Abstract
OBJECTIVE
Previous work has shown a correlation between β-cell number in cultured islet cell grafts and their ability to induce C-peptide secretion after intraportal implantation in C-peptide–negative type1 diabetic patients. In this cross-sectional study, we examined the minimal functional β-cell mass (FBM) in the implant that induces metabolic improvement.
RESEARCH DESIGN AND METHODS
Glucose clamps assessed FBM in 42 recipients with established implants. C-peptide release during each phase was expressed as percentage of healthy control values. Its relative magnitude during a second hyperglycemic phase was most discriminative and therefore selected as a parameter to be correlated with metabolic effects.
RESULTS
Recipients with functioning β-cell implants exhibited average FBM corresponding to 18% of that in normal control subjects (interquartile range 10–33%). Its relative magnitude negatively correlated with HbA1c levels (r = −0.47), daily insulin dose (r = −0.75), and coefficient of variation of fasting glycemia (CVfg) (r = −0.78, retained in multivariate analysis). A correlation between FBM and CVfg <25% appeared from the receiver operating characteristic curve (0.97 [95% CI 0.93–1.00]). All patients with FBM >37% exhibited CVfg <25% and a >50% reduction of their pretransplant CVfg; this occurred in none with FBM <5%. Implants with FBM >18% reduced CVfg from a median pretransplant value of 46 to <25%.
CONCLUSIONS
Glucose clamping assesses the degree of restoration in FBM achieved by islet cell implants. Values >37% of normal control subjects appear needed to reduce glycemic variability in type 1 diabetic recipients. Further studies should examine whether the test can help guide decisions on additional islet cell transplants and on adjusting or stopping immunotherapy.
During the last decade, the results of islet cell transplantation in type 1 diabetes have improved (1). Several groups have described a relationship between indirect or composite measures of β-cell mass and glycemic control (2–6). Our clinical islet cell transplant protocol uses cultured preparations in which β-cell number and purity are routinely determined (7,8). It was thus found that intraportally injected grafts minimally needed 2.106 β-cells/kg body weight in order to consistently induce glucose-regulated C-peptide release in C-peptide–negative type 1 diabetic patients (8). In 56% of recipients, a state of insulin independence was achieved at posttransplant (PT) month 12. However, when their functional β-cell mass (FBM) was assessed by a glucose clamp, it was found to average only 25% of that in age-matched normal control subjects (8); despite this relatively low magnitude, this subgroup exhibited a significant reduction in glycemic variation as expressed by the coefficient of variation of fasting glycemia (8). The present cross-sectional study examines whether a relationship exists between the relative size of the FBM in the implant and improvements in glycemic variability and other metabolic end points such as HbA1c and insulin needs. The FBM is assessed by the hyperglycemic clamp (HGC), which is considered the gold standard for this purpose in type 2 diabetes (9) and which also allowed distinction between subpopulations in type 1 diabetes (8,10,11).
RESEARCH DESIGN AND METHODS
β-Cell recipients
Patients (median age 44 years and BMI 24 kg/m2) had type 1 diabetes for >20 years, with variable glucose control before transplantation (median HbA1c 7.7% [interquartile range {IQR} 7.0–8.4]; 46 mmol/mol [53–68]). All subjects experienced large variability of fasting glycemia (coefficient of variation of fasting glycemia [CVfg] >25% [8]) despite the use of a subcutaneous insulin pump (n = 38) or long-acting insulin analogs (n = 3) (Supplementary Table 1).
At the time of their first clamp posttransplantation (median 6 months PT [IQR 6–18]), their diabetes was better controlled compared with pretransplantation, as illustrated by an HbA1c ≤7% (≤53 mmol/mol) in 35 of 42 (P < 0.001) and a CVfg <25% in 27 of 42 patients (P < 0.001) (Supplementary Table 1). This better glycemic control was due to a partial restoration of endogenous insulin secretion (random C-peptide >0.5 ng/dL in 34 of 42 patients). At the time of the clamp, 18 patients were off insulin, 14 on insulin pump (median insulin dose 0.32 units/kg/day), and 10 on insulin analogs (median insulin dose 0.28 units/kg/day).
Transplant protocol
Cultured islet cell grafts were prepared as previously described. Patients received in total 2.2–9.0. 106 β-cells/kg body weight either in one (n = 9) or two (n = 33) injections with a median interval of 2.7 months (IQR 2.3–3.1). The islet cell graft was infused into the portal vein over 5–6 min. Access to the portal vein occurred through a catheter inserted in the umbilical vein (n = 19) (8) or through subcutaneous transhepatic puncture under ultrasound guidance (n = 23) (8). Thus far, we did not find a different loss of FBM after infusion into the portal vein by laparoscopic or radiological access (P.G., G. Delvaux, G. Maleux, B.K., unpublished observations). The immune-suppressive regimen consisted of induction therapy with antithymocyte globulin (ATG; Fresenius) at the time of the first implant, followed by a maintenance immune-suppression regimen of mycophenolate mofetil (Cellcept) plus tacrolimus (Prograft) (8).
