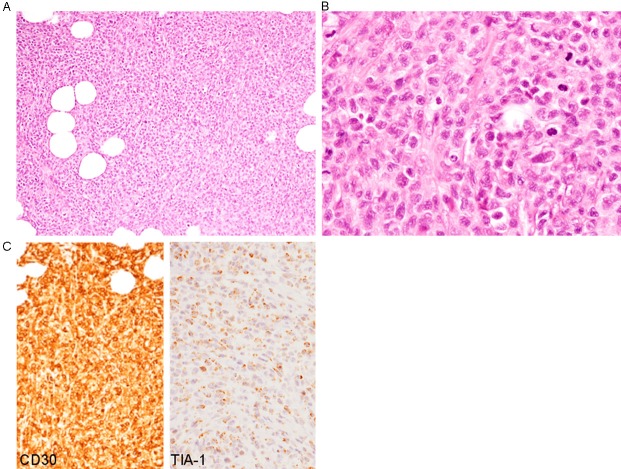
Diffuse or cohesive sheet-like proliferation of large-sized lymphoid cells was observed, and no salivary gland component was present (Figure 5A). Lymphoid cells had moderate amount of slightly eosinophilic cytoplasm and irregular-shaped large nuclei containing small nucleoli, and mitotic figures were scattered (Figure 5B).
Figure 5.
Histopathological and immunohistochemical features of the parotid gland tumor. A: Diffuse or cohesive sheet-like proliferation of large-sized lymphoid cells, HE, x 100. B: Lymphoid cells have large irregular-shaped nuclei containing small nucleoli, HE, x 400. C: CD30 and TIA-1 are diffusely expressed, x 200.
Immunohistochemically, these lymphoid cells were diffusely positive for CD30 and CD4 (Figure 5C), and some of these cells were also positive for epithelial membrane antigen. TIA-1 was expressed in most of the lymphoid cells (Figure 5C), and granzyme B was also expressed in some of these cells. CD3, CD8, CD10, CD15, CD20, CD79a, and ALK were not expressed. Moreover, in situ hybridization revealed no positive signal for EBER.
Accordingly, a diagnosis of ALK-negative ALCL was made.
Discussion
In this report, we described the first documented case of ALCL following IgG4-related autoimmune pancreatitis and cholecystitis and DLBCL. Only 7 cases of extraocular malignant lymphoma occurring in patients with IgG4-related sclerosing disease with detailed clinicopathological features have been reported in the English literature [27-31]. Table 1 summarizes the clinicopathological features of the previously reported cases as well as the present one. This condition mainly affects middle-aged to elderly males (average age 63.6 years (range 41-76), male/female 7/1). The histopathological subtypes of malignant lymphoma were DLBCL (5 cases including the present case), follicular lymphoma (1 case), small lymphocytic lymphoma (1 case), B-cell lymphoma (subtype was not available in 1 case), and ALCL (1 case). The occurrence in extranodal sites is common. The relationship between malignant lymphoma and IgG4-related sclerosing disease is variable. In two of 8 cases, IgG4-related sclerosing disease developed after the occurrence of malignant lymphoma [27,28]. In the other 4 cases, malignant lymphoma occurred during the medical follow-up of IgG4-related sclerosing disease [29,30]. Moreover, a case showing concurrent occurrence of malignant lymphoma and IgG4-related autoimmune pancreatitis has been reported [31]. The present case is very unique because we show for the first time that IgG4-related sclerosing diseases (cholecystitis, autoimmune pancreatitis, and rhinitis) developed after DLBCL, which resulted in death by ALCL. This is the first documented case of two different histopathological subtype of malignant lymphoma occurring before and after IgG4-related sclerosing disease. These results suggest that malignant lymphoma can develop both before and after the occurrence of IgG4-related sclerosing disease.
Table 1.
