Abstract
OBJECTIVE
To evaluate whether etiologic diabetes type is associated with the degree of albuminuria in children with diabetes.
RESEARCH DESIGN AND METHODS
SEARCH is an observational, longitudinal study of children with diabetes. Youth with newly diagnosed diabetes were classified according to diabetes autoantibody (DAA) status and presence of insulin resistance. We defined insulin resistance as an insulin sensitivity score <25th percentile for the United States general youth population. DAA status was based on positivity for the 65-kD isoform of glutamate decarboxylase and insulinoma-associated protein 2 antigens. The four etiologic diabetes type groups were as follows: DAA+/insulin-sensitive (IS) (n = 1,351); DAA+/insulin-resistant (IR) (n = 438); DAA−/IR (n = 379); and DAA−/IS (n = 233). Urinary albumin:creatinine ratio (UACR) was measured from a random urine specimen. Multivariable regression analyses assessed the independent relationship between the four diabetes type groups and magnitude of UACR.
RESULTS
Adjusted UACR means across the four groups were as follows: DAA+/IS = 154 μg/mg; DAA+/IR = 137 μg/mg; DAA−/IR = 257 μg/mg; and DAA−/IS = 131 μg/mg (P < 0.005). Only DAA−/IR was significantly different. We performed post hoc multivariable regression analysis restricted to the two IR groups to explore the contribution of DAA status and insulin sensitivity (continuous) to the difference in UACR between the IR groups. Only insulin sensitivity was significantly associated with UACR (β = −0.54; P < 0.0001).
CONCLUSIONS
In youth with diabetes, the DAA−/IR group had a greater UACR than all other groups, possibly because of the greater magnitude of insulin resistance. Further exploration of the relationships between severity of insulin resistance, autoimmunity, and albuminuria in youth with diabetes is warranted.
Previous reports suggested that the clinical course and factors contributing to the development and progression of diabetic nephropathy did not differ by diabetes type and that diabetic nephropathy was preceded by albuminuria that worsened over time (1,2). More recent data have further elucidated the natural history of diabetic kidney disease. In the absence of albuminuria, a significant number of people with diabetes, especially type 2, still develop a decline in glomerular filtration rate (3,4). Thus, there may be identifiable differences in the natural history of nephropathy inherent to the underlying diabetes type. Multiple pediatric diabetes cohorts have found a higher prevalence of albuminuria and progressive kidney failure in youth with a clinical diagnosis of type 2 diabetes than with type 1 diabetes (5–7). Such data suggest that insulin resistance, a key component of the pathophysiology of type 2 diabetes, may be an important contributor to diabetic nephropathy in youth with diabetes.
The epidemic of overweight and obesity has made it increasingly difficult to clinically diagnose diabetes type, because insulin resistance and autoimmunity often coexist (8,9). Cohort studies of youth with type 1 diabetes have found a significant increase in microvascular and macrovascular diseases in those with concurrent insulin resistance (10–12). The prevalence of albuminuria in insulin-resistant (IR) individuals with type 1 diabetes has not been compared with individuals with type 2 diabetes. Thus, the role of autoimmunity and insulin resistance across the spectrum of diabetes types and the risk for microvascular complications warrant investigation. Herein, we investigate the magnitude of albuminuria according to the status of autoimmunity and insulin resistance in youth with newly diagnosed type 1 and type 2 diabetes.
RESEARCH DESIGN AND METHODS
Study population
The SEARCH for Diabetes in Youth Study is a multicenter, observational study of youth with incident diabetes who are followed-up longitudinally. A detailed description of study methods has been published previously (13). In brief, SEARCH is an ongoing study that began in 2001 to conduct population-based ascertainment of cases of diabetes in youth younger than 20 years of age. The study protocol was reviewed and approved by local institutional review boards that had jurisdiction over the local study populations. Cases were ascertained from geographically defined populations in Ohio, Colorado, South Carolina, and Washington, among enrollees in several health plans in California and Hawaii, and Indian Health Service beneficiaries from four American Indian populations. Youth identified with incident nonsecondary diabetes were invited to a baseline study visit. Self-reported race and ethnicity were collected using the 2000 United States Census questions (14).
