Abstract
Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma (ALK-positive LBCL) is an extremely rare distinct clinicopathological subtype of LBCL, characterized by the presence of ALK-positive monomorphic large immunoblast-like neoplastic B cells. Herein, we describe the first cytological report on ALK-positive LBCL in the pleural effusion. A 69-year-old Japanese male with a past history of malignant lymphoma of the cecum presented with progressive dyspnea and pleural effusion. Removal of the pleural effusion and aspiration of bone marrow were performed. May-Grünwald-Giemsa stain of the pleural fluid revealed abundant single or small aggregates of large-sized round cells. These cells had centrally-located large round to oval nuclei. The peculiar finding was the presence of pseudopodial cytoplasmic projections, and some neoplastic cells had eosinophilic pseudopodial cytoplasmic projections, which resembled “flaming plasma cells”. Histopathological and immunohistochemical studies of the bone marrow demonstrated CD138+, ALK1+, CD20-, CD79a-, CD30-, and IgA+ large-sized neoplastic cells. Therefore, a diagnosis of ALK-positive LBCL was made. The peculiar finding of the present case was that most of the neoplastic cells had pseudopodial cytoplasmic projections, and some of them had eosinophilic pseudopodial cytoplasmic projections that resembled “flaming plasma cells”, which has been recognized as the characteristic finding of IgA myeloma. Therefore, tumor cells that resembled “flaming plasma cells” in the pleural effusion may have had IgA in the cytoplasm. Albeit extremely rare, ALK-positive LBCL shows aggressive clinical course, thus, recognition of the cytomorphological features of this type of malignant lymphoma is important for early and correct diagnosis.
Keywords: ALK-positive large B-cell lymphoma, pleural effusion, pseudopodial cytoplasmic projection
Introduction
Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma (ALK-positive LBCL) is an extremely rare distinct clinicopathological variant of LBCL, which was first described by Delsol et al. in 1997 [1], and is recognized as a separate entity in the recent World Health Organization Classification [2]. This disease is characterized histopathologically and immunohistochemically by the presence of ALK-positive monomorphic large immunoblast-like neoplastic B cells with or without plasmablastic differentiation, and shows an aggressive clinical course [2]. There have been only two cytological reports on ALK-positive LBCL detected by aspiration cytology from the lymph nodes [3,4]. Herein, we describe the first cytological report on ALK-positive LBCL in the pleural effusion.
Case report
A 69-year-old Japanese male without an immunocompromised status presented with progressive dyspnea. He had a past history of primary malignant lymphoma of the cecum 2 years earlier, and chemotherapy (CHOP and IVAC regimens) had been administered. Computed tomography revealed massive pleural effusion in the right thoracic cavity and multiple small nodular lesions in the thoracic walls. Removal of the pleural effusion and aspiration of the bone marrow were performed.
Cytological findings of the pleural fluid specimen
May-Grünwald-Giemsa staining revealed abundant single or small aggregates of large-sized round cells in a hemorrhagic background (Figure 1A, 1B). These cells had centrally-located large round to oval nuclei with coarse chromatin with or without conspicuous nucleoli (Figure 1A, 1B). The peculiar finding of these cells was the presence of pseudopodial cytoplasmic projections (Figure 1A, 1B), and moreover, some neoplastic cells had eosinophilic pseudopodial cytoplasmic projections, which resembled “flaming plasma cells” (Figure 1C). Multinucleated atypical cells were also observed. Mitotic figures were scattered (Figure 1B, inset).
Figure 1.
