Abstract
OBJECTIVE
Preclinical data suggest that linagliptin, a dipeptidyl peptidase-4 inhibitor, may lower urinary albumin excretion. The ability of linagliptin to lower albuminuria on top of renin-angiotensin-aldosterone system (RAAS) inhibition in humans was analyzed by pooling data from four similarly designed, 24-week, randomized, double-blind, placebo-controlled, phase III trials.
RESEARCH DESIGN AND METHODS
A pooled analysis of four completed studies identified 217 subjects with type 2 diabetes and prevalent albuminuria (defined as a urinary albumin-to-creatinine ratio [UACR] of 30−3,000 mg/g creatinine) while receiving stable doses of RAAS inhibitors. Participants were randomized to either linagliptin 5 mg/day (n = 162) or placebo (n= 55). The primary end point was the percentage change in geometric mean UACR from baseline to week 24.
RESULTS
UACR at week 24 was reduced by 32% (95% CI −42 to −21; P < 0.05) with linagliptin compared with 6% (95% CI −27 to +23) with placebo, with a between-group difference of 28% (95% CI −47 to −2; P = 0.0357). The between-group difference in the change in HbA1c from baseline to week 24 was −0.61% (−6.7 mmol/mol) in favor of linagliptin (95% CI −0.88 to −0.34% [−9.6 to −3.7 mmol/mol]; P < 0.0001). The albuminuria-lowering effect of linagliptin, however, was not influenced by race or HbA1c and systolic blood pressure (SBP) values at baseline or after treatment.
CONCLUSIONS
Linagliptin administered in addition to stable RAAS inhibitors led to a significant reduction in albuminuria in patients with type 2 diabetes and renal dysfunction. This observation was independent of changes in glucose level or SBP. Further research to prospectively investigate the renal effects of linagliptin is underway.
The increasing prevalence of chronic kidney disease (CKD), defined as the presence of increased urinary albumin excretion and/or decreased glomerular filtration rate (GFR), is a major public health issue affecting ∼13% of the U.S. population (1,2). Diabetic kidney disease is the leading cause of end-stage renal disease (ESRD) in developed countries, and both the incidence and prevalence are increasing dramatically worldwide. The development of albuminuria is a key step in the progression of diabetic kidney disease, and worsening of albuminuria is a significant predictor of progressive renal disease (3,4). Epidemiological data indicate that 39 and 10% of subjects with type 2 diabetes have micro- or macroalbuminuria, respectively (5). In addition, albuminuria (both in low and high ranges) predicts cardiovascular (CV) risk in patients with type 2 diabetes and in the general population (3,4,6,7). Guidelines recommend the annual assessment of albuminuria in all patients with type 2 diabetes starting at diagnosis, and current recommendations for the treatment of kidney disease in patients with type 2 diabetes are directed toward a multifactorial intervention, including lowering blood pressure, improving glycemic and lipid control, and reducing albuminuria (1,8).
Inhibitors of the renin-angiotensin-aldosterone system (RAAS) provide renal and CV protection beyond their ability to lower blood pressure (9,10), and the beneficial effects of these agents have been linked to concomitant changes in albuminuria. Thus, reductions in albuminuria in patients with type 2 diabetes were associated with a significant reduction in the risk of progression to ESRD (11–13). These findings suggest that albuminuria may be an important therapeutic target for preventing the progression of diabetic kidney disease and might also offer CV protection. However, despite treatment with current recommended standard therapy for CKD, including RAAS inhibitors, many patients with type 2 diabetes have significant residual albuminuria and continue to progress toward ESRD (14,15). Additional treatment options that would complement the benefit of existing therapies remain an important unmet medical need.
