Abstract
Since the discovery of the connection between ovarian hormones and breast cancer, endocrine therapy has been an integral adjuvant treatment for patients with hormone-dependent breast cancers. Oestrogen receptor (ER) plays a central role in mediating the effects of endogenous hormones and therapeutic agents. ER serves as a prognostic marker for responsiveness to endocrine therapy and is targeted either directly by selective oestrogen receptor modulators (SERMs) and pure antagonists or indirectly by aromatase inhibitors (AIs) that block oestrogen production. A significant number of ER-positive patients, however, fail to respond to therapy or develop resistance over time. This review focuses on the current understanding of ER functions and recent advances in genomic technologies and research that have provided a global perspective on hormone and ER activity and led to a number of significant discoveries, including the roles of co-regulatory factors and non-coding RNAs. Mechanistic insights into normal ER functions and therapeutic actions of SERMs and AIs will enable the development of better predictive markers and more effective target mechanisms and ultimately facilitate improvements in disease outcomes and patient survival.
Keywords: breast cancer, hormonal carcinogenesis, endocrine therapy, oestrogen receptor
Introduction
A lady with growth neoplastic
thought castration was just a bit drastic.
She preferred that her ill could be cured with a pill.
Today it’s no longer fantastic.
This quatrain, composed by Elwood Jensen and V. Craig Jordan, amusingly and succinctly summarises one of the great triumphs in breast cancer research and treatment [1]. In 1896, George Beatson reported the beneficial effects of oophorectomy, the female equivalent of castration, in two of his patients with inoperable breast cancer [2]. From his earlier studies of ovulation and lactation, Beatson astutely made the connection between ovarian functions and influences, subsequently shown to be the ovarian hormone oestrogen, with phenotypic changes in mammary tissues and possible link to cancer. He took the first steps in testing this hypothesis, and his seminal discovery provided the first evidence for hormonal carcinogenesis and the potential efficacy of targeting ovarian and hormonal functions. With contributions by Jensen, Jordan, and many others, endocrine therapy, using pills that block oestrogen production or activity, is now routinely applied in the treatment of breast cancer. Other examples of targeted therapy in breast cancer include the use of monoclonal antibodies (trastuzumab) and small molecule receptor tyrosine kinase inhibitors (lapatinib) in targeting the HER2/neu growth factor receptor-positive tumours [3]. This review focuses on the role and mechanisms of action of oestrogen receptors (ERs) in mediating the effects of oestrogen and endocrine therapeutic agents and discusses current challenges and opportunities in targeting ER and oestrogen signalling in the prevention and treatment of breast cancer.
Discovery and characterisation of ERs
Jensen and Jacobson were the first to observe the retention of radiolabelled oestrogen in hormone-responsive target tissues [4]. Subsequently, work by Jensen, Gorski, and their respective groups demonstrated the existence of intracellular oestrogen-binding receptor proteins [5–8]. The ER gene was cloned by the Chambon group, and mutagenesis studies showed that the receptor consists of a DNA-binding domain containing zinc finger motifs and a ligand-binding domain, key structural elements of ligand-dependent transcription factors [9, 10]. Functional studies also identified the N-terminal activating function (AF-1) domain, which is involved in protein–protein interactions important for the transcriptional activity of ER [11]. The identification of other related receptors places ER in the nuclear receptor superfamily of transcriptional regulators [12]. Molecular characterisation of ER revealed that, upon ligand activation, ER regulates target gene expression by binding cis-regulatory elements termed oestrogen response elements (EREs; consensus 5′-GGTCAnnnTGACC-3′). This interaction is facilitated by the pioneering factor FOXA1 [13]. ER can also bind DNA indirectly by tethering to other transcription factors, including AP-1, Sp1, NFκB, and RUNX1. DNA-bound ER nucleates co-regulator complexes that modify chromatin and render the DNA accessible to the transcriptional machinery [14, 15]. ER co-regulators include those that enhance transcriptional activity by altering nucleosome spatial orientation (SWI/SNF) or by modifying histones through acetylation (SRC1, CBP/p300, p/CAF, and p/CIP/AIB1) and methylation (CARM1, PRMT1) [16–25]. Some co-regulators such as NCoR, SMRT, NRIP1, LCoR, and REA function as nuclear receptor co-repressors and play key roles in modulating receptor activity [26–31]. The combination of interactions among ligand, ER, other transcription factors, ERE sequences, differential recruitment of co-regulators, and the overall allosteric effects on receptor complexes allows for an intricate pattern of gene- and tissue-specific effects on target gene expression. In addition to its nuclear functions, ER has been shown to exert rapid non-genomic effects through interactions with cell membrane-associated growth factor receptors and components of downstream signal transduction pathways in the cytoplasm [32]. Post-translational modifications of ER provide additional regulatory mechanisms and enable integration of signals from multiple pathways with oestrogen signalling [33].