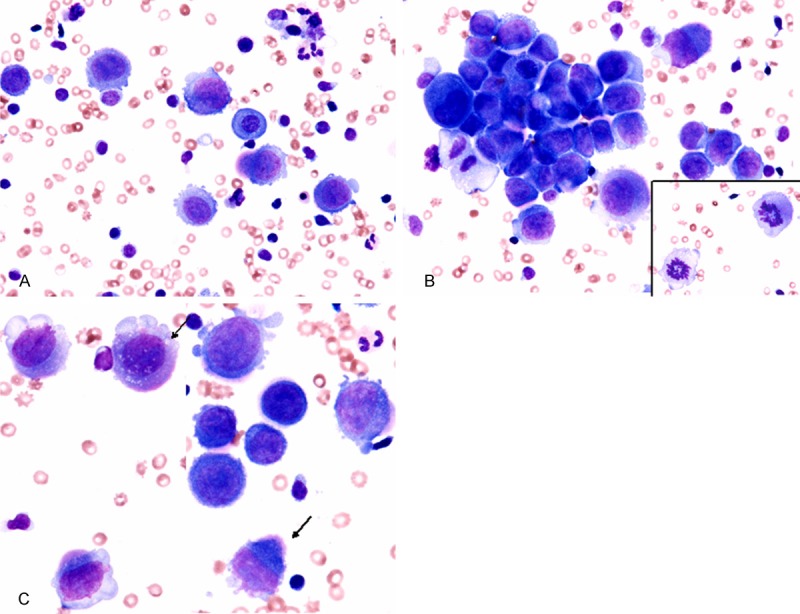
Cytological findings of the pleural effusion. A, B: Single or small aggregates of large neoplastic cells. These neoplastic cells have centrally located large oval to round nuclei containing coarse chromatin with or without conspicuous nucleoli. The presence of pseudopodial cytoplasmic projections is characteristic. Mitotic figures are scattered (inset), May-Grünwald-Giemsa stain, x 400. C: Some neoplastic cells have eosinophilic pseudopodial cytoplasmic projections (arrows), which resemble “flaming plasma cells”, May-Grünwald-Giemsa stain, x 400.
Flow cytometric analysis of the pleural fluid
A population of large-sized cells showing CD3-/CD19-/CD38-/CD138+ was predominant.
Histopathological and immunohistochemical findings of the bone marrow aspiration
Microscopic examination revealed aggregates of large-sized neoplastic cells (Figure 2A). These cells had large round to oval nuclei containing coarse chromatin with or without conspicuous nucleoli and slightly eosinophilic cytoplasm (Figure 2A). The neoplastic cells with pseudopodial cytoplasmic projections were characteristic (Figure 2A). Moreover, plasmablastic cells, which had eccentrically-located large nuclei and perinuclear pale cytoplasm, were also observed (Figure 2A, arrows). Mitotic figures were scattered.
Figure 2.
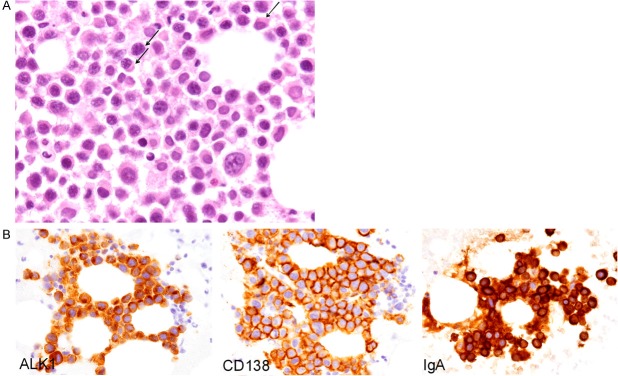
Histopathological and immunohistochemical features of the bone marrow aspiration. A: Proliferation of the large-sized neoplastic cells containing large round to oval nuclei with coarse chromatin with or without conspicuous nucleoli, and slightly eosinophilic cytoplasm. Most of the neoplastic cells have pseudopodial cytoplasmic projections. Plasmablastic cells are also observed (arrows), H&E, x 400. B: Immunohistochemically, the neoplastic cells are positive for CD138, ALK1 (cytoplasmic), and IgA, x 400.
Immunohistochemical and in situ hybridization analyses were performed using an autostainer (XT System Benchmark, Ventana Medical System, Tucson, AZ, USA) according to the manufacturer’s instruction by the same method as previously reported [5-9]. These neoplastic cells were positive for CD138, MUM-1, and ALK1 (cytoplasmic staining pattern) (Figure 2B), but negative for CD3, CD15, CD20, CD30, CD38, CD79a, bcl-2, bcl-6, and HHV-8. IgA was expressed in the cytoplasm of the neoplastic cells (Figure 2B), but IgG was not expressed. In situ hybridization analyses revealed that the neoplastic cells were positive for lambda-chain, but negative for kappa-chain and EBER.
According to these results, an ultimate diagnosis of ALK-positive LBCL was made.
