Abstract
OBJECTIVE
To assess the association of hypoglycemic treatment regimens with cardiovascular adverse events and mortality in a large population of type 2 diabetic patients at increased cardiovascular risk.
RESEARCH DESIGN AND METHODS
This analysis included 8,192 overweight patients with type 2 diabetes from the Sibutramine Cardiovascular Outcomes (SCOUT) trial randomized to lifestyle intervention with or without sibutramine for up to 6 years. Patients were grouped according to hypoglycemic treatment at baseline. The primary end point was the time from randomization to the first occurrence of a primary outcome event (POE), nonfatal myocardial infarction, nonfatal stroke, resuscitation after cardiac arrest, or cardiovascular death. Multivariable Cox proportional hazards regression models were used to assess the impact of antiglycemic treatment on POE and all-cause mortality.
RESULTS
Treatments for type 2 diabetes were as follows: diet alone (n = 1,394 subjects), metformin monotherapy (n = 1,631), insulin monotherapy (n = 1,116), sulfonylurea monotherapy (n = 1,083), metformin plus sulfonylurea (n = 1,565), and metformin plus insulin (n = 1,000); 905 subjects experienced a POE and 708 died. Metformin monotherapy was associated with lower risk of POE than insulin (hazard ratio [HR], 0.74; 95% CI, 0.57–0.95; P = 0.02). Diet alone also was associated with lower risk of POE (HR, 0.65; 95% CI, 0.48–0.87; P = 0.004). Metformin monotherapy also was associated with lower mortality (HR, 0.73; 95% CI, 0.54–0.99; P < 0.05), whereas no other monotherapies or combination therapies were significantly associated with POE or all-cause mortality compared with insulin as monotherapy.
CONCLUSIONS
In obese patients with type 2 diabetes and high risk of cardiovascular disease, monotherapy with metformin or diet-only treatment was associated with lower risk of cardiovascular events than treatment with insulin.
The prevalence of type 2 diabetes and obesity/overweight are increasing at a disturbing rate in the western world as well as in developing countries (1,2). Both diabetes and obesity have a profound effect on the risk of cardiovascular disease (CVD) (3).
Although CVD-related mortality among patients with type 2 diabetes has been decreasing over the past few decades, these patients remain at significantly (twofold to threefold) higher risk for CVD-related mortality relative to comparable groups without diabetes (4). The influence of differing hypoglycemic treatment regimens on CVD has been of increasing clinical concern (5,6). To date, only a few randomized studies have addressed the impact of different hypoglycemic treatment regimens on the outcome of cardiovascular events and cardiovascular death (7). Mostly, clinical trials examining the efficacy of various antidiabetes drugs have focused on intermediate clinical outcomes such as changes in levels of HbA1c, serum lipids, and blood pressure (8). Several studies suggest that improved glycemic control in type 2 diabetes reduces microvascular risk (7,9). However it remains unclear whether there is a specific effect of different hypoglycemic agents on “hard” clinical outcomes from macrovascular disease and all-cause mortality. Because macrovascular disease is the leading cause of morbidity and mortality in type 2 diabetes (10), it is important to explore whether any association exists between conventional approaches to hypoglycemic therapy and cardiovascular events in patients with type 2 diabetes.
Therefore, we have examined data from the Sibutramine Cardiovascular Outcomes (SCOUT) trial (see list of participating investigators in the Supplementary Data) conducted in >10,000 overweight and obese subjects to explore possible links between hypoglycemic treatment regimens and cardiovascular events. SCOUT demonstrated that long-term treatment with sibutramine had a slightly increased risk of nonfatal myocardial infarction (hazard ratio [HR], 1.28; 95% CI, 1.04–1.57; P = 0.02) and nonfatal stroke (HR, 1.36; 95% CI, 1.04–1.77; P = 0.03), but not of cardiovascular death or death from any cause, among subjects at high cardiovascular risk. It has not been established, however, whether the type of therapy for diabetes affected the outcome.
RESEARCH DESIGN AND METHODS
SCOUT was a randomized, double-blinded, placebo-controlled, multicenter trial conducted in 300 centers in 16 countries worldwide. The protocol has been described elsewhere (11). In brief, SCOUT tested whether sibutramine (a norepinephrine and serotonin reuptake inhibitor previously approved for weight management in individuals without history of CVD or diabetes) could safely and effectively reduce the burden of cardiovascular outcomes in high-risk overweight or obese patients with preexisting CVD, type 2 diabetes, or both (CV-DM).
Eligible subjects were 55 years of age or older, with BMI of 27–45 kg/m2; subjects also were eligible if BMI was at least 25 but <27 and had a waist circumference of at least 102 cm for men and 88 cm for women. Enrolled subjects had history of CVD (previous myocardial infarction, previous coronary revascularization, or otherwise proven atherosclerotic disease such as peripheral arterial occlusive disease or stroke) or type 2 diabetes (or both) with an additional cardiovascular risk factor (hypertension, hyperlipidemia, current smoking, or diabetic nephropathy).