Clinicopathological features of malignant lymphoma in patients with IgG4-related sclerosing disease
| Case No. | Age/Gender | Histological type of ML (location) | Relationship between ML and IgG4-related sclerosing disease | Reference |
|---|---|---|---|---|
| Case 1 | 59/Male | Diffuse large B-cell lymphoma (supraclavicular lymph node) | Submandibular lymph node biopsy revealed IgG4-related sclerosing disease 10 years after ML. | [27] |
| Case 2 | 41/Male | Follicular lymphoma (colon) | Renal biopsy revealed IgG4-related tubulointerstitial nephritis 14 years after ML. | [28] |
| Case 3 | 66/Male | Diffuse large B-cell lymphoma (lung, stomach, ileum, pancreas) | Autopsy revealed DLBCL after 11-year history of Mikulicz’s disease and 8-year history of inflammatory pseudotumor of the renal pelvis. | [29] |
| Case 4 | 65/Female | B-cell lymphoma (liver) | B-cell lymphoma developed 4 years after a diagnosis of autoimmune pancreatitis. | [30] |
| Case 5 | 72/Male | Diffuse large B-cell lymphoma (adrenal gland) | Diffuse large B-cell lymphoma developed 5 years after a diagnosis of autoimmune pancreatitis. | [30] |
| Case 6 | 69/Male | Diffuse large B-cell lymphoma (kidney) | Diffuse large B-cell lymphoma developed 3 years after a diagnosis of chronic parotitis. | [30] |
| Case 7 | 76/Male | Small lymphocytic lymphoma (retroperitoneal lymph node) | Concurrent autoimmune pancreatitis and small lymphocytic lymphoma. | [31] |
| Present Case | 61/Male | Diffuse large B-cell lymphoma (abdominal lymph node) and anaplastic large cell lymphoma (parotid gland) | Autoimmune pancreatitis, IgG4-related cholecystitis and rhinitis occurred 5 years after a diagnosis of diffuse large B-cell lymphoma. After one year, anaplastic large cell lymphoma developed. |
ML, Malignant lymphoma.
Takahashi et al. analyzed the incidence of malignant lymphoma in patients with IgG4-related sclerosing disease [30]. In 111 patients with IgG4-related sclerosing disease, three cases developed malignant lymphoma [30]. They concluded that patients with IgG4-related sclerosing disease may be at an increased risk of developing malignant lymphoma [30]. Although the mechanism of development of malignant lymphoma in patients with IgG4-related sclerosing disease remains unresolved, an association between autoimmune diseases, such as Sjögren syndrome, and the development of malignant lymphoma is well recognized [42]. It has been speculated that dysregulation of B lymphocytes associated with autoimmune disease leads to abnormal B lymphocytes proliferation, resulting in the occurrence of malignant B-cell lymphoma [43]. Moreover, an association between ocular adnexal marginal zone B-cell lymphoma and IgG4-related dacryoadenitis has recently demonstrated [31-35]. In addition, Mitsui et al. reported a very interesting case of IgG4-related sclerosing disease 10 years after chemotherapy for DLBCL [27]. Retrospective analysis of the lymph node biopsy specimen diagnosed as DLBCL revealed that many IgG4-positive plasma cells had infiltrated outside of the DLBCL lesion, therefore, they speculated that subclinical IgG4-related disease was present before development of DLBCL, and non-neoplastic IgG4-producing plasma cells survived after chemotherapy, which led to the development of IgG4-related sclerosing disease [27]. Therefore, these results suggest that a history of IgG4-related sclerosing disease may be a predisposing condition for the development of malignant lymphoma [28], and clinical follow-up is important in patients with IgG4-related sclerosing disease.
ALK-negative ALCL is included in the recent World Health Organization Classification as a provisional entity, and is defined as a CD30-positive T-cell neoplasm that is not reproducibly distinguishable on morphological grounds from ALK-positive ALCL, although it lacks ALK protein [44]. This type of malignant lymphoma mainly affects middle-aged persons in contrast to ALK-positive ALCL, which occurs most commonly in children and young adults, and shows more aggressive clinical course than ALK-positive ALCL [44,45]. Histopathologically, ALK-negative ALCL is characterized by solid, cohesive sheets of large-sized neoplastic cells with abundant eosinophilic to clear cytoplasm and large irregular-shaped nuclei, sometimes containing prominent nucleoli [44]. Immunohistochemically, these neoplastic cells are strongly positive for CD30, and CD15 is not expressed. Loss of T-cell markers can occur, however, more than half of cases express one or more T-cell markers. CD4 is positive in a significant proportion of cases, whereas CD8-positive cases are rare [44]. A substantial minority of cases is positive for epithelial membrane antigen [44]. Moreover, many cases express cytotoxic markers, such was TIA-1 and granzyme B [44]. In the present case, the neoplastic cells of the parotid gland tumor were large-sized and had irregular-shaped large nuclei. Immunohistochemically, these neoplastic cells were positive for CD30 and CD4, and some of these cells were also positive for epithelial membrane antigen. Moreover, cytotoxic markers were expressed in most of these cells, and ALK was negative. These features corresponded to ALK-negative ALCL. Further, this case had a fatal outcome.