Sample selection, design, and measurements
This is a cross-sectional analysis exploring the association between diabetes etiologic group and the magnitude of albuminuria. Inclusion criteria included having a baseline visit during which fasting blood was drawn, urine was collected, and anthropometric measurements were taken. These were used to measure diabetes autoantibodies (DAAs) and covariates used to calculate the insulin sensitivity score (waist circumference, triglycerides [TG], hemoglobin A1c [HbA1c]) and to assess urine albumin:creatinine ratio (UACR). Individuals using ACE inhibitors or angiotensin receptor blockers were excluded from these analyses (n = 38).
The study visit included measurement of waist circumference using the National Health and Nutrition Examination Survey protocol (15), systolic blood pressure, diastolic blood pressure, height, and weight, as previously described (16). Height and weight were measured to calculate BMI (kg/m2), which was then converted to z-scores using the standard Centers for Disease Control and Prevention approach (17).
Blood and urine samples were obtained under conditions of metabolic stability, defined as no episodes of diabetic ketoacidosis in the preceding month and the absence of fever and acute infections. Urine was not collected from girls who were menstruating. Participants excluded from analysis because of missing urine sample (n = 1,064) had similar sociodemographic characteristics as the analyzed cohort (data not shown). Specimens were processed locally and shipped within 24 h to the central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, Seattle, WA). Measurements of serum cholesterol, TG, and HDL cholesterol were performed using Roche reagent on a Roche Module P autoanalyzer (Roche Diagnostics, Indianapolis, IN). HbA1c was measured by a dedicated ion-exchange high-performance liquid chromatography instrument (TOSOH Bioscience).
Random spot urine samples were collected. Urinary creatinine was measured by the Jaffe method using Roche reagent on the Roche Modular P autoanalyzer. Two quality-control samples were analyzed in each run, and the interassay coefficient of variation was consistently <2%. Urine albumin was measured immunochemically using Siemens reagent on a Siemens BNII nephelometer. The sensitivity of the assay was also 0.2 mg/dL. The interassay coefficient of variation is <5% for the high-level and <6.5% for the low-level quality-control sample. Albuminuria was defined as a UACR ≥30 μg/mg as recommended by the American Diabetes Association guidelines (18) and National Kidney Disease Outcomes Quality Initiative (19).
Definitions of DAAs and insulin sensitivity or insulin resistance
Blood samples taken at the baseline visit were analyzed for the 65-kD isoform of glutamate decarboxylase antibodies (GADA) and insulinoma-associated protein 2 antibodies (IA-2A) using the National Institute of Diabetes and Digestive and Kidney Diseases standardized method (20). The cutoff values for positivity were 33 units/mL for GADA and 5 units/mL for IA-2A. The specificity and sensitivity were 97 and 76%, respectively, for GADA and 99 and 64%, respectively, for IA-2A (20). DAA positivity (DAA+) was defined by positive titers for either GADA or IA-2A. Because many participants were treated with insulin, analysis of insulin autoantibodies was not performed.
The insulin sensitivity score was calculated from variables measured at the study visit using the following equation:
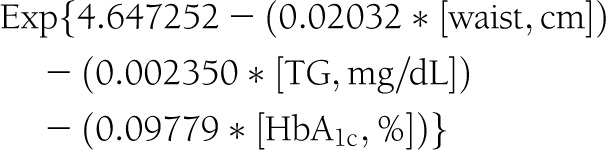 |
This equation was developed and validated using direct measurements of glucose disposal rate from euglycemic-hyperinsulinemic clamps conducted among 85 of the 2,401 SEARCH participants included in this report and 22 matched nondiabetic control subjects (21). As previously reported, we defined insulin resistance among SEARCH participants in this study as an insulin sensitivity score value <25th percentile for the United States general youth population (insulin sensitivity <8.15) (22).