Exclusion criteria were symptoms of heart failure greater than New York Heart Association functional class II, blood pressure >160/100 mmHg, pulse ≥100 bpm, scheduled cardiac surgery or coronary angioplasty, or weight loss of >3 kg within the previous 3 months.
Lower than expected overall primary outcome event (POE) rate enforced an adjustment to inclusion criteria after 15 months, restricting recruitment of CV-DM subjects to the highest cardiovascular risk group. As a consequence, the CV-DM group constituted 60% of the overall study group (11).
For safety reasons (to identify subjects with early and persistent increases in blood pressure or pulse, or both), all subjects underwent a 6-week lead-in period with single blinding (of the subject) in which 10 mg sibutramine was administered together with advice on diet and exercise. Patients eligible after this single-blind phase were randomly assigned to sibutramine 10 mg daily or placebo in a 1:1 ratio.
Patients had a physical examination performed at the initial screening and at every follow-up visit when information on body weight, vital signs, blood biochemistry, and hematology were obtained. This occurred on a monthly basis for the first 3 months and every 3 months thereafter for subjects using study medication, or annually after subjects had discontinued study medication. During follow-up, investigators could modify a subject's medication at any time after randomization baseline; they were permitted to add another class or to change drug class to achieve optimum management of all medical conditions. These changes were recorded.
For the present analysis, we evaluated the cohort of patients with type 2 diabetes (DM only and CV-DM) who had complete data for glucose-lowering treatment regimens at randomization baseline. We assessed the relative risks of a POE, defined as the time from randomization to the first occurrence of nonfatal myocardial infarction, nonfatal stroke, resuscitation after cardiac arrest, or cardiovascular death. Death from any cause was a secondary outcome.
Ethics
All participating patients gave informed written consent before participation. All relevant approvals were obtained from relevant ethical committees and the study was performed in compliance with the Declaration of Helsinki.
Statistical analysis
The population was grouped according to baseline use of the following hypoglycemic therapies: insulin as monotherapy, metformin as monotherapy, sulfonylureas as monotherapy, diet only, sulfonylureas plus insulin, sulfonylureas plus metformin, sulfonylureas plus metformin plus insulin, and “other” (comprising a heterogeneous mix of agents including thiazolidinediones). Insulin monotherapy was used as the reference in comparative analyses. This was chosen because the insulin treatment group was large and because, of all the hypoglycemic agents, insulin was considered to have the least direct influence on cardiovascular risk itself (i.e., metformin is considered to have beneficial effects on cardiovascular risk profile and sulfonylureas are considered to be potentially harmful). Furthermore, analyses of the risks associated with overall use compared with no use of metformin, sulfonylureas, and insulin were performed as sensitivity analyses.
Continuous variables are presented as means (±SD) and discrete variables are presented as percentages. Unadjusted time-to-event curves were generated using the Kaplan-Meier method and groups were compared by the log-rank test. The Cox proportional hazards models were used to calculate HRs with 95% CIs. Models were adjusted for the following covariates: age, smoking habits, diabetes duration, congestive heart failure, history of hypertension, BMI, sex, history of CVD, tobacco use, HDL concentrations, LDL concentrations, HbA1c levels, heart rate, actual systolic and diastolic blood pressure values, and sibutramine usage. Additional time-dependent Cox analyses were performed to adjust for changes in BMI, HbA1c values, and hypoglycemic therapy during follow-up. All statistical analyses were performed with the use of SAS software (version 9.2; Cary, NC). All statistical tests were two-tailed. P < 0.05 was considered to indicate statistical significance. No statistical imputation was performed for missing data.