Adding to the mechanistic complexity and refinement of oestrogen signalling, a second ER gene was discovered in 1996 by Gustafsson and Kuiper and was named ERβ [34]. The original ER was renamed ERα. ERα and ERβ share a 56% similarity in their ligand-binding domains, and both bind the predominant endogenous oestrogen 17β-estradiol. The differences in their ligand-binding domains, however, also result in selective binding of natural and synthetic ligands and allow for selective targeting of each receptor subtype. The two receptors have nearly identical DNA binding domains and share affinity for the canonical ERE. Studies of ERα-positive MCF7 breast cancer cells engineered to express ERβ have confirmed a substantial overlap of DNA-binding sites between the two receptors [35–37]. Intriguingly, their similarities in DNA binding resulted in different gene expression profiles with only a minority of ERβ-regulated genes also regulated by ERα [36, 38–41]. These functional differences may be due to the low conservation of their respective N-terminal AF-1 domains and their different abilities to interact with co-regulators [42]. When co-expressed, ERα and ERβ can function as both homodimers and heterodimers; these complexes appear to have their own transcriptional activities and regulate distinct gene sets [43, 44].
While both receptors are found in the normal breast, ERβ expression appears to be more widespread in mammary tissues [45, 46]. In both the rodent mammary gland and in the human normal breast, ERβ is found in epithelial and stromal cells, while ERα is only expressed in a subset of epithelial cells [46–48]. Nonetheless, ERα is the main mediator of the oestrogen-regulated ductal elongation and growth at puberty and during the menstrual cycle, although this is at least partly a systemic effect through the hypothalamic/pituitary axis [49, 50]. ERβ knockout mice have normal ductal and alveolar development [51], but ERβ is involved in the final terminal differentiation of the mammary gland [47].
ERα is upregulated in the majority of breast cancers, and its expression is a hallmark of hormone-dependent tumour growth. ERβ levels, in contrast, are decreased in tumour cells [52–57]. Whereas ERα is clearly linked to prognosis and response to endocrine therapy, there is no clear evidence that ERβ expression is linked to clinical parameters in breast cancer. This may be due to difficulties in accurately quantifying ERβ protein levels using existing reagents and techniques [58]. While oestrogen treatment of ERα-positive breast cancer cells stimulates proliferation, exogenously introduced ERβ in some studies suppresses ERα-induced proliferation and transcriptional activity while also inducing independent transcriptional and functional changes [40, 41, 59–62]. Related to these anti-proliferative effects, it has also been reported that ERβ-positive tumours may respond more favourably to tamoxifen, and ERβ agonist treatment of ERα-positive breast cancer cell lines appear to enhance their sensitivity to tamoxifen [63, 64]. Re-introduction of ERβ in more invasive ERα-negative breast cancers can, however, increase cell proliferation [65, 66]. The body of data correlating ERβ to both anti-proliferative and proliferative parameters suggests a bifurcated role for ERβ breast cancer biology, but the exact function of ERβ in tumourigenesis and disease progression remains to be determined [66].
Targeting ER and oestrogen signalling in breast cancer prevention and treatment
For several decades following Beatson’s initial published report, castration by surgical means or by irradiation was used to treat premenopausal women with recurrent or distant metastatic breast cancer. In some postmenopausal women, high doses of androgen or, paradoxically, the synthetic non-steroidal oestrogen diethylstilbestrol was effective in the treatment of advanced diseases [67–69]. Identification of ERα and the development of methodology to detect its expression by hormone binding assays in tumour samples enabled the clinical studies required that ERα be established as a prognostic marker for response to hormone therapy, and determining the ERα-status of tumour samples is now standard practice in clinical oncology [7].
The major breakthrough in targeting oestrogen signalling and ERα came from the development of non-steroidal anti-oestrogens using derivatives of triphenylethylenes by the pharmaceutical industry. The goal of these efforts was to develop anti-oestrogenic compounds that can be used in contraception. One compound, ICI 46, 474, had modest effects on fertility but showed promise as an anti-cancer agent with comparable effects with castration or hormone therapy [70]. This compound, later named tamoxifen, was shown to bind ERα, disrupt the binding of oestrogen, and block hormone-dependent breast cancer cell proliferation and tumour formation [71–73]. Following extensive pre-clinical and clinical studies, tamoxifen was approved for the treatment of ERα-positive breast cancers and for the prevention of breast cancer in high-risk individuals.