HGC
The clamp procedure was a modification of the method of DeFronzo, Tobin, and Andres (9) as previously described (12). In short, the evening before the clamp a standard meal was taken between 5.30 and 6.30 p.m. In patients treated with long-acting insulin, the day before the clamp, this insulin type was replaced by intermediate-acting insulin, while in patients treated with pump short-acting insulin, insulin was stopped 1 h before start of the clamp so that virtually no exogenous insulin was present during the clamp procedure. Subjects remained fasted until the test with free water intake. Intake of all medications except the immunosuppressive drugs was deferred to after the clamp procedure. During the first 14 min of the clamp, a priming dose of glucose was infused to reach a glycemia level of 180 mg/dL. This priming dose was adjusted to body surface area and was, respectively, 870 mg/m2 ⋅ min from 0 to 5 min, 350 mg/m2 ⋅ min from 5 to 10 min, and 210 and 180 mg/m2 ⋅ min after 14 min. From 15 to 170 min, glycemia was maintained at 180 mg/dL. Whole blood glucose was measured with Hemocue (Hemocue, Ängelholm, Sweden) every 5 min. The glucose infusion rate was adapted at 5- to 10-min intervals. At 150 min, 1 mg glucagon (GlucaGen HypoKit; Novo Nordisk, Bagsvaerd, Denmark) was injected intravenously to complementarily stimulate insulin secretion via the cAMP pathway. Blood was drawn for measurement of glycemia and C-peptide at −30, −15, and 0 min (basal); at 5, 7.5, and 10 min (first-phase release); at 120, 135, and 150 min (second-phase release); and at 155 and 160 min (third-phase insulin release).
Calculations
C-peptide secretion was measured during the different phases of the HGC test in β-cell graft recipients. The same test was conducted in 12 age- and weight-matched healthy volunteers, and different measures were expressed as a percentage of the mean of healthy control subjects. Plasma C-peptide levels were used to calculate area under the curve (AUC) during the four phases (basal, first, second, and postglucagon phases). The AUC was divided by its time interval resulting in a concentration/minute to facilitate comparison between intervals. In a post hoc analysis of relationship with glycemic variability, phase 2 HGC as index of FBM was divided in 4 quartiles (Q 1, 2, 3 and 4), from the lowest quartile of secretion (Q1) to the highest quartile (Q4). CVfg was calculated on the basis of 120 home blood glucose measurements in a two month period around the HGC. CVfg was expressed by an absolute value in %. A decrease by >50% of the pretreatment CVfg value was indicated as “reduction by more than 50%”.
Chemical analysis
Plasma glucose was measured on Vitros 950 IC until 2006 and Vitros 5.1FS from 2007 onwards (Ortho Clinical Diagnostics, Rochester, NY), C-peptide and proinsulin by time-resolved fluoroimmunoassay (11). The proinsulin assay was considered to measure total proinsulin immunoreactive material (13). As there is 100% cross-reactivity of proinsulin in the C-peptide assay, free C-peptide concentrations were obtained by subtracting the proinsulin concentration from the measured total C-peptide result.
Statistical analysis
Since the aim of this study was to examine the relationship between FBM, measured with HGC, and parameters of glycemic control and glycemic variability we have chosen to use only 1 data point per β-cell recipient in a cross-sectional design. All patients who received a HGC posttransplantation (n = 42) were included with a wide range of FBM (between 1 and 71% of healthy controls). Data are presented as median (IQR) unless otherwise stated. Normality was tested for by using the Shapiro-Wilk test. Comparison of patient subgroups used Kruskal-Wallis H test and if significant the Mann-Whitney test for quantitative variables and Fisher exact test for binary variables. Statistical difference between repeated measurements were examined with the Friedman test and, if significant, with the Wilcoxon signed rank test. Analysis of correlations was performed using the Spearman rank correlation test. Multiple linear regression including all parameters with P < 0.25 was used for multivariate analyses using HbA1c, CVfg, mean fasting glycemia, insulin dose (U/d and units/kg/d) and presence of insulin independence as explanatory variables and second phase C-peptide secretion as outcome variable. Both a complete model and a forward selection approach were used. Differences were considered significant for P values < 0.05. The use of AUC C-peptide during the second phase of the HGC test as predictor of CVfg <25% and reduction of pretransplant CVfg by >50% was evaluated using receiver operating characteristic (ROC) curves. All analyses were performed using SPSS statistics, 20.0. No adjustments were made for multiplicity.