RESULTS
Characteristics at baseline
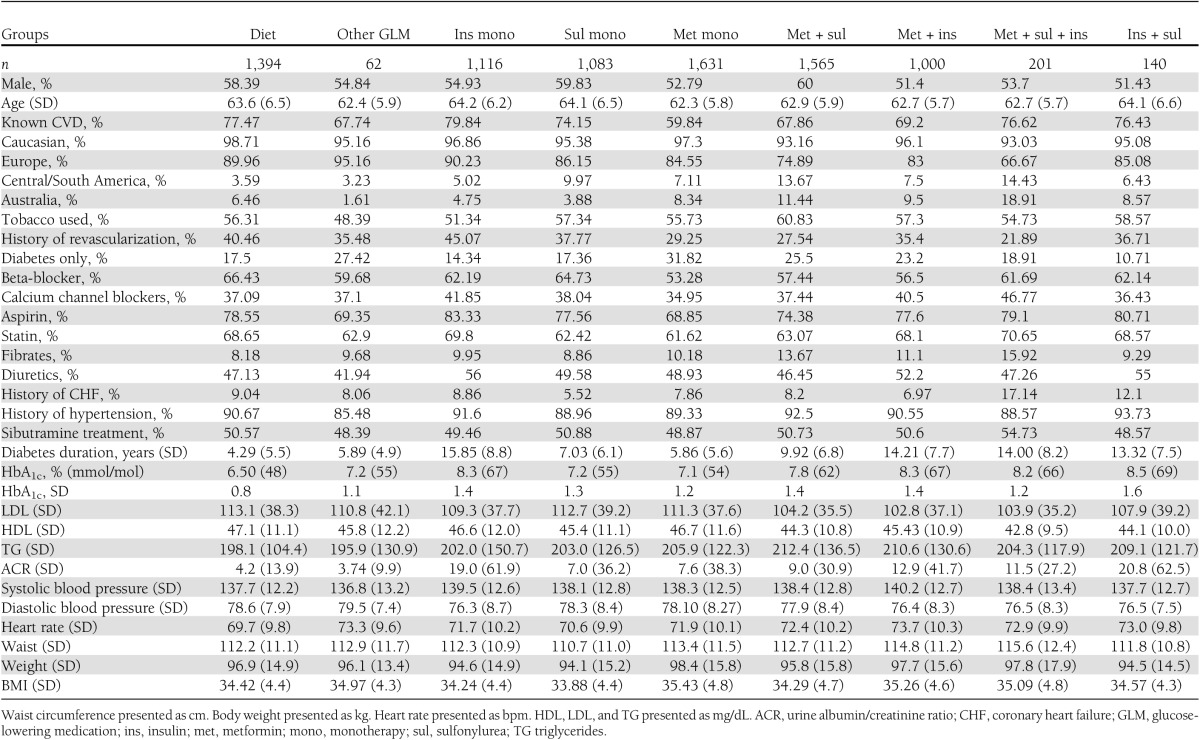
Of the 10,744 included in the lead-in period, 9,804 were randomly assigned to sibutramine or placebo. Of these, 8,192 (84%) had preexisting type 2 diabetes and had data available regarding previous glucose-lowering treatment regimens and comprised the study population. Overall, 96% were Caucasian, 55% were male, mean age was 63.2 years, and mean BMI was 34.8 kg/m2. More than 75% had history of CVD, and ∼34% had history of revascularization. Tobacco was or had been used by 56%.
Patients using insulin or several glucose-lowering agents had higher HbA1c levels and longer duration of diabetes compared with those using diet-only therapy or metformin monotherapy. Table 1 shows further baseline characteristics.
Table 1.
Baseline characteristics according to glucose-lowering medication
Primary outcome event
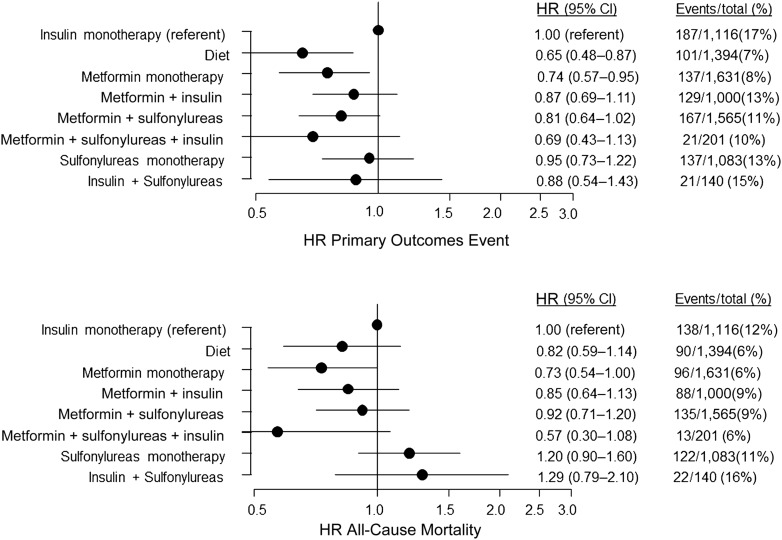
There were 905 (11%) POEs. The POEs by treatment groups are shown in Fig. 1 together with the secondary end points.
Figure 1.
HRs and number of events for POE (upper) and all-cause mortality (lower). HRs for POE (nonfatal myocardial infarction, nonfatal stroke, resuscitation after cardiac arrest, and cardiovascular death) and all-cause mortality are shown by different treatment groups compared with insulin monotherapy as reference. Events per treatment group divided by number of subjects in individual treatment group are given as percentage (%).
The association between hypoglycemic treatment regimen and cardiovascular events from a multivariable-adjusted model using insulin treatment as the reference is summarized in Fig. 1.
Age, chronic heart failure, baseline heart rate, arterial hypertension, baseline HbA1c, and male sex all were associated with an increased (P < 0.005) event rate. Metformin monotherapy (HR, 0.74; 95% CI, 0.57–0.95; P = 0.02) and diet alone (HR, 0.65; 95% CI, 0.48–0.87; P = 0.004) were associated with lower POE rates compared with insulin monotherapy. The combination therapy metformin–sulfonylurea was weakly linked with a lower and statistically nonsignificant POE rate (adjusted HR, 0.81; 95% CI, 0.64–1.015; P = 0.07).
All-cause mortality
During follow-up, 708 individuals died. When adjusted for other variables, only metformin monotherapy (HR, 0.73; 95% CI, 0.54–1.00; P < 0.05) was associated with reduced mortality (Fig. 1).