Recent experimental studies have suggested beneficial renal effects of incretin-based therapies (16–22). In a murine model of renal vascular damage (endothelial nitric oxide synthase knockout mice), coadministration of linagliptin, an oral and highly selective dipeptidyl peptidase-4 (DPP-4) inhibitor, and the angiotensin receptor blocker (ARB) telmisartan synergistically decreased albuminuria and reduced glomerulosclerosis. These results occurred independent of any changes in glucose metabolism because the β-cell response to linagliptin was alleviated as a result of previous administration of streptozotocin, a β-cell toxin, to these mice (16). However, clinical evidence regarding the renal effects of incretin-based therapies in patients with type 2 diabetes is scarce (23,24), and conclusive evidence to translate the findings of animal models to humans has yet to emerge from dedicated randomized clinical trials. Nevertheless, urinary albumin excretion, assessed by the albumin-to-creatinine (Cr) ratio (UACR), is often collected in clinical development programs involving people with diabetes. Indeed, one advantage of the databases collected during drug development is the opportunity to pool data from individual studies, which significantly increases the available power for further exploratory analyses. In this study, we used data collected during the development of linagliptin to test the hypothesis that linagliptin may reduce albuminuria in patients with type 2 diabetes and renal dysfunction.
RESEARCH DESIGN AND METHODS
This retrospective analysis used data from the global linagliptin development program. It included four phase III clinical trials conducted between January 2008 and May 2010 to assess the safety and efficacy of linagliptin in patients with type 2 diabetes (25–28) (Supplementary Table 1). These four trials were all randomized, double-blind, and placebo-controlled with identical study duration, primary end point definition, and safety assessments, which allowed data to be pooled appropriately (Supplementary Fig. 1). The design and results of these four individual trials have been described previously in detail (25–28).
In brief, patients were eligible for each of these four trials if they were aged 18−80 years, had type 2 diabetes, had a BMI ≤40 kg/m2, and were either treatment-naïve (HbA1c levels of 7.0−11.0% [53−97 mmol/mol] at screening) or had previously received one or two oral glucose-lowering therapies (HbA1c levels of 6.5−10.5% [48−91 mmol/mol] at screening). Eligible study participants were randomized to receive either placebo or linagliptin 5 mg in addition to background therapy from their original study. The primary efficacy outcome of all four studies was change in HbA1c from baseline to week 24.
Safety assessments in all four studies were identical and predefined. Respective safety results have been reported previously (25–28). Renal function assessments consisted of estimated GFR (eGFR) as determined by the Modification of Diet in Renal Disease formula and estimated Cr clearance as determined by the Cockcroft-Gault formula. Albuminuria was determined by UACR from a spot urine sample at baseline and after 12 and 24 weeks of treatment. All assessments of urine and blood were performed at a central laboratory (MDS Pharma Services Central Laboratories and Covance Laboratories). Microalbuminuria was defined as a baseline UACR of 30−300 mg/g Cr, and macroalbuminuria was defined as a baseline UACR of 300−3,000 mg/g Cr. Stages of CKD were categorized based on the classification system established by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (1).
Safety analyses included the frequency and intensity of adverse events (AEs), vital signs (e.g., systolic blood pressure [SBP] and diastolic blood pressure), clinical laboratory measures, and evaluation of hypoglycemia. Repeated blood pressure measurements were performed at each study visit with the subject in a seated position after a minimum of 5 min rest.
End points
The primary efficacy end point of this pooled analysis was specified as the percentage change in geometric mean UACR from baseline to week 24. Secondary efficacy end points were changes in HbA1c and fasting plasma glucose (FPG) from baseline to week 24.
Statistical methods
The pooled population consisted of all randomized individuals (n = 2,472) who received at least one dose of study drug (treated set: placebo group, n = 679; linagliptin group, n = 1,793). Among this population, subjects were included in the primary pooled analysis set if they met the following criteria: a baseline UACR between 30 and 3,000 mg/g Cr, a baseline eGFR ≥30 mL/min/1.73 m2, and receiving stable doses of ACE inhibitors (ACEIs), ARBs, or both for at least 4 weeks before the study and from baseline to the date of the last UACR on-treatment measurement within the 24-week treatment period.