RESULTS
Secretory capacity of islet cell implants during HGC
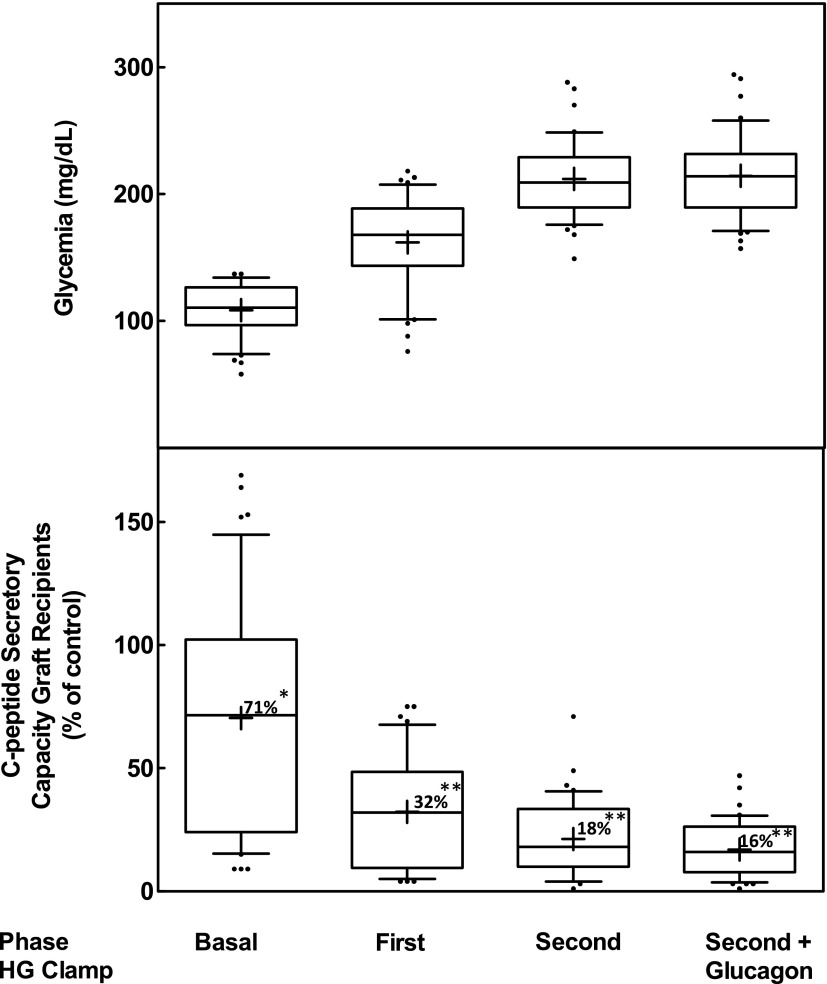
In this cross-sectional study in 42 patients, secretory capacity of the implants was measured during the different phases of the HGC test. During the basal (nonstimulated) phase, secretory capacity reached 71% of that in healthy control subjects (Fig. 1). During the stimulatory phases of the HGC, median C-peptide secretion was, however, significantly lower than measurements in healthy control subjects (Fig. 1). After acute (first phase) and chronic (second phase) glucose stimulation, secretory capacity reached 45% and 18% of that in healthy control subjects, respectively. Adding glucagon showed the same secretory capacity compared with healthy control subjects as during the second phase. The secretory capacity during the second phase of the HGC was therefore used as an index of FBM. The observation that basal secretory capacity approached that of healthy control subjects is attributed to its response to higher basal glucose levels: median 110 mg/dL (IQR 97–126) vs. 84 mg/dL (79–89); P < 0.001. In contrast, glycemic levels achieved during the first phase (168 mg/dL [IQR 143–189] vs. 145 mg/dL [126–152]; P = 0.14), second phase (209 mg/dL [189–229] vs. 193 mg/dL [178–196]; P = 0.09), and postglucagon phase (214 mg/dL [189–232] vs. 193 mg/dL [177–213]; P = 0.64) were not different from levels of healthy control subjects.
Figure 1.
Secretory capacity of implants expressed as percent of that of normal control subjects. Measurement during different phases of HGC test (HG clamp). Numbers in boxes are median values compared with healthy control subjects. Statistical difference compared with healthy control subjects: *P < 0.05; **P < 0.001.