An early concern regarding the application of anti-oestrogens is their potential impact on the beneficial effects of oestrogen on bone density and cardioprotection. Interestingly, while blocking the effects of oestrogen in breast cancer cells, tamoxifen treatment actually improved bone density and reduced circulating levels of the harmful low-density lipoproteins. One of the negative effects of this selective action is that tamoxifen increases endometrial cell proliferation and risk for endometrial cancers [74]. Another non-steroidal anti-oestrogen candidate, keoxifene, later renamed raloxifene, was demonstrated to be effective in treating osteoporosis and was also approved for the prevention of breast cancer. Compared with tamoxifen, raloxifene does not have an effect on endometrial cell growth and proliferation. Tamoxifen and raloxifene are the first members of a class of drugs, termed selective oestrogen receptor modulators (SERMs). They exhibit both oestrogenic and anti-oestrogenic effects in a tissue-specific manner and raise the possibility of simultaneously targeting multiple endocrine-related diseases or conditions. An alternative approach for directly targeting ER in breast cancer treatment is through the use of pure anti-oestrogens. Fulvestrant, initially designated as ICI 182,780, is a steroidal compound with high affinity for ERα. In addition to blocking ER activity, treatment with fulvestrant also leads to the rapid degradation of ER proteins. Consequently, treatment completely disrupts ER activity, as compared with the SERMs. This drug is particularly effective as second-line treatment when tumour cells develop resistance to tamoxifen but still require ER for continuing proliferation [75].
As the role of oestrogen became apparent in hormonal carcinogenesis and disease progression in the majority of breast cancers, an alternative strategy for targeting oestrogen signalling and ER functions emerged. Aromatase is a key enzyme involved in the conversion of androgen to oestrogen by catalysing the aromatisation of the A ring in testosterone. Inhibition of aromatase activity indirectly targets ER functions by effectively starving hormone-dependent tumour cells of locally produced oestrogens. Steroidal (exemestane) and non-steroidal (anastrozole, letrozole) aromatase inhibitors (AIs) have been developed to selectively target aromatase enzymes. These compounds either bind and inactivate aromatase or compete with endogenous substrates to reduce oestrogen production. In clinical trials, AIs showed improved efficacy as compared with treatments with tamoxifen, and these drugs are now approved for use in the adjuvant therapy of postmenopausal patients with ER-positive tumours [76–78].
Challenges and opportunities
ERα protein level, as noted previously, is the major marker for potential response to endocrine therapy. Progesterone receptor (PR), an ERα target gene, expression is an additional marker for responsiveness. Not all tumours that are classified as ERα-positive, however, respond to treatments. Resistance to endocrine therapy is estimated at about 40% [79]. The evolutionary history and specific somatic mutations that gave rise to the primary tumours may have rendered them non-responsive prior to diagnosis and subsequent treatment. Moreover, the selective pressures of long-term endocrine treatment may drive the evolution of resistant tumour cells and recurrent tumours. Mechanisms of resistance to endocrine therapy include hypersensitivity to low levels of oestrogen following treatments with AIs, alternative activation of ERα via growth factor-mediated pathways and mechanisms, and complete oestrogen- and ERα-independent growth and proliferation of tumour cells [80]. Another challenge in the application of endocrine therapy is the treatment of premenopausal patients where disruption of hormone production and ER functions may be less effective and desirable and also introduces side effects, which may increase susceptibility to other diseases following long-term treatments [81]. In spite of the benefits of current endocrine therapeutic options, further scientific and technical breakthroughs are required to fully realise the potential of targeting endocrine-related mechanisms and reducing the morbidity and mortality associated with hormone-dependent breast cancers.