All efficacy analyses were performed on the full analysis set. This included randomized subjects who received at least one dose of study treatment, had a baseline measurement of the relevant end point, and had at least one on-treatment measurement of the relevant end point. Missing data were handled using the last observation carried forward approach, with observations after the start of rescue therapy accepted as observed cases. Because of their skewed distribution, the UACR data were log10-transformed before analysis and changes from baseline were analyzed by ANCOVA and adjusted for log10-transformed baseline values and trial effect. Results of the analysis were back-transformed to obtain geometric means of the UACR ratios of the 24-week value to the baseline value and corresponding 95% CI; the values then were expressed as percentage change in adjusted geometric mean of the UACR ratios of the 24-week value to the baseline value, as previously reported (29). The UACR results were calculated for the primary pooled analysis set and the four individual studies. Furthermore, subgroup analyses were performed for the following factors: race (Asian, white), baseline HbA1c (less than mean, more than or equal to mean), and baseline SBP (less than mean, more than or equal to mean). All subjects in the primary pooled analysis set received concomitant ACEIs, ARBs, or both. To further evaluate whether concomitant RAAS blockade could influence the effect of linagliptin on albuminuria, an additional sensitivity analysis set was defined to test the primary end point and included subjects who were not treated with RAAS inhibitors but who met criteria for UACR and eGFR at baseline.
The changes in HbA1c from baseline to week 24 were assessed using ANCOVA, with treatment, study, and washout as fixed classification effects and baseline HbA1c as a covariate. The same model with the addition of the covariate baseline FPG was used to assess changes in FPG from baseline to week 24. The reductions in HbA1c within the linagliptin group were stratified into quartiles based on the change from baseline to week 24: <0.1, 0.1−0.59, 0.60−0.99, and ≥1.0% (<1.1, 1.1−6.5, 6.6−10.8, and ≥10.9 mmol/mol). Effects on SBP after treatment within the linagliptin group were stratified into three categories based on the change from baseline to the last value on treatment: SBP decrease of >1.0 mmHg, stable SBP with only minimal changes between −1.0 and +1.0 mmHg, and SBP increase of >1.0 mmHg. The difference between percentage changes in geometric mean UACR across HbA1c quartiles and SBP categories was tested using an ANOVA F-test to determine whether they were statistically significantly different. Safety assessments were performed on the pooled treated set. All analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
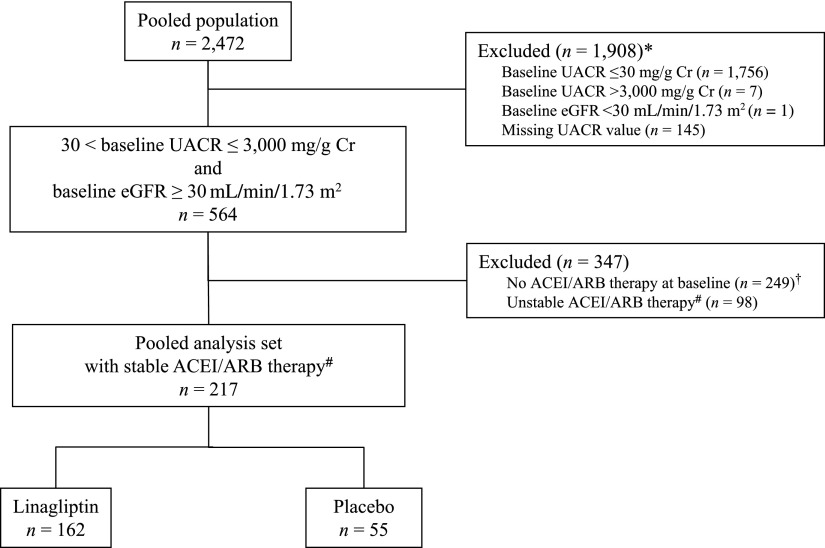
Patient disposition is shown in Fig. 1. Of the 2,472 subjects who were included in the treated set of the four clinical studies, 564 subjects had albuminuria (UACR 30−3,000 mg/g Cr) and an eGFR ≥30 mL/min/1.73 m2 at screening. Furthermore, of these 564 subjects, 217 subjects were receiving stable doses of ACEIs and/or ARBs at baseline and during the 24-week treatment period and were used in the primary analysis set (Fig. 1). Of note, only seven patients were excluded from the analysis because of a UACR >3,000 mg/g Cr. The sensitivity analysis set included 249 subjects with type 2 diabetes and prevalent albuminuria who were not previously treated with RAAS inhibitors (Fig. 1).