Correlation between HGC index of FBM and metabolic control
The average phase 2 HGC value was 18% of that in healthy control subjects—however, ranging from 1 to 71%. A wide variation was also observed in associated metabolic parameters such as HbA1c, fasting glycemia, CVfg, and insulin dose (Supplementary Table 1). A correlation was observed between the phase 2 HGC values and HbA1c, CVfg, and insulin need but not with fasting glycemia (Table 1 and Supplementary Fig. 1). In the multivariate analysis, only CVfg remained independently correlated with phase 2 HGC (Table 1). The same was seen when only insulin-dependent patients were studied (Table 1). For further analysis of this correlation, patients were divided according to their FBM values into four quartiles (Fig. 2), i.e., quartile 1 with a relative FBM of 6% (range 1–10), quartile 2 with 13% (11–18), quartile 3 with 23% (19–33), and quartile 4 with 38% (34–71). All quartiles correlated with a decrease in CVfg as measured pretransplantation (P = 0.02 for quartile 1; P = 0.005 for quartiles 2 and 3; P = 0.003 for quartile 4). Patients in quartiles 3 and 2 had undergone a stronger decrease in CVfg than, respectively, those in quartiles 2 and 1, while patients in quartiles 3 and 4 showed no statistical difference in the effects on CVfg (Fig. 2).
Table 1.
Correlation between FBM implant and metabolic parameters in recipients
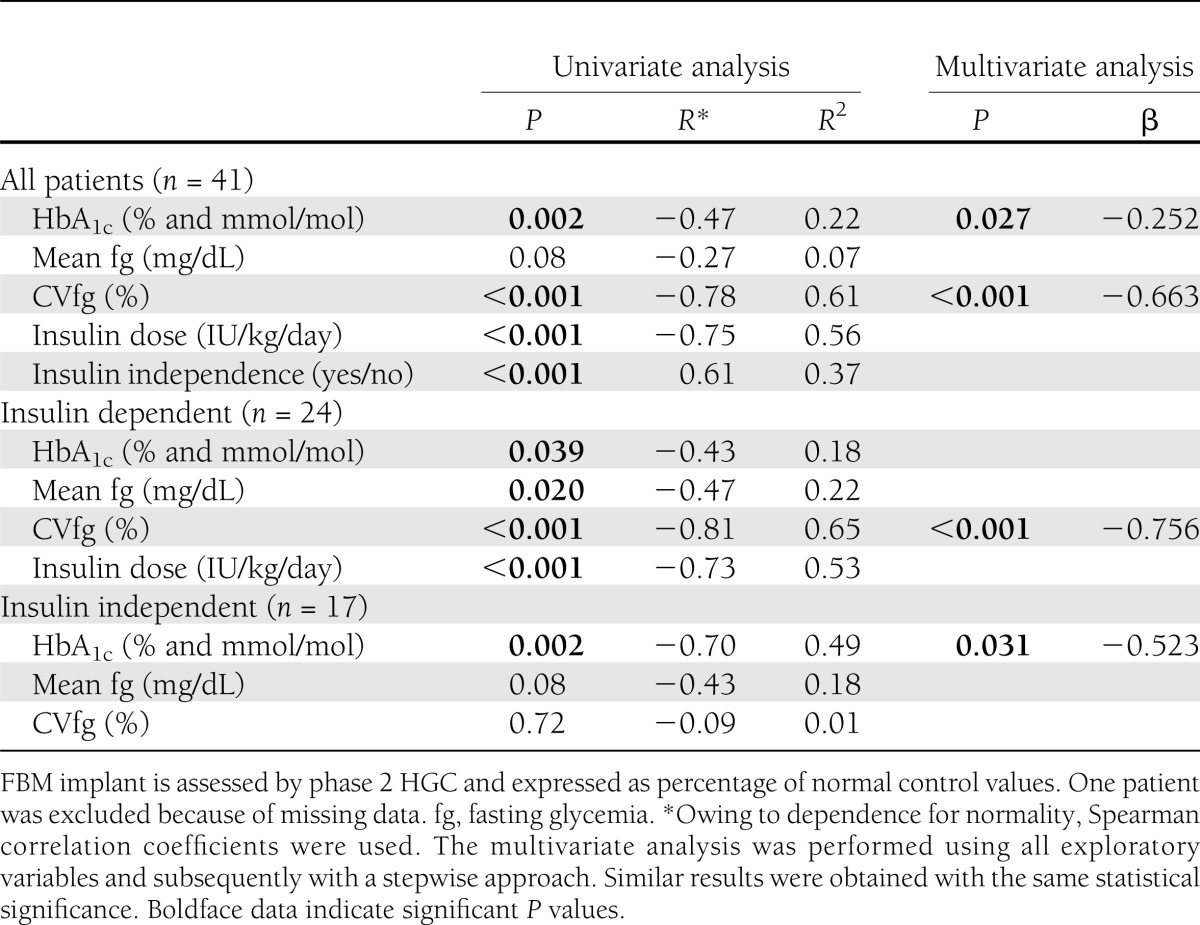
Figure 2.