Neither the combination of metformin–sulfonylurea (HR, 0.90; 95% CI, 0.69–1.17; P = 0.44) nor the other monotherapies or combination therapies showed a significant association with all-cause mortality. There was a weak trend for the combination of metformin, sulfonylurea, and insulin to be associated with lower all-cause mortality (HR, 0.57; 95% CI, 0.30–1.08; P = 0.08).
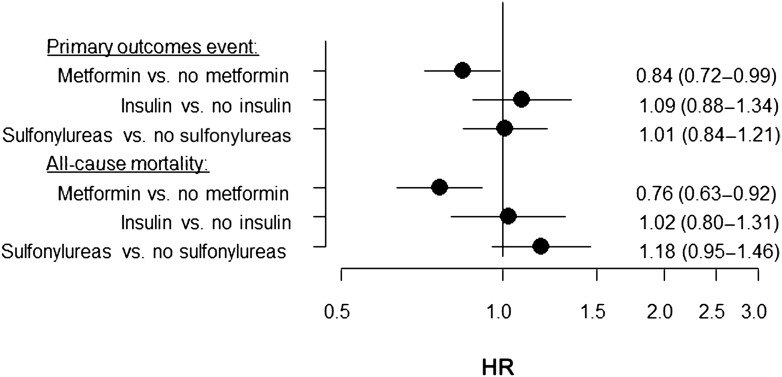
Analyses of risks associated with overall use of metformin, sulfonylureas, and insulin, compared with no use of each specific agent, showed beneficial outcomes associated with use of metformin (compared with no use of metformin) for both end points and showed neutral outcomes associated with insulin (compared with no use of insulin) and sulfonylureas (compared with no use of sulfonylureas) for both end points (Fig. 2). The effects associated with metformin, sulfonylureas, or insulin were not modified by concomitant use of other agents (all P for tests for interactions > 0.1), HbA1c levels (P > 0.4 for all agents), or diabetes duration (P > 0.1 for all agents) for both end points.
Figure 2.
HRs for POE (nonfatal myocardial infarction, nonfatal stroke, resuscitation after cardiac arrest, and cardiovascular death) and all-cause mortality by overall use of metformin, sulfonylureas, and insulin compared with no use of the specific agent.
Time-dependent analyses
Analyses adjusting for changes in BMI and HbA1c over time are shown in Table 2. These analyses showed similar associations as the main analyses.
Table 2.
POE and all-cause mortality adjusted for changes in BMI and HbA1c during follow-up
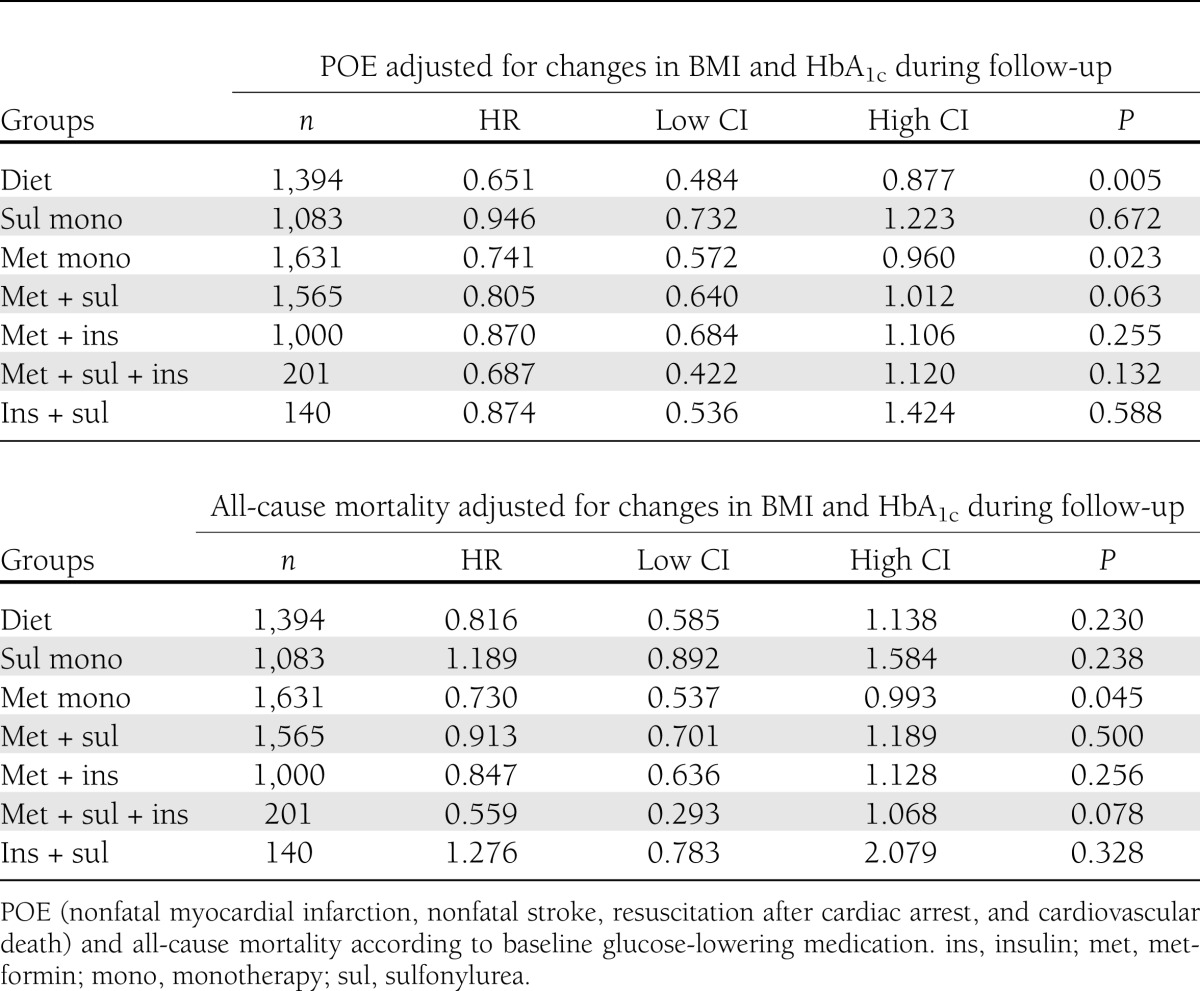
Treatment changes were made as follows: 522 subjects had addition of metformin, 358 had addition of sulfonylureas, 305 had addition of insulin, and 263 were assigned to other glucose-lowering medication during follow-up. The diet-only group (HR, 0.66; 95% CI, 0.49 – 0.88; P = 0.005) and metformin-only group (HR 0.74, 95% CI 0.58 – 0.96; P = 0.02) continued to be significantly associated with lower POE compared with insulin monotherapy when controlled for changes in treatment class during follow-up. However, neither modality was significantly associated with improved all-cause mortality (results not shown). For all analyses, there was no significant interaction between use of sibutramine and glucose-lowering medications (P > 0.05 for all).
CONCLUSIONS
We found that in this group of patients with type 2 diabetes at high cardiovascular risk and undergoing therapeutic weight loss, treatment with diet alone and metformin as monotherapy were associated with lower POE rates compared with insulin monotherapy. Although this confirms previous evidence of the benefit of metformin on all-cause mortality, we also found neither benefit nor harm from sulfonylureas, alone or in any combination, compared with insulin monotherapy.
