Abstract
Objective: To investigate the correlation between CD133-positive non-small cell lung cancer (NSCLC) and clinicopathological features and its impact on survival. Methods: A search in the Pubmed, Embase and Wanfang databases (up to July 15, 2013) was performed. Only articles in which CD133 antigen was detected in situ localization by immunohistochemical staining were included. This meta-analysis was done using RevMan 5.2 software. Outcomes included overall survival and various clinicopathological features. Results: A total of 1004 NSCLC patients from 11 studies were included. Meta-analysis showed that CD133 expression patients had a significant worse 5-year overall survival compared to the low expression ones (RR = 3.19, 95% CI: 2.05-4.98, P<0.0001 fixed random). With respect to clinicopathological features, CD133 expression by IHC method was closely correlated with tumor T stage (OR = 0.91, 95% CI: 0.59-1.39, P = 0.67 fixed-effect) and tumor grade (OR = 1.20, 95% CI: 0.80-1.79, P = 0.37 fixed-effect). Conclusion: CD133-positive NSCLC patients had worse prognosis, and was associated with common clinicopathological poor prognostic factors.
Keywords: Non-small cell lung cancer, cancer stem cells, CD133, prognosis
Introduction
Non-small cell lung cancer (NSCLC) has a relatively poor prognosis and is a leading cause of cancer death worldwide. A substantial proportion of NSCLC patients suffer a recurrence following curative tumor resection, even when they have early stage disease [1]. The current challenge is to identify new therapeutic targets and strategies and to incorporate them into existing treatment regimens with the goal of improving therapeutic gain. Identifying reliable markers predictive of clinical outcome is also desirable to establish therapeutic strategies and select suitable treatment options for individual NSCLC patients.
Recently, a rare subpopulation cancer cells, termed cancer stem cells (CSC) have been thought to be responsible for the initial, progression, metastasis and ultimately recurrence of cancer, for they have the exclusive properties of self-renew and could giving rise to all the heterogeneous lineages of cancer cells that eventually constitute tumor bulk [2]. CD133 is a trans-membrane glycoprotein, its expression in cell surface down-regulates quickly as cell differentiated [3]. CD133 has been used widely as a marker to identify CSC in colon, lung, brain, pancreatic cancer and so on [4-6]. Its prognostic value for cancer patients has also been found in many cancers [7,8].
With respect to lung cancer, the correlation between CD133 and clinicopathological features of NSCLC and its prognostic value is relatively unclear. Thus a systematic review of published literatures was conducted to clarify the relationship between CSC marker CD133 and NSCLC cancer based on current evidences.
Methods
Literature search strategy
A comprehensive literature search of electronic databases PubMed, Embase and Wanfang was performed up to July 15, 2013. The following search terms were used: (CD133 or prominin or AC133) and (outcome or survival or prognosis) and (lung cancer or lung carcinoma or carcinoma of lung) and (Neoplastic Stem Cells or cancer stem cell or tumor-initiating cell). The citation lists associated with all the studies retrieved in the search were used to identify other potentially relevant publications. Review articles were also scanned to find additional eligible studies. The title and abstract of each study identified in the search was scanned to exclude any clearly irrelevant ones. The remaining articles were browsed to determine whether they contained information on the topic of interest.
Selection criteria
Diagnosis of NSCLC was proven by histopathological methods. Studies of CD133 expression based on primary lung cancer tissue (via either surgical or biopsy), rather than serum or any other kinds of specimen were included. All studies on the correlation of CD133 overexpression with clinicopathological markers and the association of CD133 overexpression on overall survival of NSCLC were included. There was no limitation on language as well as the minimum patients of every single study. When there were multiple articles by the same group based on similar patients and using same detection methods, only the largest or the most recently article was included.