Advances in genomics and genomic technologies have contributed significantly to biomedical research in general and provided a number of mechanistic insights into ER biology in breast cancer cells. These insights have resulted in candidate markers and target mechanisms in endocrine therapy. For example, gene expression profiling studies using microarrays have identified hundreds of oestrogen responsive genes, both transcriptional targets as well as those downstream of ER-regulated signalling pathways, which can be exploited as both markers of oestrogen responsiveness in tumour cells and as targetable genes and gene networks, which specifically regulate tumour cell proliferation [82–84]. Comparative analysis of sensitive and resistant cells may further elucidate markers and mechanisms of resistance. Similar gene expression studies in clinical samples have identified gene sets and signatures that define clinical subtypes and predict response to endocrine therapy and may also suggest potential resistant mechanisms [85]. Genome-wide mapping studies of ER binding sites and computational modelling of sequence motifs have identified co-localising transcription factors such as FOXA1, GATA3, and AP-2γ that are required for ER transcriptional regulatory activity and represent additional candidate markers and therapeutic targets [13, 86, 87]. Improvements and innovations in proteomic technologies also contribute to our understanding of the ER complex, including associated co-regulators and transcription factors and may define potential markers and targets [88].
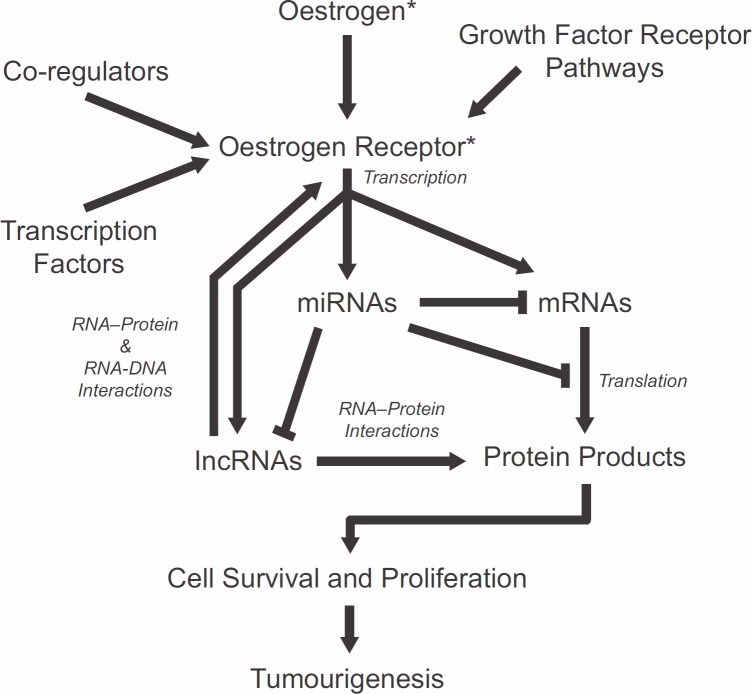
Genomic studies have also highlighted the emerging importance of non-coding RNAs in basic and translational research. Small microRNAs (miRNAs) serve as key regulators of gene expression by targeting genes for degradation or by blocking their translation. ERα-positive breast cancers display a distinct miRNA-expression profile compared with ERα-negative breast cancers [89–92]. Whether ERα directly regulates miRNA is not clear, but miRNA regulations are nonetheless likely to occur indirectly via other oestrogen-responsive genes or through ERα interaction with the miRNA processing machinery [93–95]. In addition, several miRNAs, including miR-206, have been shown to regulate ERα expression by targeting the 3′ untranslated region of its mRNA [96, 97]. Transcriptome-wide nuclear run-on studies have identified long non-coding RNAs (lncRNAs) as early targets of activated ER [98]. These transcripts share the same features as protein-coding RNAs such as capping, splice sites, and polyadenylation but encode extremely short open-reading frames. Functionally, lncRNAs participate in RNA–protein, RNA–RNA, and RNA–DNA interactions in molecular processes, including those that are involved in cancer-related functions [99, 100]. Recent report by Li and colleagues shows that a specific type of lncRNAs transcribed from enhancer regions of ER target genes and named enhancer RNAs, function in the looping of chromatin that facilitates interactions between distal regulatory sites with promoters of target genes [101]. Non-coding RNAs can be specifically targeted by complementary RNAs, and their expression and function disrupted by the cellular RNA interference mechanisms [102]. The rapid progress in understanding the roles of RNAs in oestrogen signalling and ER functions suggests the potential of applying RNA therapeutics, singly or in combination with existing chemo- and endocrine therapy drugs, in improving the specificity and efficacy of endocrine therapy in breast cancer prevention and treatment. Mechanisms of oestrogen signalling and ER action and potential markers and targets are summarised in Figure 1.
Figure 1. Summary of molecular interactions and mechanisms involved in oestrogen signalling and oestrogen receptor functions. Each component represents potential markers and target mechanisms for endocrine therapy. *Targets of current endocrine therapeutics.