Because it seems unlikely that a large-scale clinical trial to test the impact of existing hypoglycemic pharmacotherapies on CVD will be performed, data from observational studies may provide important information to guide clinical practice. Few comparative, prospective, clinical, observational trials have addressed the impact of various hypoglycemic treatment regimens on cardiovascular outcome in such a large cohort. The strength of SCOUT (and of this analysis) is that it provides data for a large number of type 2 diabetic obese patients at increased cardiovascular risks comprehensively studied in a cardiovascular outcome trial in which all events were ascertained and independently adjudicated.
The favorable effect of metformin in obese and nonobese individuals with type 2 diabetes previously has been shown in observational studies and from post hoc analyses of randomized trials investigating intensive glucose-lowering treatments (12–14).
Post hoc analysis of the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction 2 (DIGAMI 2) trial found that insulin treatment significantly increased the risk of nonfatal myocardial infarction and stroke, whereas metformin was protective (although it did not reduce mortality) (12). Study populations and time of follow-up might account for this discrepancy. DIGAMI 2 recruited diabetic patients with acute myocardial infarction, whereas the SCOUT population comprised patients with wider ranges of cardiovascular risk factors. Neither study was designed to compare the potential effects of insulin against metformin and therefore may have an inherent selection bias.
The UK Prospective Study Group (UKPDS 34) also showed that patients using metformin had reduced outcomes (diabetes-related end points such as fatal or nonfatal myocardial infarction, heart failure, stroke) compared with patients treated with insulin or sulfonylurea. Despite a modest hypoglycemic effect (HbA1c reduced by 0.6%), myocardial infarction was reduced by 39% and all-cause mortality was reduced by 36% (13,15). Proposed explanations for the benefits of metformin are its effects on weight (weight-neutral), ability to reduce insulin resistance, and low risk of hypoglycemia. In contrast, hypoglycemic drugs that increase weight, especially central adiposity, have been shown to be atherogenic (16,17).
A recent meta-analysis (18) of randomized clinical trials reporting the effect of metformin on cardiovascular events confirmed results of a previous study (8) and concluded that metformin was associated with neither significant harm nor benefit when compared with an active comparator. However, when compared with placebo or no therapy, metformin was associated with lower cardiovascular event rates, and when administered in combination with sulfonylureas it was associated with reduced survival (Mantel-Haenszel odds ratio, 1.432; 95% CI, 1.068–1.918; P = 0.016). The authors further stated that metformin appeared to be associated with reduction of cardiovascular events in trials of longer duration.