Data extraction
Data tables were made to extract all relevant data from texts, tables and figures of each included studies, including author, publication year, patient’s country, tumor stage, number of patients, research technique used, cutoff value of CD133, clinicopathological features, positive rates of CD133 overexpression, as well as the expression-related survival. In case the prognosis was only plotted as Kaplan-Meier curve in some articles, the software GetData Graph Digitizer 2.24 (http://getdata-graph-digitizer.com/) was applied to digitize and extract the data.
Statistical analysis
ORs with 95% CI were used to evaluate the association between the stem cell markers CD133 and the clinicopathological features for lung cancer, including tumor grade and stage, tumor differentiation and lymph node status. The RR was used for assessing the association of CD133 and the survival outcome combined over studies. For those RRs that were not given directly in the published articles, the published data and figures from original papers were used to assess the RR according to the methods described by Parmar et al. [9] Heterogeneity across studies was evaluated with the Q test and P values. ORs and RRs were calculated by a random-effects model when the P value was less than 0.05. Otherwise, a fixed-effects model was used. The Begg and Egger funnel plot was used to assess publication bias. Statistical analyses were estimated using Review manager software 5.2 (updated in March 2012 by the Cochrane Collaboration). P values were two-sided, with significance at P<0.05.
Results
Description of studies
A total of 11 publications met the criteria for this analysis [10-20] (Figure 1). The total number of patients was 1004, ranging from 50 to 161 patients per study. Main characteristics of the eligible studies were summarized in Table 1. Eleven articles dealt with clinicopathological factors. Five studies determined with OS. Six studies only reported the association between SOX2 expression and clinicopathological factors without OS analysis. There was one kind of method used to evaluate CD133 expression in lung cancer specimens: immunohistochemistry (IHC). Table 1 show all the studies included in the meta-analysis in detail.
Figure 1.
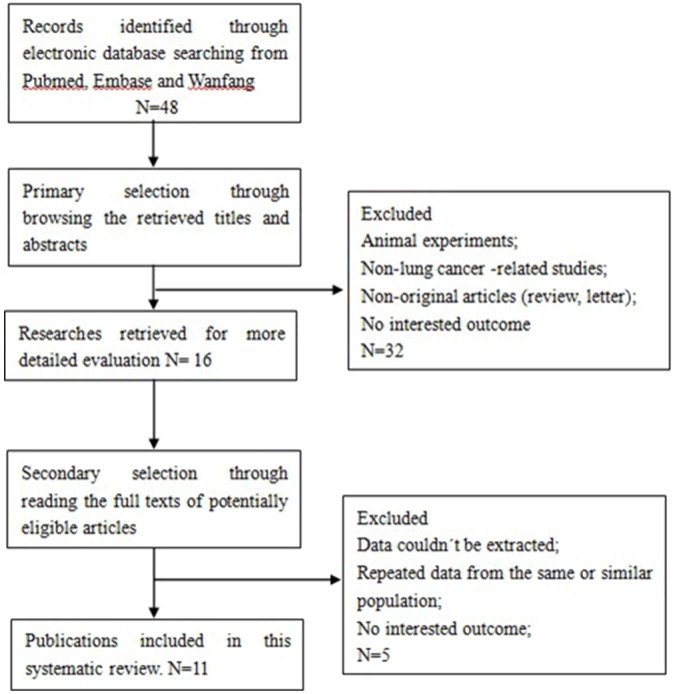
Literature search and selection of articles.
Table 1.