Participants were assigned to one of four diabetes etiologic groups, according to the status of autoimmunity and insulin resistance at their baseline visit. These four groups were as follows: DAA+/insulin-sensitive (IS); DAA+/IR; DAA−/IR; and DAA−/IS.
Statistical analyses
Statistical analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC) and S-PLUS software version 6.0 (Insightful, Seattle, WA). Each minority group was limited in sample size; hence, for the present report, all racial/ethnic groups other than non-Hispanic white were combined into a single “ethnic minority” category. The distribution of each potential covariate was evaluated and, when necessary, logarithmically transformed for normalization of the distribution. The means and percents of covariates were compared across the four etiologic groups using χ2 and ANOVA tests when appropriate. Multivariable regression analyses assessed the relationship between the four etiologic groups and the magnitude of UACR. Both the Shapiro–Wilk test and Kolmogorov–Smirnov test indicated that the residuals did not deviate significantly from a normal distribution. A plot of residuals against the predicted values of the outcome variable found no evidence that the variance of the residuals changed across the range of predicted values. Covariates included in the model were age at visit, sex, race/ethnicity, parental education and insurance type, clinic site, diabetes duration, HbA1c, systolic blood pressure z-score, and BMI z-score. Results were considered significant if P < 0.05.
RESULTS
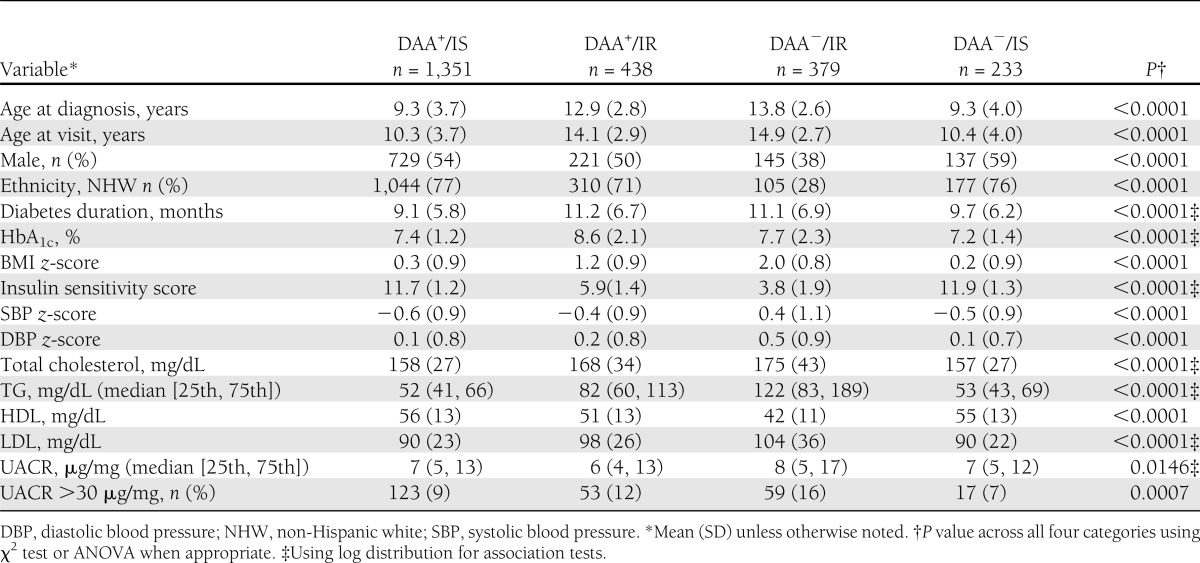
The sociodemographic and clinical characteristics of the 2,401 participants, according to the four etiologic groups, are depicted in Table 1. The ethnic minority group comprised of 323 Hispanics, 312 non-Hispanic blacks, 99 Asians/Pacific Islanders, and 23 Native Americans/Alaska Natives. There were significant differences across the four etiologic groups for all covariates. The largest differences were in the DAA−/IR group, which, in comparison with the other three groups, demonstrated a preponderance of ethnic minorities and elevated systolic blood pressure, diastolic blood pressure, and TG levels. Elevated UACR (≥30 μg/mg) was prevalent in 16% of the DAA−/IR group, which was significantly higher than that of all other groups (P = 0.0007).