In contrast to our findings, UKPDS 34 found that addition of metformin to sulfonylureas increased risk for diabetic-related deaths and all-cause mortality in nonoverweight and overweight patients (13). This discrepancy most likely can be explained by differences in study populations, follow-up time, and reference groups (sulfonylurea monotherapy in UKPDS and insulin monotherapy in our analysis, respectively).
A recent, randomized, clinical trial showed that the combination of extended-release metformin and sulfonylureas was significantly more effective in lowering HbA1c and glucose (19). Epidemiological studies confirm that the levels of glucose and HbA1c are strong predictors of long-term morbidity and mortality (20–23). Furthermore, observations from 1,294 participants in the Fremantle Diabetes Study concluded that combination therapy with metformin–sulfonylurea appeared to be safe and not associated with increased cardiovascular risk in the most conservative model (24). Another recent meta-analysis of combination therapy with metformin–sulfonylureas found that although there was no significant association with either cardiovascular mortality or all-cause mortality, there was a significant increase in the risk for a composite end point of cardiovascular hospitalization or mortality (25).
Because many type 2 diabetic patients receive metformin and sulfonylurea in combination to improve their glycemic control, our findings that the combination did not cause adverse outcomes in this high cardiovascular risk group are of importance.
Despite the theoretical possibility that sulfonylureas, by closing ATP-sensitive K+ channels and hence reducing the protective effects of ischemic preconditioning, might exert a deleterious role on cardiovascular events (26–28), our analysis found no detrimental association of sulfonylurea monotherapy compared with insulin. Conflicting data exist regarding cardiovascular outcomes in patients with diabetes using sulfonylurea monotherapy (29). University Group Diabetes Program study comparing insulin with placebo (30) reported that tolbutamide was associated with higher cardiovascular mortality, whereas the UKPDS found no differences in the rates of myocardial infarction between patients treated with sulfonylurea compared with insulin. Whereas most concern has been in relation to older first-generation sulfonylureas, two large retrospective studies found that both first-generation and second-generation sulfonylurea use was associated with a significant excess risk of all-cause mortality compared with metformin (31,32). Our findings point to the notion of nonharmful effects of sulfonylureas on cardiovascular outcome.
The duration of diabetes previously has been associated with increased cardiovascular risk and mortality (33). Although it might be assumed that the groups receiving diet-only or metformin monotherapy had shorter duration of diabetes and, hence, lower probability of cardiovascular risk and mortality compared with insulin users, diabetes duration was not associated with either POE or all-cause mortality in our population (23).
BMI correlates robustly with ischemic heart disease in subjects with long-standing type 2 diabetes (34). Caterson et al. (35) showed in the current study population (of overweight/obese subjects with preexisting CVD and/or type 2 diabetes) that modest weight loss is associated with lower POE and lower cardiovascular mortality over 4–5 years. We therefore adjusted for the changes in BMI during follow-up and confirmed that metformin monotherapy and diet-only monotherapy were still associated with lower POE and all-cause mortality compared with insulin monotherapy.
Another important issue is the “severity” of diabetes associated with CVD in the separate treatment groups. Patients receiving insulin might be presumed to be at higher initial cardiovascular risk compared with those receiving diet-only or metformin-only treatment. Traditionally, severity of diabetes is classified according to the level of glycemic control measured by HbA1c (36). Andersson et al. (23) recently described, again in the current study population, that high baseline HbA1c concentration was associated with increasing cardiovascular and all-cause mortality risks. When we adjusted for changes in HbA1c during follow-up, the metformin monotherapy treatment group was still significantly associated with lower POE and all-cause mortality compared with insulin monotherapy. The diet-only treatment group was solely associated with lower POE after adjustment for HbA1c changes.
There is conflicting evidence regarding whether exogenous insulin and hyperinsulinemia in diabetes may exert adverse effects on CVD risk (37). Animal experiments indicate insulin to be atherogenic (38). Two large randomized studies (22,39) showed no macrovascular benefit from an intensive glucose control regimen that included insulin, and one suggested increase in all-cause and CVD mortality (40). However, follow-up in the UKPDS 34 in subjects with type 2 diabetes in the intensive treatment group, which included insulin, showed a significant reduction in myocardial infarction and all-cause mortality (38).
This article is based on well-phenotyped patients studied with the rigorous standards of a cardiovascular outcome trial. The findings we report here, however, are based on post hoc associative analyses and are subject to confounding by indication that can be only partially accounted for by adjustment and use of propensity analyses. Unfortunately, we were not able to subdivide the sulfonylurea group into first-generation and second-generation classes, which differ in pharmacokinetics and in their cardiovascular risk profile in some (31,32), but not all, studies.
We do not have information about hypoglycemic event rates, which have been proposed, but not confirmed (22,39), as an important risk for cardiovascular events. Furthermore, we did not have data regarding endogenous insulin production such as C-peptide concentrations. This would have been interesting because the roles of endogenous versus exogenous insulin production in CVD are as yet unclear (37). Thus, we cannot preclude that the findings may be reflective of other ongoing processes, rather than just of the method of treatment used to control blood glucose levels among those with endogenous insulin production.