CVfg in patients pretransplantation (PreTx) and posttransplantation (PostTx). Posttransplantation CVfg values are shown from the lowest (Q1) to the highest (Q4) quartiles of C-peptide secretory capacity during the second phase of the HGC. Statistical difference between quartiles: *P < 0.05; **P < 0.005. CVfg of all quartiles improved significantly (P < 0.05) compared with before transplantation. Data are means ± SEM. All recipients had a CVfg >25% before transplantation (dotted line). Q, quartile.
A strong correlation was measured between CVfg and episodes of fasting hypoglycemia <70 mg/dL (r = 0.78, P < 0.001).
FBM as predictor of variability of fasting glycemia
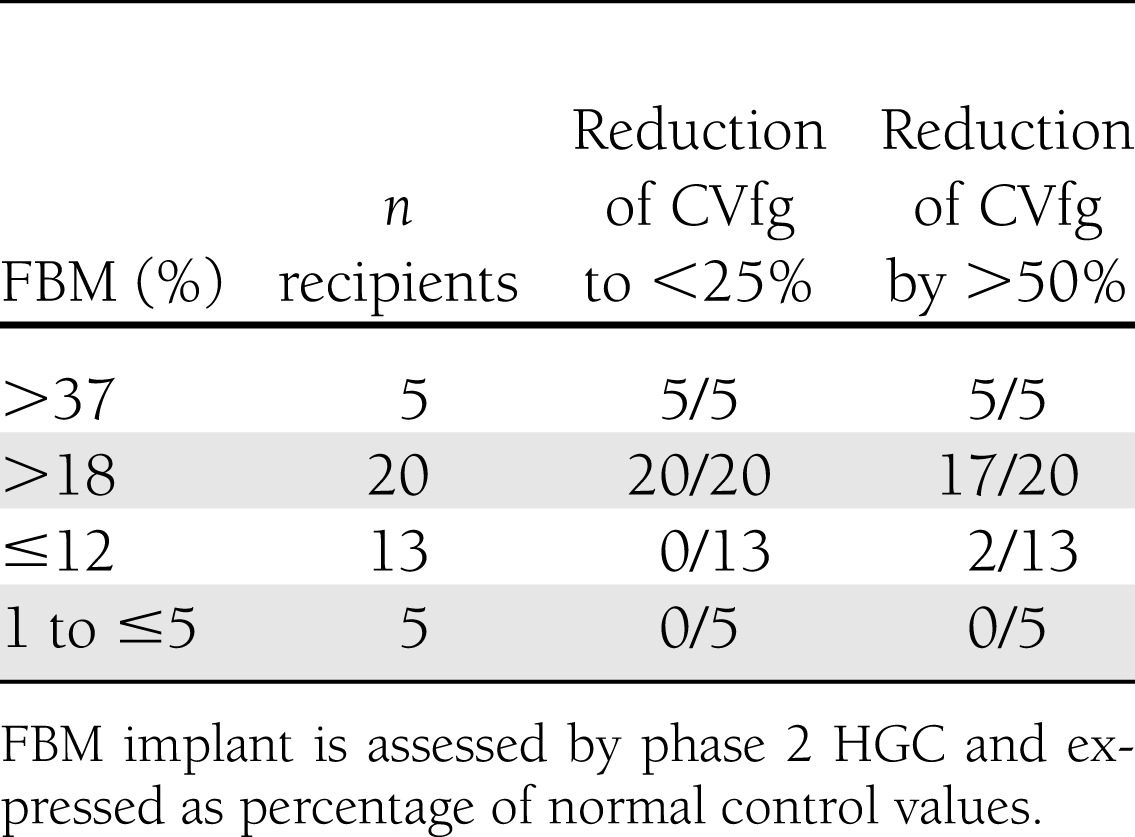
An ROC analysis was performed in order to investigate whether the phase 2 HGC values are associated with a specific metabolic effect, in particular bringing the CVfg <25% or inducing a reduction by >50% in pretransplant CVfg (Supplementary Fig. 2). The latter two criteria were achieved in all patients with an implant FBM >37% of control subjects, while this occurred in none of the patients with an FBM <5% (Table 2). CVfg was consistently reduced <25% in all patients with FBM >18% of control subjects (positive predictive value 100%); this end point was never reached for an FBM <12% (negative predictive value 100%). As indicated by the area under the ROC curve (0.97 [95% CI 0.93–1.00]), PT FBM distinguishes each patient with CVfg <25% from one with CVfg >25% (Supplementary Fig. 2).