In conclusion, we report the first documented case of ALK-negative ALCL following IgG4-related autoimmune pancreatitis and cholecystitis and DLBCL. It has been suggested that the incidence of malignant lymphoma may be high in patients with IgG4-related sclerosing disease, therefore, intense medical follow-up is important in patients with IgG4-related sclerosing disease.
References
- 1.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 2.Sato Y, Notohara K, Kojima M, Takata K, Masaki Y, Yoshino T. IgG4-related disease: historical overview and pathology of hematological disorders. Pathol Int. 2010;60:247–258. doi: 10.1111/j.1440-1827.2010.02524.x. [DOI] [PubMed] [Google Scholar]
- 3.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 4.Zen Y, Nakanuma Y. IgG4-related disease. A cross-sectional study of 114 cases. Am J Surg Pathol. 2010;34:1812–1819. doi: 10.1097/PAS.0b013e3181f7266b. [DOI] [PubMed] [Google Scholar]
- 5.Zen Y, Nakanuma Y. Pathogenesis of IgG4-related disease. Curr Opin Rheumatol. 2011;23:114–118. doi: 10.1097/BOR.0b013e3283412f4a. [DOI] [PubMed] [Google Scholar]
- 6.Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008;14:3948–3955. doi: 10.3748/wjg.14.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193–1203. doi: 10.1097/01.pas.0000136449.37936.6c. [DOI] [PubMed] [Google Scholar]
- 8.Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, Nakazawa K, Shimojo H, Kiyosawa K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–1404. doi: 10.1016/s0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- 9.Zen Y, Kitagawa S, Minato H, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Fujimura M, Nakanuma Y. IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell granuloma) of the lung. Hum Pathol. 2005;36:710–717. doi: 10.1016/j.humpath.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa S, Zen Y, Harada K, Sasaki M, Sato Y, Minato H, Watanabe K, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Tsuneyama K, Saito K, Haratake J, Takagawa K, Nakanuma Y. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Küttner’s tumor) Am J Surg Pathol. 2005;29:783–791. doi: 10.1097/01.pas.0000164031.59940.fc. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Shinomura Y, Imai K. A new conceptualization for Mikulicz’s disease as an IgG4-related plasmacytic disease. Mod Rheumatol. 2006;16:335–340. doi: 10.1007/s10165-006-0518-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasashima S, Zen Y, Kawashima A, Konishi K, Sasaki H, Endo M, Matsumoto Y, Kawakami K, Kasashima F, Moriya M, Kimura K, Ohtake H, Nakanuma Y. Inflammatory abdominal aortic aneurysm: close relationship to IgG4-related periaortitis. Am J Surg Pathol. 2008;32:197–204. doi: 10.1097/PAS.0b013e3181342f0d. [DOI] [PubMed] [Google Scholar]
- 13.Kasashima S, Zen Y, Kawashima A, Endo M, Matsumoto Y, Kasashima F. A new clinicopathological entity of IgG4-related inflammatory abdominal aortic aneurysm. J Vasc Surg. 2009;49:1264–1271. doi: 10.1016/j.jvs.2008.11.072. [DOI] [PubMed] [Google Scholar]
- 14.Ishida M, Hotta M, Kushima R, Asai T, Okabe H. IgG4-related inflammatory aneurysm of the aortic arch. Pathol Int. 2009;59:269–273. doi: 10.1111/j.1440-1827.2009.02363.x. [DOI] [PubMed] [Google Scholar]
- 15.Kasashima S, Zen Y, Kawashima A, Endo M, Matsumoto Y, Kasashima F, Ohtake H, Nakanuma Y. A clinicopathologic study of immunoglobulin G4-related sclerosing disease of the thoracic aorta. J Vasc Surg. 2010;52:1587–1595. doi: 10.1016/j.jvs.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 16.Kasashima S, Zen Y. IgG4-related inflammatory abdominal aortic aneurysm. Curr Opin Rheumatol. 2011;23:18–23. doi: 10.1097/BOR.0b013e32833ee95f. [DOI] [PubMed] [Google Scholar]