Conclusion
Current successes in the treatment of hormone-dependent breast cancers still leave room for significant improvements in the specificity and efficacy of current endocrine therapeutic approaches and in overcoming resistant tumours. Accumulating insights regarding oestrogen signalling and mechanisms of action of ligands and ER provide opportunities for the development of novel markers, targets, and therapeutic strategies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Jensen E. A conversation with Elwood Jensen. Interview by David D. Moore. Annu Rev Physiol. 2012;74:1–11. doi: 10.1146/annurev-physiol-020911-153327. [DOI] [PubMed] [Google Scholar]
- 2.Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet. 1896;2:104–7. doi: 10.1016/S0140-6736(01)72307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen DL, Andersson M, Kamby C. HER2-targeted therapy in breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35(2):121–36. doi: 10.1016/j.ctrv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Jensen EV, Jacobson HI. Fate of steroid estrogens in target tissues. In: Pincus G, Vollmer EP, editors. Biological Activities of Steroids in Relation to Cancer. New York: Academic Press; 1960. pp. 161–74. [Google Scholar]
- 5.Jensen EV, Suzuki T, Kawashima T, Stumpf WE, Jungblut PW, DeSombre ER. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci USA. 1968;59(2):632–8. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci USA. 1966;55(6):1574–81. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toft D, Shyamala G, Gorski J. A receptor molecule for estrogens: studies using a cell-free system. Proc Natl Acad Sci USA. 1967;57(6):1740–3. doi: 10.1073/pnas.57.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Malley BW, Means AR. Female steroid hormones and target cell nuclei. Science. 1974;183(4125):610–20. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- 9.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320(6058):134–9. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Green S, Staub A, Chambon P. Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. Embo J. 1986;5(9):2231–6. doi: 10.1002/j.1460-2075.1986.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warnmark A, Treuter E, Wright AP, Gustafsson JA. Activation functions 1 and 2 of nuclear receptors: molecular strategies for transcriptional activation. Mol Endocrinol. 2003;17(10):1901–9. doi: 10.1210/me.2002-0384. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson S, Gustafsson JA. Estrogen receptor action. Crit Rev Eukaryot Gene Expr. 2002;12(4):237–57. doi: 10.1615/CritRevEukaryotGeneExpr.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- 13.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122(1):33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103(6):843–52. doi: 10.1016/S0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 15.Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, et al. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115(6):751–63. doi: 10.1016/S0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 16.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277(5328):965–8. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 17.Phelan ML, Sif S, Narlikar GJ, Kingston RE. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol Cell. 1999;3(2):247–53. doi: 10.1016/S1097-2765(00)80315-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Cote J, Xue Y, Zhou S, Khavari PA, Biggar SR, et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. Embo J. 1996;15(19):5370–82. [PMC free article] [PubMed] [Google Scholar]
- 19.Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994;264(5164):1455–8. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- 20.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87(5):953–9. doi: 10.1016/S0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 21.Onate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270(5240):1354–7. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 22.Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64(2):435–59. doi: 10.1128/MMBR.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, et al. Regulation of transcription by a protein methyltransferase. Science. 1999;284(5423):2174–7. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 24.Lin WJ, Gary JD, Yang MC, Clarke S, Herschman HR. The mammalian immediate-early TIS21 protein and the leukemia-associated BTG1 protein interact with a protein-arginine N-methyltransferase. J Biol Chem. 1996;271(25):15034–44. doi: 10.1074/jbc.271.25.15034. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Huang ZQ, Xia L, Feng Q, Erdjument-Bromage H, Strahl BD, et al. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science. 2001;293(5531):853–7. doi: 10.1126/science.1060781. [DOI] [PubMed] [Google Scholar]