Main characteristics of the eligible studies
| Study | Patient’s country | Year | Tumor stage (UICC) | Technique | Number of patients | Cut off (IHC) |
|---|---|---|---|---|---|---|
| Zhang | China | 2007 | I–IV | IHC | 77 | >10% |
| Wei | China | 2008 | I–IV | IHC | 77 | >10% |
| Salnikov | Germany | 2009 | I–III | IHC | 88 | >20% |
| Tirino | Italy | 2009 | I–IV | IHC | 89 | ND |
| Xu | China | 2010 | I–IV | IHC | 102 | >10% |
| Chen | China | 2010 | I–IV | IHC | 65 | >10% |
| Ni | China | 2010 | I–IV | IHC | 50 | >10% |
| Li | China | 2011 | I | IHC | 145 | >1% |
| Sun | China | 2012 | I–IV | IHC | 67 | >10% |
| Wang | China | 2012 | I–IV | IHC | 83 | >10% |
| Mizugaki | Japan | 2013 | I–IV | IHC | 161 | moderate to strong staining intensity |
Correlation of stem cell markers with clinicopathological parameters
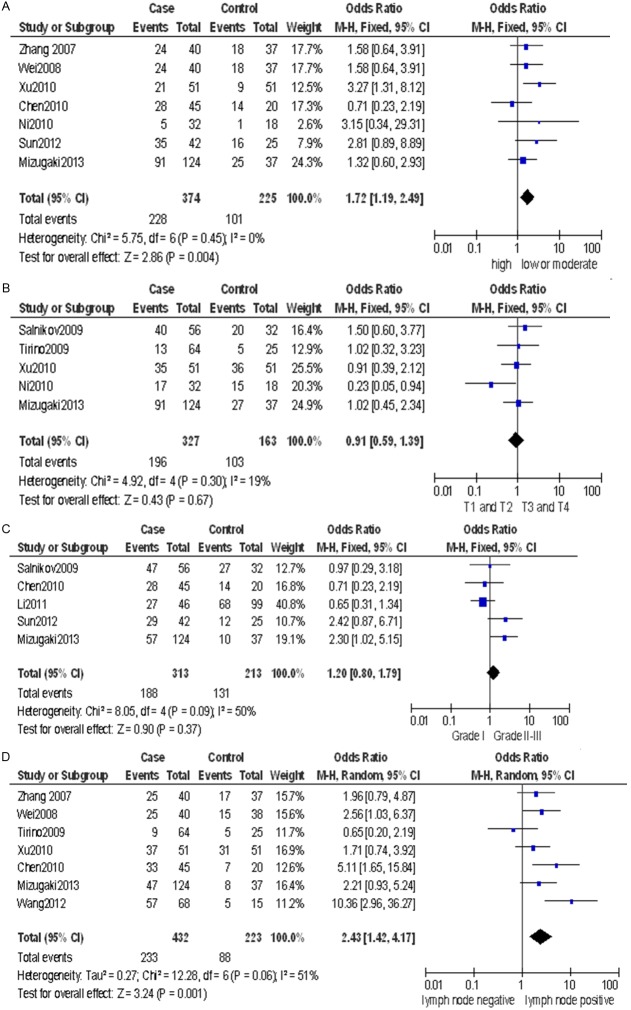
In the total analyses, the expression of stem cell markers was not associated with clinical parameters such as tumor T stage (pooled OR = 0.91, 95% CI: 0.59-1.39, P = 0.67 fixed-effect) or tumor grade (pooled OR = 1.20, 95% CI: 0.80-1.79, P = 0.37 fixed-effect) (Figure 2B, 2C). However, the expression of CD133 was associated with biologically aggressive phenotypes such as low tumor differentiation (pooled OR = 1.72, 95% CI: 1.19-2.49, P = 0.004 fixed-effect) and lymph node metastasis (pooled OR = 2.43, 95% CI: 1.42-4.17, P = 0.001 random-effect) (Figure 2A, 2D).
Figure 2.
Forest plot of OR was assessed for association between CD133 and clinical pathologic features, such as tumor differentiation (A), tumor T stage (B) tumor grade (C) lymph node status (D).
Impact of CD133 on OS of NSCLC
The meta-analysis was performed on five studies investigating the association of CD133 expression and OS. The pooled RR was calculated using the methods described above. CD133 expression (RR = 3.19, 95% CI: 2.05-4.98, P<0.0001 in the fixed-effect) was highly correlated with poor OS (Figure 3). This indicated that CD133 was independent prognostic factors in NSCLC.