Table 1.
Sociodemographic and clinical characteristics of 2,401 youth with type 1 or type 2 diabetes according to etiologic group: SEARCH for Diabetes in Youth Study
Multivariable analysis suggested that the etiologic groups significantly contributed to the variability of UACR (P = 0.004). The adjusted mean UACR for the DAA−/IR group was significantly higher than those of the other three groups (Table 2). All other pairwise comparisons were nonsignificant (data not shown).
Table 2.
UACR least-square means and pairwise comparisons with DAA−/IR group in the SEARCH population
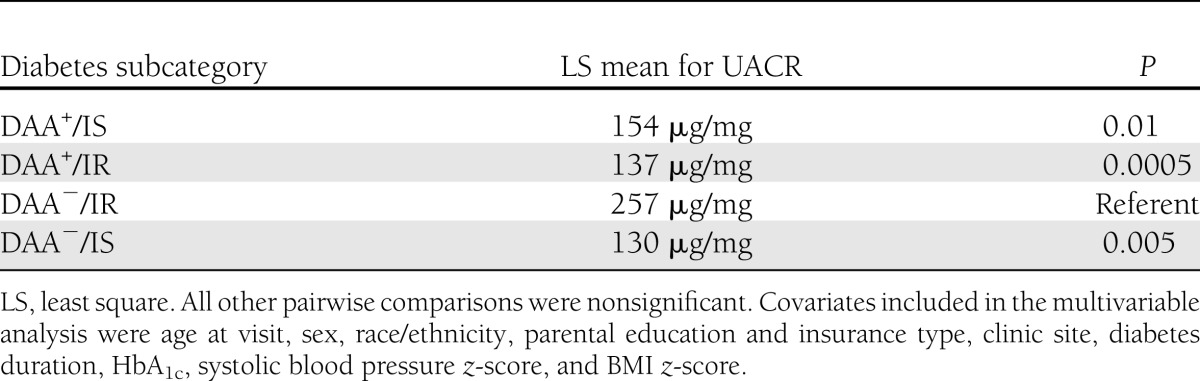
To explore reasons for the difference in UACR between the two IR groups, we performed a post hoc t test on the means of the insulin sensitivity scores and found them to be significantly different (P < 0.0001). We then assessed the contribution of DAA status and insulin sensitivity to the difference in UACR between the two IR groups by performing a post hoc multivariable analysis restricted to the IR participants. The regression equation used the original model but incorporated DAA status and insulin sensitivity (continuous) in place of the four etiologic diabetes type groups. DAA status was not statistically significant (β = 0.18; P = 0.08), whereas insulin sensitivity was significantly and inversely associated with UACR (β = −0.54; P < 0.0001).
CONCLUSIONS
This is the first study to compare the magnitude of albuminuria in youth with diabetes classified according to markers of the underlying etiology of diabetes using measures of autoimmunity and insulin resistance. We found that in youth with recently diagnosed autoimmune-mediated diabetes, there was no difference in UACR between those who were IS compared with IR. There was, however, a significantly higher UACR in youth without autoimmunity but with IR over all other subgroups. There were significant difference in covariates that could be confounders or mediators of the effect of etiologic subgroup; however, we statistically controlled for this issue in our multivariable analysis. We hypothesized that the difference in albuminuria between the two IR groups could be attributable to a greater severity of insulin resistance in the DAA−/IR group. Post hoc analyses showed insulin sensitivity to be significantly associated with UACR in the IR groups.
Our finding that there was no difference in UACR between youth with autoimmune-mediated diabetes who were IS compared with IR was unexpected. The hypothesis that insulin resistance in addition to autoimmunity could increase the risk of microvascular complications of diabetes was proposed 20 years ago (23). Several studies have since identified increases in both microvascular and macrovascular complications in persons with type 1 diabetes with versus without insulin resistance (11,12,24,25). It is difficult to compare these studies with ours because of differences in study population and methodologies, especially our pediatric cohort with newly diagnosed diabetes and estimation of insulin resistance.