Table 2.
Correlation between FBM implant and reduction in glycemic variability in recipients
CONCLUSIONS
This cross-sectional study shows a correlation between the FBM of established islet cell implants and their ability to correct three parameters of metabolic control (HbA1c, CVfg, and insulin dose), the strongest relationship being with glycemic variability. We selected glucose clamps to assess the secretory capacity of the implants and compare it with that in nondiabetic control subjects. Other β-cell stimulation tests exhibit a higher intraindividual variability (14–16).
Hyperglycemic glucose clamps have shown a wide range of residual β-cell function in type 1 diabetic patients (8,10,11). Their second secretory phase has indicated that intraportal islet cell implants exhibit a functional capacity that varies between 12 and 45% of that in age- and body weight–matched healthy control subjects (8). The current study confirms this variability in a larger group of recipients, including both insulin-dependent and -independent patients. It argues against using the basal phase or the first hyperglycemic phase as an index of FBM, as these appear significantly less sensitive for detecting differences from the control values, thus overestimating the relative potency of implants. The average secretory capacity during the second phase was significantly lower than in control subjects, with equal levels in the absence (18% of control subjects) and in the presence (16%) of glucagon. Both segments of the second phase can thus serve as an index of FBM. The higher-than-normal basal glycemia in some transplant patients may lead to an underestimation of the first-phase insulin release because of a presumed higher degree of degranulation of the insulin granules and to a lesser extent of the second phase of the hyperglycemic phase in these patients. Because of side effects after glucagon administration in some patients and no added value of the postglucagon response in this study, we conclude that in studies with intraportal human implants the glucagon segment can be omitted. It may, however, still be useful when porcine or human stem cell–derived cells will be tested in future trials.
In our protocol, we did not perform a euglycemic or isoglycemic-hyperinsulinemic clamp that allows fine assessment of glucose disposal and thus estimation of insulin resistance. It is assumed that in lean type 1 diabetic recipients, graft insulin secretion is more important than insulin resistance in determining overall glycemia. Moreover, an FBM with an IQR variability of 10–33% makes it difficult to study insulin action and insulin resistance with an iso- or euglycemic clamp technique. However, the HGC technique is an adequate method to determine graft insulin secretory capacity, which is the purpose of this study. In order to examine the effects of insulin secretion on variables like average glucose control, glycemic variability, and insulin needs, it is imperative to include subjects with an important variation in function.
The relative magnitude of the second phase was selected as an index of the FBM of the implants. It strongly correlates with the reduction in glycemic variability, which is clinically relevant since glycemic variability is widely recognized as the main limiting factor in obtaining and maintaining optimal HbA1c with intensive insulin therapy in type 1 diabetic patients (17).
The current study was conducted in patients who were C-peptide negative before transplantation, with a history of hypoglycemia and important fasting glycemic variability, defined as CVfg >25%. Even on insulin analogs or insulin pump, most type 1 diabetic patients have CVfg >25% (18–22). They are at higher risk for hypoglycemia (23) and therefore frequently kept at higher HbA1c targets with its associated risks for chronic complications. There is no universally accepted gold standard method for quantifying glycemic variability (24). We used daily home blood glucose monitoring because it has the advantage of providing real-life data on a continuous basis with the lowest risk of study-related changes in patient behavior. With these data, glycemic variability can be easily estimated by calculating the SD or, better, the CV (8,17,21,25). In future studies, continuous glucose monitoring may become an even better tool to study glycemic variability, although only 64% of a selected group of adult patients and 19% of adolescents are willing to use this new methodology on a permanent basis beyond month 6 (26).
FBM of established implants correlated with their ability to reduce CVfg but not with decreased mean fasting glucose concentrations, indicating that all patients received appropriate insulin doses overnight. In pathophysiological terms, FBM determines and thus predicts glycemic variability. This was indeed shown in the ROC analysis demonstrating a strong predictive value of FBM on reduction of glycemic variability. An FBM of at least 18% is needed to achieve a CVfg <25%. A value of at least 37% brings CVfg <25% plus reduces it by >50% of pretransplantation values in all patients. The latter criterion is currently used in our center to decide whether an additional implant is to be performed. It is only met in a small number of patients, which underlines the need for further improvements in the preparation of grafts and the transplant protocols.