- 26.Treuter E, Albrektsen T, Johansson L, Leers J, Gustafsson JA. A regulatory role for RIP140 in nuclear receptor activation. Mol Endocrinol. 1998;12(6):864–81. doi: 10.1210/me.12.6.864. [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Lazar MA. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402(6757):93–6. doi: 10.1038/47069. [DOI] [PubMed] [Google Scholar]
- 28.Webb P, Anderson CM, Valentine C, Nguyen P, Marimuthu A, West BL, et al. The nuclear receptor corepressor (N-CoR) contains three isoleucine motifs (I/LXXII) that serve as receptor interaction domains (IDs) Mol Endocrinol. 2000;14(12):1976–85. doi: 10.1210/me.14.12.1976. [DOI] [PubMed] [Google Scholar]
- 29.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377(6548):454–7. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes I, Bastien Y, Wai T, Nygard K, Lin R, Cormier O, et al. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol Cell. 2003;11(1):139–50. doi: 10.1016/S1097-2765(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 31.Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc Natl Acad Sci USA. 1999;96(12):6947–52. doi: 10.1073/pnas.96.12.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin ER, Pietras RJ. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res Treat. 2008;108(3):351–61. doi: 10.1007/s10549-007-9618-4. [DOI] [PubMed] [Google Scholar]
- 33.Anbalagan M, Huderson B, Murphy L, Rowan BG. Post-translational modifications of nuclear receptors and human disease. Nucl Recept Signal. 2012;10:e001. doi: 10.1621/nrs.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93(12):5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao C, Gao H, Liu Y, Papoutsi Z, Jaffrey S, Gustafsson JA, et al. Genome-wide mapping of estrogen receptor-beta-binding regions reveals extensive cross-talk with transcription factor activator protein-1. Cancer Res. 2010;70:5174–83. doi: 10.1158/0008-5472.CAN-09-4407. [DOI] [PubMed] [Google Scholar]
- 36.Grober OM, Mutarelli M, Giurato G, Ravo M, Cicatiello L, De Filippo MR, et al. Global analysis of estrogen receptor beta binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor alpha for target gene regulation. BMC Genomics. 2011;12:36. doi: 10.1186/1471-2164-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS. Genome-wide dynamics of chromatin binding of estrogen receptors alpha and beta: mutual restriction and competitive site selection. Mol Endocrinol. 2010;24:47–59. doi: 10.1210/me.2009-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tee MK, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, et al. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell. 2004;15:1262–72. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) {alpha} or ER{beta} in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology. 2004;145(7):3473–86. doi: 10.1210/en.2003-1682. [DOI] [PubMed] [Google Scholar]
- 40.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of Estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147(10):4831–42. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 41.Williams C, Edvardsson K, Lewandowski SA, Strom A, Gustafsson J-A. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2008;27:1019–32. doi: 10.1038/sj.onc.1210712. [DOI] [PubMed] [Google Scholar]
- 42.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392(1):49–53. doi: 10.1016/0014-5793(96)00782-X. [DOI] [PubMed] [Google Scholar]
- 43.Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S, Spelsberg TC. Estrogen receptor {alpha} and {beta} heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol. 2005;19(6):1555–68. doi: 10.1210/me.2004-0381. [DOI] [PubMed] [Google Scholar]
- 44.Papoutsi Z, Zhao C, Putnik M, Gustafsson J, Dahlman-Wright K. Binding of estrogen receptor alpha/beta heterodimers to chromatin in MCF-7 cells. J Mol Endocrinol. 2009;43(2):65–72. doi: 10.1677/JME-08-0177. [DOI] [PubMed] [Google Scholar]
- 45.Speirs V, Skliris GP, Burdall SE, Carder PJ. Distinct expression patterns of ER alpha and ER beta in normal human mammary gland. J Clin Pathol. 2002;55(5):371–4. doi: 10.1136/jcp.55.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S, Han B, Liu G, Ouellet J, Labrie F, Pelletier G. Immunocytochemical localization of sex steroid hormone receptors in normal human mammary gland. J Histochem Cytochem. 2010;58:509–15. doi: 10.1369/jhc.2009.954644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng G, Weihua Z, Warner M, Gustafsson J-A. Inaugural article: estrogen receptors ER{alpha} and ER{beta} in proliferation in the rodent mammary gland. PNAS. 2004;101(11):3739–46. doi: 10.1073/pnas.0307864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmieri C, Saji S, Sakaguchi H, Cheng G, Sunters A, O’Hare MJ, et al. The expression of ERb and its variants, but not ERa, in adult human mammary fibroblasts. J Mol Endocrinol. 2004;33(1):35–50. doi: 10.1677/jme.0.0330035. [DOI] [PubMed] [Google Scholar]
- 49.Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, et al. Induction of mammary gland development in estrogen receptor-a knockout mice. Endocrinology. 2000;141:2982–94. doi: 10.1210/en.141.8.2982. [DOI] [PubMed] [Google Scholar]