In conclusion, we demonstrated that, compared with insulin monotherapy, treatment with metformin monotherapy was associated with decreased risk of cardiovascular events in obese type 2 patients with diabetes with known or increased risk of CVD. Furthermore, metformin monotherapy was associated with a lower incidence of all-cause mortality compared with insulin monotherapy. These findings are consistent with recent published studies. Providing glycemic control is adequate, our data reinforce the safety and benefit of metformin regarding cardiovascular risks. In addition, we found no evidence to support previous concerns with the use of sulfonylureas. In the absence of prospective, randomized, controlled trials in these diabetic individuals at high cardiovascular risk, our data provide important clinical information to inform treatment choices.
Supplementary Material
Acknowledgments
The SCOUT trial was funded by Abbott Laboratories.
I.C. and C.T.-P. have served on the steering committee of the SCOUT trial and have given presentations sponsored by Abbott. C.A. was funded by an independent research grant from the Danish agency for science, technology, and innovation (grant number FSS-11-120873). No other potential conflicts of interest relevant to this article were reported.
The funding source had no influence on the design of the present analyses, content, or the decision to publish the manuscript.
A.A.G. wrote the initial draft of the manuscript, revised the manuscript for important intellectual content, and took part in the interpretation of data. L.K. revised the manuscript for important intellectual content and took part in the interpretation of data. N.F. contributed to acquisition of data, revised the manuscript for important intellectual content, and took part in the interpretation of data. W.P.T.J. performed data analyses, revised the manuscript for important intellectual content, and took part in the interpretation of data. A.M.S. revised the manuscript for important intellectual content and took part in the interpretation of data. I.C. performed data analyses, revised the manuscript for important intellectual content, and took part in the interpretation of data. W.C. revised the manuscript for important intellectual content and took part in the interpretation of data. L.F.V.G. revised the manuscript for important intellectual content and took part in the interpretation of data. C.T.-P. performed data analyses, revised the manuscript for important intellectual content, and took part in the interpretation of data. C.A. performed data analyses, revised the manuscript for important intellectual content, and took part in the interpretation of data. All authors contributed to study design and approved the final version of the manuscript. C.T.-P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Abbott Laboratories for providing assistance in the writing process and statistical expertise.
Footnotes
Clinical trial reg. no. NCT00234832, www.clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0027/-/DC1.
References
- 1.Powell TM, Khera A. Therapeutic approaches to obesity. Curr Treat Options Cardiovasc Med 2010;12:381–395 [DOI] [PubMed] [Google Scholar]
- 2.Schwarz PE, Greaves CJ, Lindström J, Yates T, Davies MJ. Nonpharmacological interventions for the prevention of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:363–373 [DOI] [PubMed] [Google Scholar]
- 3.Klein S, Burke LE, Bray GA, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism Clinical implications of obesity with specific focus on CVD: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 2004;110:2952–2967 [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT. Preventing cardiovascular events in patients with diabetes mellitus. Am J Med 2004;116(Suppl. 5A):39S–46S [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen CH, Gislason GH, Andersson C, et al. Effects of oral glucose-lowering drugs on long term outcomes in patients with diabetes mellitus following myocardial infarction not treated with emergent percutaneous coronary intervention—a retrospective nationwide cohort study. Cardiovasc Diabetol 2010;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson C, Olesen JB, Hansen PR, et al. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia 2010;53:2546–2553 [DOI] [PubMed] [Google Scholar]
- 7.Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147:386–399 [DOI] [PubMed] [Google Scholar]
- 8.Selvin E, Bolen S, Yeh HC, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med 2008;168:2070–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarich SW. Potential of glucose-lowering drugs to reduce cardiovascular events. Curr Diab Rep 2009;9:87–94 [DOI] [PubMed] [Google Scholar]
- 10.Terry T, Raravikar K, Chokrungvaranon N, Reaven PD. Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes? Insights from ACCORD, ADVANCE, and VADT. Curr Cardiol Rep 2012;14:79–88 [DOI] [PubMed] [Google Scholar]
- 11.James WP, Caterson ID, Coutinho W, et al. SCOUT Investigators Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010;363:905–917 [DOI] [PubMed] [Google Scholar]
- 12.Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L, DIGAMI 2 Investigators The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008;29:166–176 [DOI] [PubMed] [Google Scholar]