Figure 1.
Patient disposition. *Patients might have had more than one exclusion criterion. †183 participants receiving linagliptin; 66 participants receiving placebo. #For at least 4 weeks before the study and from baseline to the date of the last UACR measurement within the 24-week treatment period.
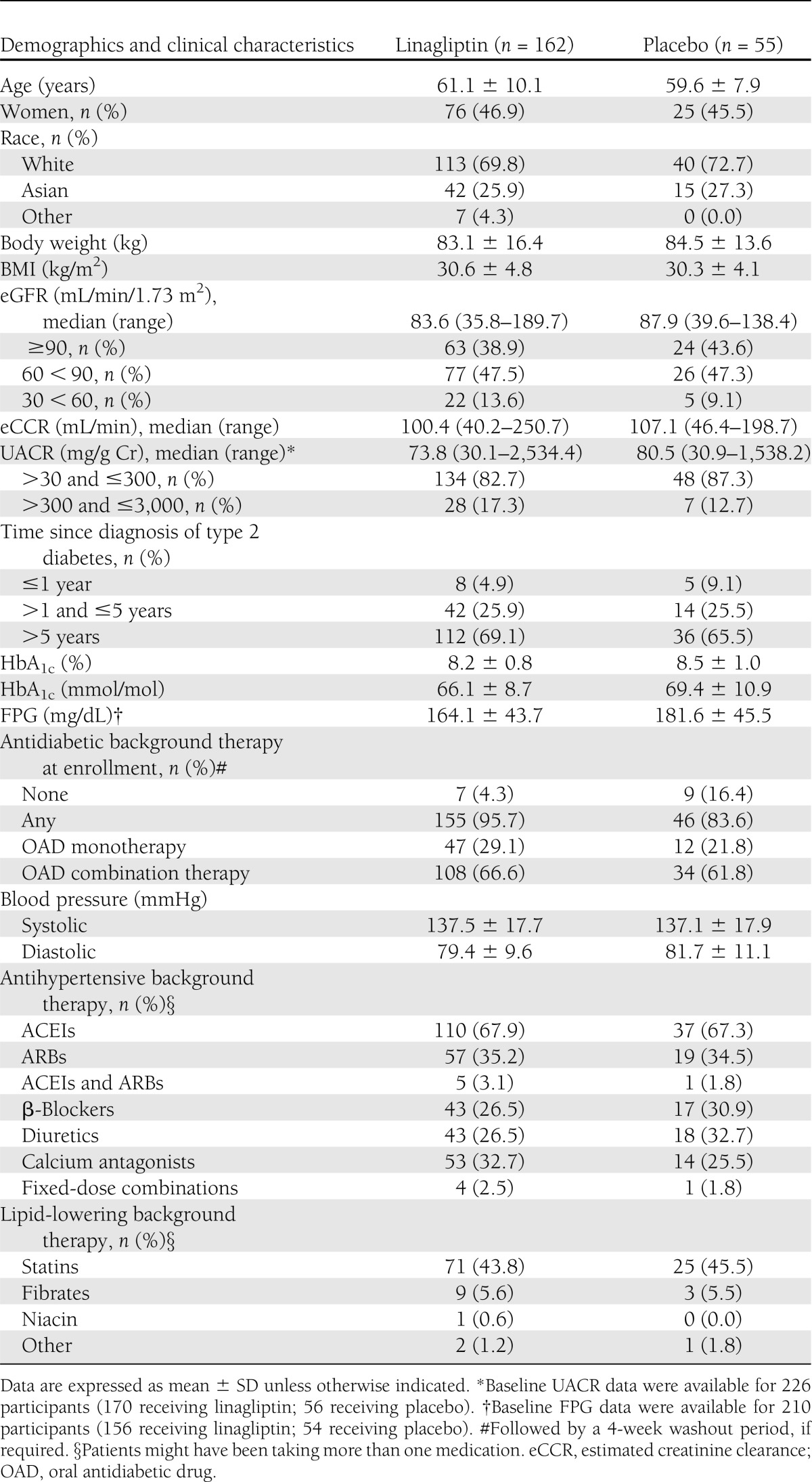
Baseline demographic, clinical, and biochemical characteristics, as well as concomitant background therapies, were balanced between the two treatment groups (Table 1). Overall, the majority of the subjects (71%) were white. Mean age and baseline HbA1c of the study population were 60.7 ± 9.6 years and 8.3 ± 0.9% (67.2 ± 9.8 mmol/mol), respectively, and 68% of subjects had type 2 diabetes for more than 5 years. At study entry, most individuals had microalbuminuria (84%) and mild or no renal impairment (88%); 68 and 35% of participants received ACEIs or ARBs at screening, respectively, with only 3% of participants receiving dual RAAS blockade.