The significant difference in UACR between the DAA+/IR and DAA−/IR groups also was unexpected. Post hoc analysis restricted to the two IR groups indicated that differences in the degree of IS between the two groups was associated with the higher UACR in the DAA−/IR group. This finding, along with the lack of difference in UACR between the DAA+/IR and the two IS groups, suggest a threshold effect of IR on the magnitude of albuminuria. Our study design imposes a logical yet arbitrary cutpoint for IR to define the four diabetes etiologic groups. Future studies exploring the shape of the relationship between insulin sensitivity and UACR are needed to clarify their relationship.
We are not the first to find an association between the degree of insulin resistance and the degree of albuminuria (26–28). The components of metabolic syndrome have long been identified as risk factors for albuminuria, even in the absence of diabetes (27,29). Recently, several theories have been proposed regarding the mechanisms with which insulin resistance could lead to albuminuria as well as other microvascular and macrovascular complications (30–32). Visceral adipose tissue is unique in its ability to function as a metabolic and endocrinologic organ (32). Macrophage infiltration and adipokine production result in an inflammatory and hormonal cascade that has been found to have direct effects on endothelial and podocyte functions within the glomerulus (30,31,33). Moreover, in the presence of adipokines, podocytes have been noted to develop altered insulin signaling that renders them more susceptible to apoptosis, leading to albuminuria (30,31). It is also important to consider that the relationships of diabetic nephropathy lesions and albuminuria are more complex in type 2 versus type 1 diabetes. A substantial subset of microalbuminuric type 2 patients may have glomerular structural parameters in the normal range, whereas this is rare among type 1 patients (34). Thus, the prognostic significance of the albuminuria findings in the current study will require long-term follow-up.
There are several limitations to our study. Most importantly, we collected only a single random urine specimen. Orthostatic proteinuria is fairly common in children, as is intraindividual variation in UACR (35,36). However, both of these situations would result in nondifferential misclassification, which would bias the results toward the null hypothesis. Another limitation is the use of only two antibodies, IA-2A and GADA, for identification of autoimmune-mediated diabetes. Recently, autoantibodies to zinc transporter 8 have been identified in the pathogenesis of type 1 diabetes (37). Although there are plans to measure this autoantibody in SEARCH, it is not yet available. This could have resulted in misclassification in the DAA−/IR group; however, again, this would bias our results toward the null hypothesis. In addition, we estimated insulin sensitivity rather than measuring it with the euglycemic-hyperinsulinemic clamp. Euglycemic-hyperinsulinemic clamp studies are impractical for use in a large cohort of children because of the invasiveness and high cost. Our estimating equation was developed and validated in a subset of 85 SEARCH participants and 22 nondiabetic controls who underwent euglycemic-hyperinsulinemic clamp study and was found to explain 74% of the variance of glucose disposal rate (21). This is superior to a previously published estimating equation that used only 24 participants and found their equation to explain 57% of the variance in glucose disposal rate (38). We defined insulin resistance using an arbitrary cutpoint of the 25th percentile in the insulin sensitivity score in nondiabetic children. Previously, we performed sensitivity analyses using higher and lower cutpoints of the IS score and found the groups to be extremely similar (22).
Strengths of our study include the large sample size and ethnic and geographic diversity of the SEARCH cohort, which make our findings very generalizable. Moreover, SEARCH is a unique and valuable resource because it includes youth with newly diagnosed type 1 and type 2 diabetes. No other study has had the capability to explore the impact of insulin resistance on microvascular complications across the diabetes etiologic spectrum in a pediatric population.
In summary, using a novel approach to etiologic classification of diabetes type, we have been able to explore the association of diabetes etiologic subgroups with the severity of albuminuria in newly diagnosed youth with diabetes. Our results suggest that rather than the presence of diabetes autoimmunity, it may be the severity of insulin resistance that more strongly associates with albuminuria. This finding increases the urgency for investigators to determine the pathogenesis and prognostic significance of albuminuria in these young diabetic patients, and for clinicians and public health experts to address the overweight and obesity epidemic in youth. Further analyses of the spectrum of insulin resistance and thresholds that may increase the risk for diabetic kidney disease are warranted.