- 50.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1:467–75. doi: 10.1016/S1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 51.Forster C, Makela S, Warri A, Kietz S, Becker D, Hultenby K, et al. Involvement of estrogen receptor beta in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci USA. 2002;99:15578–83. doi: 10.1073/pnas.192561299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roger P, Sahla ME, Mäkelä S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61(6):2537–41. [PubMed] [Google Scholar]
- 53.Palmieri C, Cheng G, Saji S, Zelada-Hedman M, Warri A, Weihua Z, et al. Estrogen receptor beta in breast cancer. Endocr Relat Cancer. 2002;9(1):1–13. doi: 10.1677/erc.0.0090001. [DOI] [PubMed] [Google Scholar]
- 54.Shaaban AM, O’Neill PA, Davies MP, Sibson R, West CR, Smith PH, et al. Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am J Surg Pathol. 2003;27:1502–12. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Saji S, Hirose M, Toi M. Clinical significance of estrogen receptor beta in breast cancer. Cancer Chemother Pharmacol. 2005;56:21–6. doi: 10.1007/s00280-005-0107-3. [DOI] [PubMed] [Google Scholar]
- 57.Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen receptor beta: an overview and update. Nucl Recept Signal. 2008;6(e003) doi: 10.1621/nrs.06003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haldosen LA, Zhao C, Dahlman-Wright K. Estrogen receptor beta in breast cancer. Mol Cell Endocrinol. 2013 doi: 10.1016/j.mce.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–10. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 60.Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson J-A. Estrogen receptor {beta} inhibits 17{beta}-estradiol-stimulated proliferation of the breast cancer cell line T47D. PNAS. 2004;101(6):1566–71. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartman J, Lindberg K, Morani A, Inzunza J, Strom A, Gustafsson J-A. Estrogen receptor {beta} inhibits angiogenesis and growth of T47D Breast cancer xenografts. Cancer Res. 2006;66(23):11207–13. doi: 10.1158/0008-5472.CAN-06-0017. [DOI] [PubMed] [Google Scholar]
- 62.Matthews J, Wihlen B, Tujague M, Wan J, Strom A, Gustafsson J-A. Estrogen receptor (er) {beta} modulates er{alpha}-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol. 2006;20(3):534–43. doi: 10.1210/me.2005-0140. [DOI] [PubMed] [Google Scholar]
- 63.Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, et al. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26(22):3727–34. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 64.Lattrich C, Schuler S, Haring J, Skrzypczak M, Ortmann O, Treeck O. Effects of a combined treatment with tamoxifen and estrogen receptor beta agonists on human breast cancer cell lines. Arch Gynecol Obstet. 2013 doi: 10.1007/s00404-013-2977-7. [DOI] [PubMed] [Google Scholar]
- 65.Tonetti DA, Rubenstein R, DeLeon M, Zhao H, Pappas SG, Bentrem DJ, et al. Stable transfection of an estrogen receptor beta cDNA isoform into MDA-MB-231 breast cancer cells. J Steroid Biochem Mol Biol. 2003;87(1):47–55. doi: 10.1016/j.jsbmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 66.Leygue E, Murphy LC. A bi-faceted role of estrogen receptor beta in breast cancer. Endocr Relat Cancer. 2013;20(3):R127–39. doi: 10.1530/ERC-12-0389. [DOI] [PubMed] [Google Scholar]
- 67.Kennedy BJ. Hormone therapy for advanced breast cancer. Cancer. 1965;18(12):1551–7. doi: 10.1002/1097-0142(196512)18:12<1551::AID-CNCR2820181206>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 68.Haddow A, Watkinson JM, Paterson E, Koller PC. Influence of synthetic oestrogens on advanced malignant disease. Br Med J. 1944;2(4368):393–8. doi: 10.1136/bmj.2.4368.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peethambaram PP, Ingle JN, Suman VJ, Hartmann LC, Loprinzi CL. Randomized trial of diethylstilbestrol vs. tamoxifen in postmenopausal women with metastatic breast cancer. An updated analysis. Breast Cancer Res Treat. 1999;54(2):117–22. doi: 10.1023/A:1006185805079. [DOI] [PubMed] [Google Scholar]
- 70.Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25(2):270–5. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gottardis MM, Robinson SP, Jordan VC. Estradiol-stimulated growth of MCF-7 tumors implanted in athymic mice: a model to study the tumoristatic action of tamoxifen. J Steroid Biochem. 1988;30(1–6):311–4. doi: 10.1016/0022-4731(88)90113-6. [DOI] [PubMed] [Google Scholar]
- 72.Jordan VC, Koerner S. Inhibition of oestradiol binding to mouse uterine and vaginal oestrogen receptors by triphenylethylenes. J Endocrinol. 1975;64(1):193–4. doi: 10.1677/joe.0.0640193. [DOI] [PubMed] [Google Scholar]
- 73.Lippman ME, Bolan G. Oestrogen-responsive human breast cancer in long term tissue culture. Nature. 1975;256(5518):592–3. doi: 10.1038/256592a0. [DOI] [PubMed] [Google Scholar]
- 74.Jordan VC, Gottardis MM, Satyaswaroop PG. Tamoxifen-stimulated growth of human endometrial carcinoma. Ann NY Acad Sci. 1991;622:439–46. doi: 10.1111/j.1749-6632.1991.tb37886.x. [DOI] [PubMed] [Google Scholar]