Figure 3.
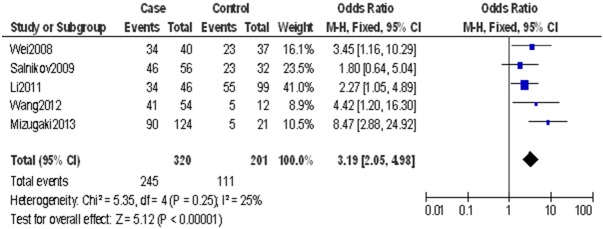
Forest plot of RR for OS among included studies. It shows the combined RR which is calculated by a fixed-effects mode, and it demonstrates that the CD133 can work as prognostic factor on OS in lung cancer patients.
Publication bias
The shapes of Begg’s funnel plots seemed to have no evidence of obviously asymmetrical in results of meta-analyses of CD133 expression for above clinicopathological parameters and 5-year OS (figures not shown), and the results of Egger’s test still suggest no evidence of publication bias (Table 2).
Table 2.
Egger’s test of funnel plot asymmetry
| Clinicopathological parameters | t value | df | P value |
|---|---|---|---|
| Degree of differentiation | 2.11 | 7 | 0.368 |
| tumor grade | 2.21 | 4 | 0.142 |
| Lymph node metastasis | 0.63 | 7 | 0.176 |
| T stage | 0.39 | 4 | 0.624 |
| overall survival | 0.21 | 4 | 1 |
Discussion
The present meta-analysis is the first study to systematically estimate the association between stem cell marker CD133 and NSCLC survival. The presence of both significant and non-significant studies addressing the importance of stem cells in NSCLC made it necessary to perform a quantitative aggregation of the survival results. The present results indicate that stem cell marker CD133 was significantly associated with tumor differentiation and lymph node metastasis, as well as OS. The results suggest that this marker could be developed for clinical applications.
CD133 is a Pentaspan, transmembrane protein that was first identified in mouse neuroepithelial stem cells [21] and later described in human hematopoietic stem cells [22]. Although its exact biological function remains unclear, CD133 is considered a putative stem cell marker in diverse hematopoietic and nonhematopoietic tissues and cancers [23]. In addition, it has been reported that the presence of CD133-positive cells compared with CD133-negative cells was associated with a significantly poorer prognosis in colorectal cancer, brain tumor, and gastric adenocarcinoma [7,8,24]. It is notable that this association is observed in our meta-analysis of CD133 phenotype and tumor differentiation and lymph node metastasis, as well as OS, suggesting that this marker can be developed for clinical applications.
For future studies, co-expression of lung cancer CSC markers associated with patient survival may be more meaningful for clinical application in lung cancer. Several studies have shown that CSC-related factors, including ALDH1 and CD44, are associated with lung cancer progression [25]. In addition, CSCs have major phenotypic and functional heterogeneity which may help distinguish them from cancer cells, and may be of potential benefit in the development of anti-cancer therapies to improve clinical outcomes.
To be sure, there were some potential limitations in this study. First, in prognostic factors meta-analyses, variability in definitions, outcomes, measurements, and experimental procedures might contribute to between-study heterogeneity [26]. In the current meta-analysis, despite the fact that we tried to optimize standardization, some remaining variability in definitions was unavoidable. Second, as reported above, potential publication bias was a concern. We restricted our review to articles published in English or Chinese language because other languages were not accessible to the readers. This selection could favor the positive studies that are more often published in English while the negative ones tend to be more often reported in native languages [27].