- 17.Zen Y, Kasashima S, Inoue D. Retroperitoneal and aortic manifestations of immunoglobulin G4-related disease. Semin Diagn Pathol. 2012;29:212–218. doi: 10.1053/j.semdp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Kawano M, Mizushima I, Yamaguchi Y, Imai N, Nakashima H, Nishi S, Hisano S, Yamanaka N, Yamamoto M, Takahashi H, Umehara H, Saito T, Saeki T. Immunohistochemical characteristics of IgG4-related tubulointerstitial nephritis: Detailed analysis of 20 Japanese cases. Int J Rheumatol. 2012;2012:609795. doi: 10.1155/2012/609795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong S, Lam WY, Wong WK, Lee KC. Hypophysitis presented as inflammatory pseudotumor in immunoglobulin G4-related systemic disease. Hum Pathol. 2007;38:1720–1723. doi: 10.1016/j.humpath.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Cheuk W, Yuen HK, Chan JK. Chronic sclerosing dacryoadenitis: Part of the spectrum of IgG4-related sclerosing disease? Am J Surg Pathol. 2007;31:643–645. doi: 10.1097/01.pas.0000213445.08902.11. [DOI] [PubMed] [Google Scholar]
- 21.Ishida M, Hotta M, Kushima R, Shibayama M, Shimizu T, Okabe H. Multiple IgG4-related sclerosing lesions in the maxillary sinus, parotid gland and nasal septum. Pathol Int. 2009;59:670–675. doi: 10.1111/j.1440-1827.2009.02425.x. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Takahashi H, Tabeya T, Suzuki C, Naishiro Y, Ishigami K, Yajima H, Shimizu Y, Obara M, Yamamoto H, Himi T, Imai K, Shinomura Y. Risk of malignancies in IgG4-related disease. Mod Rheumatol. 2012;22:414–418. doi: 10.1007/s10165-011-0520-x. [DOI] [PubMed] [Google Scholar]
- 23.Kamisawa T, Chen PY, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A, Hishima T. Pancreatic cancer with a high serum IgG4 concentration. World J Gastroenterol. 2006;12:6225–6228. doi: 10.3748/wjg.v12.i38.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukui T, Mitsuyama T, Takaoka M, Uchida K, Matsushita M, Okazaki K. Pacnreatic cancer assocated with autoimmune pancreatitis in remission. Intern Med. 2008;47:151–155. doi: 10.2169/internalmedicine.47.0334. [DOI] [PubMed] [Google Scholar]
- 25.Ghazale A, Chari S. Is autoimmune pancreatitis a risk factor for pancreatic cancer? Pancreas. 2007;35:376. doi: 10.1097/MPA.0b013e318073ccb8. [DOI] [PubMed] [Google Scholar]
- 26.Loos M, Esposito I, Hedderich DM, Ludwig L, Fingerle A, Friess H, Klöppel G, Büchler P. Autoimmune pancreatitis complicated by carcinoma of the pancreatobiliary system: a case report and review of the literature. Pancreas. 2011;40:151–154. doi: 10.1097/MPA.0b013e3181f74a13. [DOI] [PubMed] [Google Scholar]
- 27.Mitsui T, Yokohama A, Koiso H, Ishizaki T, Uchiumi H, Saitoh T, Handa H, Hirato J, Karasawa M, Murakami H, Kojima M, Nojima Y, Tsukamoto N. Development of IgG4-related disease 10 years after chemotherapy for diffuse large B cell lymphoma and longstanding bronchial asthma. Int J Hematol. 2013;98:122–128. doi: 10.1007/s12185-013-1359-z. [DOI] [PubMed] [Google Scholar]
- 28.Oshima Y, Usui R, Manabe S, Hasegawa N, Kakuta Y, Nitta K, Hatano M. IgG4-related tubulointerstitial nephritis and lymphadenopathy after therapy for malignant lymphoma. Intern Med. 2012;51:1221–1226. doi: 10.2169/internalmedicine.51.6691. [DOI] [PubMed] [Google Scholar]
- 29.Uehara T, Ikeda S, Hamano H, Kawa S, Moteki H, Matsuda K, Kaneko Y, Hara E. A case of Mikulicz’s disease complicated by malignant lymphoma: a postmortem histopathological finding. Intern Med. 2012;51:419–423. doi: 10.2169/internalmedicine.51.5713. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi N, Ghazale AH, Smyrk TC, Mandrekar JN, Chari ST. Possible association between IgG4-associated systemic disease with or without autoimmune pancreatitis and non-Hodgkin lymphoma. Pancreas. 2009;38:523–526. doi: 10.1097/MPA.0b013e31819d73ca. [DOI] [PubMed] [Google Scholar]
- 31.Kim T, Grobmyer SR, Dixon LR, Allan RW, Hochwald SN. Autoimmune pancreatitis and concurrent small lymphocytic lymphoma: not just a coincidence? J Gastrointest Surg. 2008;12:1566–1570. doi: 10.1007/s11605-008-0543-6. [DOI] [PubMed] [Google Scholar]