- 75.Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–95. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 76.Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–9. doi: 10.1016/S0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 77.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350(11):1081–92. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 78.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 79.Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, et al. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22(47):7316–39. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 80.Normanno N, Di Maio M, De Maio E, De Luca A, de Matteis A, Giordano A, et al. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr Relat Cancer. 2005;12(4):721–47. doi: 10.1677/erc.1.00857. [DOI] [PubMed] [Google Scholar]
- 81.Emens LA, Davidson NE. Adjuvant hormonal therapy for premenopausal women with breast cancer. Clin Cancer Res. 2003;9(1 Pt 2):486S–94S. [PubMed] [Google Scholar]
- 82.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144(10):4562–74. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 83.Lin CY, Strom A, Vega VB, Kong SL, Yeo AL, Thomsen JS, et al. Discovery of estrogen receptor alpha target genes and response elements in breast tumor cells. Genome Biol. 2004;5(9):R66. doi: 10.1186/gb-2004-5-9-r66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alvarez-Baron CP, Jonsson P, Thomas C, Dryer SE, Williams C. The two-pore domain potassium channel KCNK5: induction by estrogen receptor alpha and role in proliferation of breast cancer cells. Mol Endocrinol. 2011;25(8):1326–36. doi: 10.1210/me.2011-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loi S, Piccart M, Sotiriou C. The use of gene-expression profiling to better understand the clinical heterogeneity of estrogen receptor positive breast cancers and tamoxifen response. Crit Rev Oncol Hematol. 2007;61(3):187–94. doi: 10.1016/j.critrevonc.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Kong SL, Li G, Loh SL, Sung WK, Liu ET. Cellular reprogramming by the conjoint action of ERalpha, FOXA1, and GATA3 to a ligand-inducible growth state. Mol Syst Biol. 2011;7:526. doi: 10.1038/msb.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tan SK, Lin ZH, Chang CW, Varang V, Chng KR, Pan YF, et al. AP-2gamma regulates oestrogen receptor-mediated long-range chromatin interaction and gene transcription. Embo J. 2011;30(13):2569–81. doi: 10.1038/emboj.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mohammed H, D’Santos C, Serandour AA, Ali HR, Brown GD, Atkins A, et al. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 2013;3(2):342–9. doi: 10.1016/j.celrep.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhat-Nakshatri P, Wang G, Collins NR, Thomson MJ, Geistlinger TR, Carroll JS, et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009;37(14):4850–61. doi: 10.1093/nar/gkp500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8(10):R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 92.Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, et al. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer. 2006;5:24. doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katchy A, Edvardsson K, Aydogdu E, Williams C. Estradiol-activated estrogen receptor alpha does not regulate mature microRNAs in T47D breast cancer cells. J Steroid Biochem Mol Biol. 2012;128(3–5):145–53. doi: 10.1016/j.jsbmb.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 94.Klinge CM. miRNAs and estrogen action. Trends Endocrinol Metab. 2012;23(5):223–33. doi: 10.1016/j.tem.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamagata K, Fujiyama S, Ito S, Ueda T, Murata T, Naitou M, et al. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol Cell. 2009;36(2):340–7. doi: 10.1016/j.molcel.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 96.Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21(5):1132–47. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- 97.Zhao JJ, Lin J, Yang H, Kong W, He L, Ma X, et al. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008;283(45):31079–86. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Hah N, Danko CG, Core L, Waterfall JJ, Siepel A, Lis JT, et al. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell. 2011;145(4):622–34. doi: 10.1016/j.cell.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–41. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 100.Lipovich L, Johnson R, Lin CY. MacroRNA underdogs in a microRNA world: evolutionary, regulatory, and biomedical significance of mammalian long non-protein-coding RNA. Biochim Biophys Acta. 2010;1799(9):597–615. doi: 10.1016/j.bbagrm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 101.Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498(7455):516–20. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1(5):391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]