Table 1.
Study participant demographics and baseline clinical characteristics
Efficacy
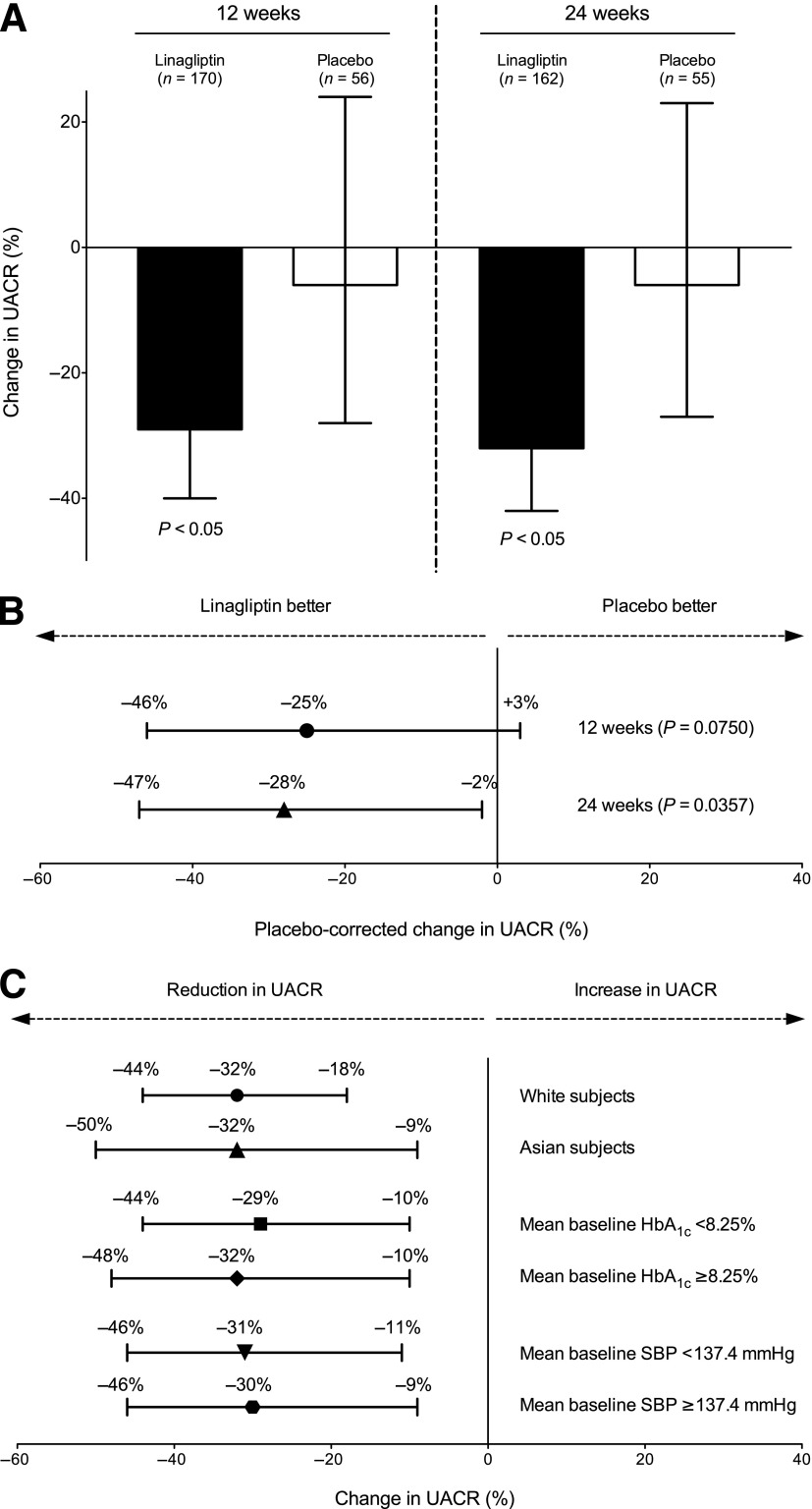
Median UACR values were similar between treatment groups at baseline: 73.8 (30.1−2,534.4) and 80.5 (30.9−1,538.2) mg/g Cr in the linagliptin and placebo groups, respectively (Table 1). After 24 weeks of treatment, the percentage change in adjusted geometric mean UACR from baseline was significantly higher with linagliptin (−32% [95% CI −42 to −21]; P < 0.05) compared with placebo (−6% [95% CI −27 to +23]), with a between-group difference of −28% (95% CI −47 to −2; P = 0.0357) (Fig. 2A and B). Notably, the magnitude of the albuminuria-lowering effect of linagliptin was already seen after 12 weeks of treatment (−29% [95% CI −40 to −17]; P < 0.05; between-group difference: −25% [95% CI −46 to +3]; P = 0.0750) (Fig. 2A and B).
Figure 2.
A: Adjusted geometric mean of percentage change in UACR from baseline to 12 and 24 weeks (■, linagliptin; □, placebo); P < 0.05 versus baseline. Error bars represent 95% CIs. B: Adjusted geometric mean of placebo-corrected percentage change in UACR from baseline to 12 (n = 226; P = 0.0750; ●) and 24 weeks (n = 217; P = 0.0357; ▲). Error bars represent 95% CIs. C: Adjusted geometric mean of percentage change in UACR from baseline to week 24 stratified by race, mean baseline HbA1c, and mean baseline SBP in the linagliptin