Discussion
ALK-positive LBCL is an extremely rare distinct clinicopathological entity of non-Hodgkin lymphoma, and less than 100 cases have been reported [10-13]. This disease commonly affects middle-aged man (male: female ratio is 3-5: 1) without an immunocompromised status. This type of lymphoma commonly presents in the lymph nodes (especially cervical and mediastinum), however, extranodal involvement has also been documented [10-13]. The prognosis is poor with 25% of patients alive at 5 years and a median survival as short as 12 months [10].
The accurate diagnosis of ALK-positive LBCL requires histopathological and immunohistochemical analyses. The neoplastic cells are characteristically composed of monomorphic large immunoblast-like cells with large round nuclei containing conspicuous nucleoli and rich cytoplasm. Plasmablastic differentiation is observed in most cases, and binucleated cells are also observed [10-13]. Immunohistochemically, the neoplastic cells are strongly positive for ALK1 with a restricted granular cytoplasmic staining pattern highly indicative of the expression of the CLTC-ALK protein. They also strongly express CD138, but not CD3, CD20, CD79a, and CD30. MUM-1 is frequently positive. EBER and HHV-8 are not detected in the neoplastic cells. Most of the neoplastic cells express cytoplasmic immunoglobulin (usually IgA, more rarely IgG) with light chain restriction [2,10-13]. In the present case, the neoplastic cells in the bone marrow showed typical histopathological and immunohistochemical features of the above-mentioned ALK-positive LBCL. Moreover, retrospective analyses of the cecum tumor revealed the same histopathological and immunohistochemical features, therefore, this case was recognized as relapse of the primary ALK-positive LBCL of the cecum in the pleural effusion and bone marrow (the initial diagnosis of the cecum tumor of the present case was plasmablastic lymphoma, and analysis of ALK expression was not performed at the initial diagnosis).
The main differential diagnostic considerations of ALK-positive LBCL include ALK-positive anaplastic large cell lymphoma (ALCL), anaplastic variant of diffuse large B-cell lymphoma, and plasmablastic lymphoma. ALK-positive ALCL is characterized by CD138- and CD30+, and anaplastic variant of diffuse large B-cell lymphoma is characterized by CD20+, CD79a+, CD30+/-, and ALK- [13]. Moreover, differentiation from plasmablastic lymphoma may be difficult because plasmablastic lymphoma and ALK-positive LBCL share many morphological and immunophenotypical features [3]. However, plasmablastic lymphoma is ALK-, and usually EBER+, and mainly occurs in immunodeficient patients. These immunohistochemical characteristics can lead to the correct diagnosis.
The previously reported cytological features of ALK-positive LBCL in needle biopsy specimens from the lymph node are as follows: i) moderate to highly cellular with single and/or clusters of large cells, and cohesive clusters with papillary configurations might be present, ii) the neoplastic cells show mostly immunoblastic and plasmablastic morphology, iii) the nuclei are round to oval with conspicuous nucleoli, and the cytoplasm was rich, and iv) multinucleated cells are present [3,4]. Although cohesive clusters of papillary configurations were not observed in the present case, the cytological features of the pleural effusion fundamentally corresponded to the above-mentioned characteristics.
The peculiar cytological finding of the present case was the presence of pseudopodial cytoplasmic projections, and moreover, some neoplastic cells had eosinophilic pseudopodial cytoplasmic projections, which resembled “flaming plasma cells” [14]. This finding has not been mentioned in the previous reports on ALK-positive LBCL, and the neoplastic cells in the bone marrow of this patient also showed this finding. “Flaming plasma cells” are characterized by the presence of fiery fringes, which are formed by pseudopodic cytoplasmic projections stained red by May-Grünwald-Giemsa staining [14]. These structures contain numerous dilated endoplasmic reticulum cisterns, and are thought to be distended with immunoglobulin. “Flaming plasma cells” have been recognized as the characteristic finding of IgA myeloma, although these cells can also be found in other types of myeloma [14]. In the present case, immunohistochemical analysis clearly demonstrated that IgA was expressed in the cytoplasm of the neoplastic cells in the bone marrow, which is characteristic feature of ALK-positive LBCL. Therefore, the tumor cells that resembled “flaming plasma cells” in the pleural effusion may have had IgA in the cytoplasm although immunocytochemical analysis was not performed in the present case.