Acknowledgments
SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site contract numbers for the study are as follows: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714); University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1); Kuakini Medical Center (U58CCU919256 and U01 DP000245); Children’s Hospital Medical Center, Cincinnati (U48/CCU519239, U01 DP000248, and 1U18DP002709); University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708-01); University of Washington School of Medicine (U58/CCU019235-4, U01 DP000244, and U18DP002710-01); and Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200-2010-35171). The authors also received support from involvement of General Clinical Research Centers (GCRC) at the South Carolina Clinical and Translational Research (SCTR) Institute at the Medical University of South Carolina (National Institutes of Health/National Center for Research Resources grant number UL1RR029882); Children’s Hospital and Regional Medical Center (grant number M01RR00037); Colorado Pediatric General Clinical Research Center (grant number M01 RR00069) and the Barbara Davis Center at the University of Colorado at Denver (DERC National Institutes of Health P30 DK57516); and the Institutional Clinical and Translational Science Award (CTSA) and National Institutes of Health/National Center for Research Resources at the University of Cincinnati (grant number 1UL1RR026314-01).
No potential conflicts of interest relevant to this article were reported.
A.K.M. designed the study, wrote the manuscript, interpreted the data, and made substantial contributions to the manuscript. A.L. processed and analyzed the data. D.D. collected the data, designed the study, interpreted the data, and made substantial contributions to the manuscript. D.M.M. interpreted the data and made substantial contributions to the manuscript. R.B.D. processed and analyzed data. L.M.D. collected the data, interpreted the data, and made substantial contributions to the manuscript. L.K.G. interpreted the data and made substantial contributions to the manuscript. J.M.L. collected data, interpreted the data, and made substantial contributions to the manuscript. B.R. collected the data. S.M.M. collected the data, interpreted the data, and made substantial contributions to the manuscript. G.I. collected the data, interpreted the data, and made substantial contributions to the manuscript. R.K.S. interpreted the data and made substantial contributions to the manuscript. M.A. interpreted the data and made substantial contributions to the manuscript. K.R. interpreted the data and made substantial contributions to the manuscript. A.D.L. collected the data. M.M. interpreted the data and made substantial contributions to the manuscript. E.J.M.-D. collected the data, designed the study, interpreted the data, and made substantial contributions to the manuscript. A.K.M. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Pugh JA, Medina R, Ramirez M. Comparison of the course to end-stage renal disease of type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetic nephropathy. Diabetologia 1993;36:1094–1098 [DOI] [PubMed] [Google Scholar]
- 2.Hasslacher C, Ritz E, Wahl P, Michael C. Similar risks of nephropathy in patients with type I or type II diabetes mellitus. Nephrol Dial Transplant 1989;4:859–863 [DOI] [PubMed] [Google Scholar]
- 3.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003;289:3273–3277 [DOI] [PubMed] [Google Scholar]
- 4.Molitch ME, Steffes M, Sun W, et al. Epidemiology of Diabetes Interventions and Complications Study Group Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010;33:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maahs DM, Snively BM, Bell RA, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care 2007;30:2593–2598 [DOI] [PubMed] [Google Scholar]
- 6.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care 2012;35:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 2006;29:1300–1306 [DOI] [PubMed] [Google Scholar]
- 8.Tripathi A, Rizvi AA, Knight LM, Jerrell JM. Prevalence and impact of initial misclassification of pediatric type 1 diabetes mellitus. South Med J 2012;105:513–517 [DOI] [PubMed] [Google Scholar]