It is not to be excluded that the exposure of B-cells to calcineurin inhibitors leads to impaired insulin secretion (27,28) while lower glomerular filtration rate in transplanted patients leads to lower C-peptide clearance and thus potentially falsely elevated basal C-peptide levels. Future studies in recipients of intraportal islet grafts under other immune-suppression or immune-suppressive doses and in de novo type 1 diabetic patients—with normal estimated glomerular filtration rate and residual endogenous pancreatic function—should determine if these observations are applicable to these populations. Likewise, we agree that the results here obtained with intraportal implants cannot be extrapolated to extrahepatic implants. However, today no clinical functional extrahepatic implants have been published in man.
Each transplant study should also determine what minimal β-cell transplant function is needed so that benefits of treatment overrule (risk of) adverse events. In our study, no clinical benefit in terms of glycemic variability was measured in patients with an FBM <5%. This finding helps us with the decision to stop tacrolimus and mycophenolate mofetil as maintenance immune suppression. As both drugs are associated with acute and long-term risks, continuation is discussed on an individual basis with patients who have a FBM <5%.
In conclusion, the FBM of islet cell implants as measured by a HGC test predicts their effect on glycemic variability in C-peptide–negative type 1 diabetic recipients of an intraportal graft. Cutoff values were identified above and below which clinical benefit was present or absent, respectively. Further studies can indicate to which extent these values can help guide and monitor decisions on conducting additional islet cell transplants or on adjusting or stopping immune therapy. The glucose clamp data may also serve to compare outcome of different islet cell transplant protocols and to assess β-cell replacement therapies using novel cell sources.
Supplementary Material
Acknowledgments
This study was supported by the JDRF (grant 4/2005/1327) and the W. Gepts fund of University Hospital Brussels. P.G. is funded by a clinical research foundation of the University Hospitals Leuven, Katholieke Universiteit Leuven. I.W., C.M., and B.K. are Senior Clinical Investigators of the Research Foundation Flanders (Fonds Wetenschappelijk Onderzoek-Vlaanderen).
No potential conflicts of interest relevant to this article were reported.
P.G. wrote the manuscript and researched data. R.H. researched data and reviewed the manuscript. U.V.d.V. researched data. Z.L. reviewed the manuscript. D.H.L. researched data. I.W., F.G., and C.D.B. reviewed the manuscript. L.K. advised in statistical analysis. C.M. reviewed the manuscript. D.P. contributed to the discussion and edited the manuscript. B.K. wrote the manuscript and researched data. P.G. and B.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 2nd Joint Artificial Insulin Delivery Pancreas and Islet Transplantation (AIDPIT)/European Pancreas and Islet Association Winter Symposium and 31st AIDPIT Workshop, Igls, Austria, 30 January 2012.
The authors thank Monique Robyn, Hilde Morobe (University Hospitals Leuven), Koen Verbeeck, Sofie Vandenhoeck, Soniya Thomas, Jenny Van Den Brande (University Hospitals Brussel), and Rie Braspenning (University Hospital Antwerp) for their work in completing all the clamp tests. The authors also thank the Eurotransplant Foundation and its transplant surgeons and coordinators for organ procurement. Finally, the authors thank the staff of the Beta-Cell Bank, the Belgian Diabetes Registry, the Diabetes Research Center of the Vrije Universiteit Brussel, and the Clinical Biology Department of University Hospital Brussels.
Footnotes
Clinical trial reg. no. NCT00623610, clinicaltrials.org.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0128/-/DC1.