In conclusion, we described the first documented case of ALK-positive LBCL in the pleural fluid. Most of the neoplastic cells had pseudopodial cytoplasmic projections, and some of them had eosinophilic pseudopodial cytoplasmic projections, which resembled “flaming plasma cells”. Albeit extremely rare, ALK-positive LBCL shows an aggressive clinical course, thus, recognition of the cytomorphological features of this type of malignant lymphoma is important for early and correct diagnosis.
References
- 1.Delsol G, Lamant L, Mariame B, Pulford K, Dastugue N, Brousset P, Rigal-Huguet F, al Saati T, Cerretti DP, Morris SW, Mason DY. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2;5 translocation. Blood. 1997;89:1483–1490. [PubMed] [Google Scholar]
- 2.Delsol G, Campo E, Gascoyne RD. ALK-positive large B-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumour of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 254–255. [Google Scholar]
- 3.Lin O, Koreishi A, Brandt SM, Arcila M, Teruya-Feldstein J. ALK+ large B-cell lymphoma: A rare variant of aggressive large B-cell lymphoma mimicking carcinoma on cytology specimens. Diagn Cytopathol. 2013;41:404–407. doi: 10.1002/dc.22830. [DOI] [PubMed] [Google Scholar]
- 4.Nakatsuka S, Oku K, Nagano T, Kimura H, Hanamoto A, Ito M, Hashimoto K. A case of anaplastic lymphoma kinase-positive large B-cell lymphoma: aspiration cytology findings. Diagn Cytopathol. 2013 doi: 10.1002/dc.22968. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Ishida M, Iwai M, Yoshida K, Kagotani A, Okabe H. Sarcomatoid carcinoma with small cell carcinoma component of the urinary bladder: A case report with review of the literature. Int J Clin Exp Pathol. 2013;6:1671–1676. [PMC free article] [PubMed] [Google Scholar]
- 6.Ishida M, Mori T, Umeda T, Kawai Y, Kubota Y, Abe H, Iwai M, Yoshida K, Kagotani A, Tani T, Okabe H. Pleomorphic lobular carcinoma in a male breast: case report with review of the literature. Int J Clin Exp Pathol. 2013;6:1441–1444. [PMC free article] [PubMed] [Google Scholar]
- 7.Nakanishi R, Ishida M, Hodohara K, Yoshida T, Yoshii M, Okuno H, Horinouchi A, Iwai M, Yoshida K, Kagotani A, Okabe H. Prominent gelatinous bone marrow transformation presenting prior to myelodysplastic syndrome: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:1677–1682. [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida M, Mochizuki Y, Iwai M, Yoshida K, Kagotani A, Okabe H. Pigmented squamous intraepithelial neoplasia of the esophagus. Int J Clin Exp Pathol. 2013;6:1868–1873. [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshii M, Ishida M, Hodohara K, Okuno Y, Nakanishi R, Yoshida T, Okabe H. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood: Report of a case with review of the literature. Oncol Lett. 2012;4:381–384. doi: 10.3892/ol.2012.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurent C, Do C, Gascoyne RD, Lamant L, Ysebaert L, Laurent G, Delsol G, Brousset P. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma: a rare clinicopathologic entity with poor prognosis. J. Clin. Oncol. 2009;27:4211–4216. doi: 10.1200/JCO.2008.21.5020. [DOI] [PubMed] [Google Scholar]
- 11.Li S. Anaplastic lymphoma kinase-positive large B-cell lymphoma: a distinct clinicopathological entity. Int J Clin Exp Pathol. 2009;2:508–518. [PMC free article] [PubMed] [Google Scholar]
- 12.Beltran B, Castillo J, Salas R, Quinones P, Morales D, Hurtado F, Riva L, Winer E. ALK-positive diffuse large B-cell lymphoma: report of four cases and review of the literature. J Hematol Oncol. 2009;2:11. doi: 10.1186/1756-8722-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan EA, Nascimento AF. Anaplastic lymphoma kinase-positive large B-cell lymphoma: an underrecognized aggressive lymphoma. Adv Hematol. 2012;2012:529572. doi: 10.1155/2012/529572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pantanowitz L, Tranovich V, Ballesteros E. Flaming plasma cells. Arch Pathol Lab Med. 2001;125:1394–1395. doi: 10.5858/2001-125-1394-FPC. [DOI] [PubMed] [Google Scholar]