- 9.Liu LL, Lawrence JM, Davis C, et al. SEARCH for Diabetes in Youth Study Group Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes 2010;11:4–11 [DOI] [PubMed] [Google Scholar]
- 10.Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 2010;95:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care 2007;30:707–712 [DOI] [PubMed] [Google Scholar]
- 12.Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int 2002;62:963–970 [DOI] [PubMed] [Google Scholar]
- 13.SEARCH Study Group SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 2004;25:458–471 [DOI] [PubMed] [Google Scholar]
- 14.Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat 2 2003. (135):1–55 [PubMed] [Google Scholar]
- 15.Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 2004;145:439–444 [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care 2006;29:1891–1896 [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002;109:45–60 [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.KDOQI KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007;49(Suppl. 2):S12–S154 [DOI] [PubMed] [Google Scholar]
- 20.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabelea D, D’Agostino RB, Jr, Mason CC, et al. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia 2011;54:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabelea D, Pihoker C, Talton JW, et al. SEARCH for Diabetes in Youth Study Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care 2011;34:1628–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teupe B, Bergis K. Epidemiological evidence for “double diabetes.” Lancet 1991;337:361–362 [DOI] [PubMed] [Google Scholar]
- 24.Chillarón JJ, Goday A, Flores-Le-Roux JA, et al. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab 2009;94:3530–3534 [DOI] [PubMed] [Google Scholar]
- 25.Olson JC, Erbey JR, Williams KV, et al. Subclinical atherosclerosis and estimated glucose disposal rate as predictors of mortality in type 1 diabetes. Ann Epidemiol 2002;12:331–337 [DOI] [PubMed] [Google Scholar]
- 26.Rowley KG, Iser DM, Best JD, O’Dea K, Leonard D, McDermott R. Albuminuria in Australian Aboriginal people: prevalence and associations with components of the metabolic syndrome. Diabetologia 2000;43:1397–1403 [DOI] [PubMed] [Google Scholar]
- 27.Mykkänen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes 1998;47:793–800 [DOI] [PubMed] [Google Scholar]
- 28.Nosadini R, Cipollina MR, Solini A, et al. Close relationship between microalbuminuria and insulin resistance in essential hypertension and non-insulin dependent diabetes mellitus. J Am Soc Nephrol 1992;3(Suppl.):S56–S63 [DOI] [PubMed] [Google Scholar]
- 29.Bonnet F, Marre M, Halimi JM, et al. DESIR Study Group Waist circumference and the metabolic syndrome predict the development of elevated albuminuria in non-diabetic subjects: the DESIR Study. J Hypertens 2006;24:1157–1163 [DOI] [PubMed] [Google Scholar]
- 30.Diez-Sampedro A, Lenz O, Fornoni A. Podocytopathy in diabetes: a metabolic and endocrine disorder. Am J Kidney Dis 2011;58:637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welsh GI, Coward RJ. Podocytes, glucose and insulin. Curr Opin Nephrol Hypertens 2010;19:379–384 [DOI] [PubMed] [Google Scholar]
- 32.Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab 2008;34:2–11 [DOI] [PubMed] [Google Scholar]
- 33.Kang YS, Song HK, Lee MH, et al. Visfatin is upregulated in type-2 diabetic rats and targets renal cells. Kidney Int 2010;78:170–181 [DOI] [PubMed] [Google Scholar]
- 34.Fioretto P, Stehouwer CD, Mauer M, et al. Heterogeneous nature of microalbuminuria in NIDDM: studies of endothelial function and renal structure. Diabetologia 1998;41:233–236 [DOI] [PubMed] [Google Scholar]
- 35.Brandt JR, Jacobs A, Raissy HH, et al. Orthostatic proteinuria and the spectrum of diurnal variability of urinary protein excretion in healthy children. Pediatr Nephrol 2010;25:1131–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McHardy KC, Gann ME, Ross IS, Pearson DW. A simple approach to screening for microalbuminuria in a type 1 (insulin-dependent) diabetic population. Ann Clin Biochem 1991;28:450–455 [DOI] [PubMed] [Google Scholar]
- 37.Énée E, Kratzer R, Arnoux JB, et al. ZnT8 is a major CD8+ T cell-recognized autoantigen in pediatric type 1 diabetes. Diabetes 2012;61:1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 2000;49:626–632 [DOI] [PubMed] [Google Scholar]