References
- 1.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care 2012;35:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vantyghem MC, Raverdy V, Balavoine AS, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) Is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β-score greater than 3). J Clin Endocrinol Metab 2012;97:E2078–E2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, Lakey JR, Bigam D, Shapiro AM. Beta-score: an assessment of beta-cell function after islet transplantation. Diabetes Care 2005;28:343–347 [DOI] [PubMed] [Google Scholar]
- 4.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA 2005;293:830–835 [DOI] [PubMed] [Google Scholar]
- 5.Rickels MR, Schutta MH, Markmann JF, Barker CF, Naji A, Teff KL. beta-Cell function following human islet transplantation for type 1 diabetes. Diabetes 2005;54:100–106 [DOI] [PubMed] [Google Scholar]
- 6.Fiorina P, Shapiro AMJ, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant 2008;8:1990–1997 [DOI] [PubMed] [Google Scholar]
- 7.Keymeulen B, Ling Z, Gorus FK, et al. Implantation of standardized beta-cell grafts in a liver segment of IDDM patients: graft and recipients characteristics in two cases of insulin-independence under maintenance immunosuppression for prior kidney graft. Diabetologia 1998;41:452–459 [DOI] [PubMed] [Google Scholar]
- 8.Keymeulen B, Gillard P, Mathieu C, et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci USA 2006;103:17444–17449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 10.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 11.Vandemeulebroucke E, Keymeulen B, Decochez K, et al. Belgian Diabetes Registry Hyperglycaemic clamp test for diabetes risk assessment in IA-2-antibody-positive relatives of type 1 diabetic patients. Diabetologia 2010;53:36–44 [DOI] [PubMed] [Google Scholar]
- 12.Gillard P, Vandemeulebroucke E, Keymeulen B, et al. Functional beta-cell mass and insulin sensitivity is decreased in insulin-independent pancreas-kidney recipients. Transplantation 2009;87:402–407 [DOI] [PubMed] [Google Scholar]
- 13.Kjems LL, Røder ME, Dinesen B, Hartling SG, Jørgensen PN, Binder C. Highly sensitive enzyme immunoassay of proinsulin immunoreactivity with use of two monoclonal antibodies. Clin Chem 1993;39:2146–2150 [PubMed] [Google Scholar]
- 14.Smith CP, Tarn AC, Thomas JM, et al. Between and within subject variation of the first phase insulin response to intravenous glucose. Diabetologia 1988;31:123–125 [DOI] [PubMed] [Google Scholar]
- 15.Gjessing HJ, Damsgaard EM, Matzen LE, Frøland A, Faber OK. Reproducibility of beta-cell function estimates in non-insulin-dependent diabetes mellitus. Diabetes Care 1987;10:558–562 [DOI] [PubMed] [Google Scholar]
- 16.Utzschneider KM, Prigeon RL, Tong J, et al. Within-subject variability of measures of beta cell function derived from a 2 h OGTT: implications for research studies. Diabetologia 2007;50:2516–2525 [DOI] [PubMed] [Google Scholar]
- 17.Rodbard D. Glycemic variability: measurement and utility in clinical medicine and research—one viewpoint. Diabetes Technol Ther 2011;13:1077–1080 [DOI] [PubMed] [Google Scholar]
- 18.Hermansen K, Fontaine P, Kukolja KK, Peterkova V, Leth G, Gall MA. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia 2004;47:622–629 [DOI] [PubMed] [Google Scholar]
- 19.Russell-Jones D, Simpson R, Hylleberg B, Draeger E, Bolinder J. Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type I diabetes mellitus using a basal-bolus regimen. Clin Ther 2004;26:724–736 [DOI] [PubMed] [Google Scholar]
- 20.Kurtoglu S, Atabek ME, Dizdarer C, Pirgon O, Isguven P, Emek S, PREDICTIVE Turkey Study Group Insulin detemir improves glycemic control and reduces hypoglycemia in children with type 1 diabetes: findings from the Turkish cohort of the PREDICTIVE observational study. Pediatr Diabetes 2009;10:401–407 [DOI] [PubMed] [Google Scholar]
- 21.Renard E, Dubois-Laforgue D, Guerci B, Variability Study Group Non-inferiority of insulin glargine versus insulin detemir on blood glucose variability in type 1 diabetes patients: a multicenter, randomized, crossover study. Diabetes Technol Ther 2011;13:1213–1218 [DOI] [PubMed] [Google Scholar]
- 22.Bolli GB, Kerr D, Thomas R, et al. Comparison of a multiple daily insulin injection regimen (basal once-daily glargine plus mealtime lispro) and continuous subcutaneous insulin infusion (lispro) in type 1 diabetes: a randomized open parallel multicenter study. Diabetes Care 2009;32:1170–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia 2007;50:2553–2561 [DOI] [PubMed] [Google Scholar]
- 24.Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr Rev 2010;31:171–182 [DOI] [PubMed] [Google Scholar]
- 25.Niskanen L, Virkamäki A, Hansen JB, Saukkonen T. Fasting plasma glucose variability as a marker of nocturnal hypoglycemia in diabetes: evidence from the PREDICTIVE study. Diabetes Res Clin Pract 2009;86:e15–e18 [DOI] [PubMed] [Google Scholar]
- 26.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care 2010;33:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strumph P, Kirsch D, Gooding W, Carroll P. The effect of FK506 on glycemic response as assessed by the hyperglycemic clamp technique. Transplantation 1995;60:147–151 [PubMed] [Google Scholar]
- 28.Radu RG, Fujimoto S, Mukai E, et al. Tacrolimus suppresses glucose-induced insulin release from pancreatic islets by reducing glucokinase activity. Am J Physiol Endocrinol Metab 2005;288:E365–E371 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.