- 32.Matsuo T, Ichimura K, Yoshino T. Local recurrence as immunoglobulin G4 (IgG4)-related disease 10 years after radiotherapy to ocular adnexal extranodal marginal zone B-cell lymphoma of mucosa-assocaited lymphoid tissue. J Clin Exp Hematop. 2011;51:125–133. doi: 10.3960/jslrt.51.125. [DOI] [PubMed] [Google Scholar]
- 33.Go H, Kim JE, Kim YA, Chung HK, Khwarg SI, Kim CW, Jeon YK. Ocular adnexal IgG4-related disease: comparative analysis with mucosa-associated lymphoid tissue lymphoma and other chronic inflammatory conditions. Histopathology. 2012;60:296–312. doi: 10.1111/j.1365-2559.2011.04089.x. [DOI] [PubMed] [Google Scholar]
- 34.Kubota T, Moritani S, Yoshino T, Nagai H, Terasaki H. Ocular adnexal marginal zone B cell lymphoma infiltrated by IgG4-positive plasma cells. J Clin Pathol. 2010;63:1059–1065. doi: 10.1136/jcp.2010.082156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato Y, Ohshima K, Ichimura K, Sato M, Yamadori I, Tanaka T, Takata K, Morito T, Kondo E, Yoshino T. Ocular adnexal IgG4-related disease has uniform clinicopathology. Pathol Int. 2008;58:465–470. doi: 10.1111/j.1440-1827.2008.02257.x. [DOI] [PubMed] [Google Scholar]
- 36.Cheuk W, Yuen HK, Chan AC, Shih LY, Kuo TT, Ma MW, Lo YF, Chan WK, Chan JK. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: a previously undescribed complication of IgG4-related sclerosing disease. Am J Surg Pathol. 2008;32:1159–1167. doi: 10.1097/PAS.0b013e31816148ad. [DOI] [PubMed] [Google Scholar]
- 37.Ishida M, Iwai M, Yoshida K, Kagotani A, Okabe H. Sarcomatoid carcinoma with small cell carcinoma component of the urinary bladder: A case report with review of the literature. Int J Clin Exp Pathol. 2013;6:1671–1676. [PMC free article] [PubMed] [Google Scholar]
- 38.Ishida M, Mori T, Umeda T, Kawai Y, Kubota Y, Abe H, Iwai M, Yoshida K, Kagotani A, Tani T, Okabe H. Pleomorphic lobular carcinoma in a male breast: case report with review of the literature. Int J Clin Exp Pathol. 2013;6:1441–1444. [PMC free article] [PubMed] [Google Scholar]
- 39.Nakanishi R, Ishida M, Hodohara K, Yoshida T, Yoshii M, Okuno H, Horinouchi A, Iwai M, Yoshida K, Kagotani A, Okabe H. Prominent gelatinous bone marrow transformation presenting prior to myelodysplastic syndrome: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:1677–1682. [PMC free article] [PubMed] [Google Scholar]
- 40.Ishida M, Mochizuki Y, Iwai M, Yoshida K, Kagotani A, Okabe H. Pigmented squamous intraepithelial neoplasia of the esophagus. Int J Clin Exp Pathol. 2013;6:1868–73. [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshii M, Ishida M, Hodohara K, Okuno Y, Nakanishi R, Yoshida T, Okabe H. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood: Report of a case with review of the literature. Oncol Lett. 2012;4:381–384. doi: 10.3892/ol.2012.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jonsson MV, Theander E, Jonsson R. Predictors for the development of non-Hodgkin lymphoma in primary Sjögren’s syndrome. Presse Med. 2012;41:e511–516. doi: 10.1016/j.lpm.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 43.Hansen A, Lipsky PE, Dörner T. B-cell lymphoproliferation in chronic inflammatory rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:561–569. doi: 10.1038/ncprheum0620. [DOI] [PubMed] [Google Scholar]
- 44.Mason DY, Harris NL, Delsol G, Stein H, Campo E, Kinney MC, Jaffe ES, Falini B. Anaplastic large cell lymphoma, ALK-negative. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 317–319. [Google Scholar]
- 45.ten Berge RL, Oudejans JJ, Ossenkoppele GJ, Meijer CJ. ALK-negative systemic anaplastic large cell lymphoma: differential diagnostic and prognostic aspects-a review. J Pathol. 2003;200:4–15. doi: 10.1002/path.1331. [DOI] [PubMed] [Google Scholar]