- 13.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 14.Ong CR, Molyneaux LM, Constantino MI, Twigg SM, Yue DK. Long-term efficacy of metformin therapy in nonobese individuals with type 2 diabetes. Diabetes Care 2006;29:2361–2364 [DOI] [PubMed] [Google Scholar]
- 15.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 16.Baldwin MD. Assessing cardiovascular risk factors and selecting agents to successfully treat patients with type 2 diabetes mellitus. J Am Osteopath Assoc 2011;111(Suppl. 5):S2–S12 [PubMed] [Google Scholar]
- 17.Ajjan RA, Grant PJ. Cardiovascular disease prevention in patients with type 2 diabetes: The role of oral anti-diabetic agents. Diab Vasc Dis Res 2006;3:147–158 [DOI] [PubMed] [Google Scholar]
- 18.Lamanna C, Monami M, Marchionni N, Mannucci E. Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2011;13:221–228 [DOI] [PubMed] [Google Scholar]
- 19.Lewin A, Lipetz R, Wu J, Schwartz S. Comparison of extended-release metformin in combination with a sulfonylurea (glyburide) to sulfonylurea monotherapy in adult patients with type 2 diabetes: a multicenter, double-blind, randomized, controlled, phase III study. Clin Ther 2007;29:844–855 [DOI] [PubMed] [Google Scholar]
- 20.Malmberg K, Rydén L, Wedel H, et al. DIGAMI 2 Investigators Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005;26:650–661 [DOI] [PubMed] [Google Scholar]
- 21.Marcinak JF, Viswanathan P, Arora V, Roebel LE, Strack TR, Leifke E. Shift from surrogate end point to outcome trials: implications for cardiovascular safety assessment in development programs for antidiabetic drugs. Clin Pharmacol Ther 2012;91:514–520 [DOI] [PubMed] [Google Scholar]
- 22.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 23.Andersson C, van Gaal L, Caterson ID, et al. Relationship between HbA1c levels and risk of cardiovascular adverse outcomes and all-cause mortality in overweight and obese cardiovascular high-risk women and men with type 2 diabetes [corrected in Diabetologia 2012;55:2860]. Diabetologia 2012;55:2348–2355 [DOI] [PubMed] [Google Scholar]
- 24.Sillars B, Davis WA, Hirsch IB, Davis TM. Sulphonylurea-metformin combination therapy, cardiovascular disease and all-cause mortality: the Fremantle Diabetes Study. Diabetes Obes Metab 2010;12:757–765 [DOI] [PubMed] [Google Scholar]
- 25.Rao AD, Kuhadiya N, Reynolds K, Fonseca VA. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality? A meta-analysis of observational studies. Diabetes Care 2008;31:1672–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeller M, Danchin N, Simon D, et al. French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction Investigators Impact of type of preadmission sulfonylureas on mortality and cardiovascular outcomes in diabetic patients with acute myocardial infarction. J Clin Endocrinol Metab 2010;95:4993–5002 [DOI] [PubMed] [Google Scholar]
- 27.Horsdal HT, Søndergaard F, Johnsen SP, Rungby J. Antidiabetic treatments and risk of hospitalisation with myocardial infarction: a nationwide case-control study. Pharmacoepidemiol Drug Saf 2011;20:331–337 [DOI] [PubMed] [Google Scholar]
- 28.Thisted H, Johnsen SP, Rungby J. Sulfonylureas and the risk of myocardial infarction. Metabolism 2006;55(Suppl. 1):S16–S19 [DOI] [PubMed] [Google Scholar]
- 29.Sullivan D, Forder P, Simes J, et al. FIELD Study Investigators Associations between the use of metformin, sulfonylureas, or diet alone and cardiovascular outcomes in 6005 people with type 2 diabetes in the FIELD study. Diabetes Res Clin Pract 2011;94:284–290 [DOI] [PubMed] [Google Scholar]
- 30.Feinglos MN, Bethel MA. Therapy of type 2 diabetes, cardiovascular death, and the UGDP. Am Heart J 1999;138:S346–S352 [DOI] [PubMed] [Google Scholar]
- 31.Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 2009;339:b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schramm TK, Gislason GH, Vaag A, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J 2011;32:1900–1908 [DOI] [PubMed] [Google Scholar]
- 33.Silbernagel G, Rosinger S, Grammer TB, et al. Duration of type 2 diabetes strongly predicts all-cause and cardiovascular mortality in people referred for coronary angiography. Atherosclerosis 2012;221:551–557 [DOI] [PubMed] [Google Scholar]
- 34.Wentworth JM, Fourlanos S, Colman PG. Body mass index correlates with ischemic heart disease and albuminuria in long-standing type 2 diabetes. Diabetes Res Clin Pract 2012;97:57–62 [DOI] [PubMed] [Google Scholar]
- 35.Caterson ID, Finer N, Coutinho W, et al. SCOUT Investigators Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial. Diabetes Obes Metab 2012;14:523–530 [DOI] [PubMed] [Google Scholar]
- 36.Wong K, Glovaci D, Malik S, et al. Comparison of demographic factors and cardiovascular risk factor control among U.S. adults with type 2 diabetes by insulin treatment classification. J Diabetes Complications 2012;26:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muis MJ, Bots ML, Grobbee DE, Stolk RP. Insulin treatment and cardiovascular disease; friend or foe? A point of view. Diabet Med 2005;22:118–126 [DOI] [PubMed]
- 38.Panicker GK, Karnad DR, Salvi V, Kothari S. Cardiovascular risk of oral antidiabetic drugs: current evidence and regulatory requirements for new drugs. J Assoc Physicians India 2012;60:56–61 [PubMed] [Google Scholar]
- 39.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 40.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.