In summary, this meta-analysis indicated that CD133 expression was associated with common clinical parameters of NSCLC, such as tumor differentiation and lymph node metastasis. Moreover, positive CD133 expression was associated with a worse outcome than CD133-negative expression, and CD133 was an independent factor associated with reduced survival. Further studies of CD133 and its potential as a marker for lung cancer prognosis in clinic are warranted.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–43. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 3.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 4.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2006;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 5.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 6.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 7.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99:1285–9. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–9. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 9.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta - analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang HZ, Wei YP, Wang M, Wu C, Yang YQ, Chen J, Cao YK. Association of CD133 and endothelin-converting enzyme expressions with prognosis of non-small cell lung carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:696–9. [PubMed] [Google Scholar]
- 11.Wei YP, et al. Expression of Tumor Stem Cell Marker CDl33 in Non-small Cell Lung Carcinoma and Its Clinical Significance. Journal of Sun Yat-sen University (Medical Sciences) 2008;29:312–316. [Google Scholar]
- 12.Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non - small cell lung cancer patients. Int J Cancer. 2010;126:950–8. doi: 10.1002/ijc.24822. [DOI] [PubMed] [Google Scholar]
- 13.Tirino V, Camerlingo R, Franco R, Malanga D, La Rocca A, Viglietto G, Rocco G, Pirozzi G. The role of CD133 in the identification and characterisation of tumour-initiating cells in non-small-cell lung cancer. Eur J Cardiothorac Surg. 2009;36:446–53. doi: 10.1016/j.ejcts.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 14.Xu YH, Zhang GB, Wang JM, Hu HC. B7-H3 and CD133 expression in non-small cell lung cancer and correlation with clinicopathologic factors and prognosis. Saudi Med J. 2010;31:980–986. [PubMed] [Google Scholar]
- 15.Chen JR, et al. Expressions of CDl33 and CDl05 in lung cancer tissue and their clinical significance. Tumor. 2010;30:334–337. [Google Scholar]
- 16.Ni JY, et al. Expression and significance of OCT4, CD133 in non-small cell lung cancer. Journal of Clinical Medicine in Practice. 2012;16:19–22. [Google Scholar]
- 17.Li F, Zeng H, Ying K. The combination of stem cell markers CD133 and ABCG2 predicts relapse in stage I non-small cell lung carcinomas. Med Oncol. 2011;28:1458–62. doi: 10.1007/s12032-010-9646-5. [DOI] [PubMed] [Google Scholar]
- 18.Sun HY, et al. Expression and significance of CD133 and ALDH1 in non-small cell lung cancer. J Clin Exp Pathol. 2012;28:813–815. [Google Scholar]
- 19.Wang SG, Zeng ZY, Yang SS, Lin JS, Yuan Y. The study of CD133 expression in 83 cases of human lung adenocarcinoma cells. Journal of Cardiovascular and Pulmonary Diseases. 2012;31:727–729. [Google Scholar]
- 20.Mizugaki H, Sakakibara-Konishi J, Kikuchi J, Moriya J, Hatanaka KC, Kikuchi E, Kinoshita I, Oizumi S, Dosaka-Akita H, Matsuno Y, Nishimura M. CD133 expression: a potential prognostic marker for non-small cell lung cancers. Int J Clin Oncol. 2013:1–6. doi: 10.1007/s10147-013-0541-x. [DOI] [PubMed] [Google Scholar]
- 21.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94:12425–30. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 23.Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–50. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 24.Zhao P, Li Y, Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC Cancer. 2010;10:218. doi: 10.1186/1471-2407-10-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okudela K, Woo T, Mitsui H, Tajiri M, Masuda M, Ohashi K. Expression of the potential cancer stem cell markers, CD133, CD44, ALDH1, and β - catenin, in primary lung adenocarcinoma—their prognostic significance. Pathol Int. 2012;62:792–801. doi: 10.1111/pin.12019. [DOI] [PubMed] [Google Scholar]
- 26.Simon R, Altman DG. Statistical aspects of prognostic factor studies in oncology. Br J Cancer. 1994;69:979. doi: 10.1038/bjc.1994.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M, Zellweger-Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet. 1997;350:326–329. doi: 10.1016/S0140-6736(97)02419-7. [DOI] [PubMed] [Google Scholar]