group. ●, white subjects (n = 113); ▲, Asian subjects (n = 45; treatment × race interaction, P = 0.7397); ■, mean baseline HbA1c <8.25% (n = 97); ♦, mean baseline HbA1c ≥8.25% (n = 65; treatment × baseline HbA1c interaction, P = 0.8100); ▼, mean baseline SBP <137.4 mmHg (n = 88);  , mean baseline SBP ≥137.4 mmHg (n = 74; treatment × baseline SBP interaction, P = 0.6475). Error bars represent 95% CIs.
, mean baseline SBP ≥137.4 mmHg (n = 74; treatment × baseline SBP interaction, P = 0.6475). Error bars represent 95% CIs.
Further subgroup analyses of the primary end point were performed for race, baseline HbA1c, and baseline SBP. For each analysis, there was no statistically significant interaction between treatment and the relevant subgroup. The overall effect of linagliptin was consistent with the results from the primary analysis (Fig. 2C and Supplementary Fig. 2).
We further explored the effect of linagliptin on the UACR in patients with renal dysfunction who were not previously treated with RAAS inhibitors. This sensitivity analysis showed a significant reduction in the UACR from baseline to week 24 with linagliptin (−30% [95% CI −40 to −19]; P < 0.05; n = 183). The between-group difference of −17% (95% CI −38 to +12) showed a similar trend as the primary analysis set but did not reach statistical significance (P = 0.2301; n = 249).
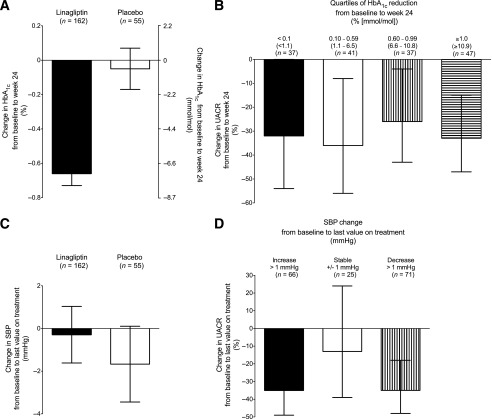
Important potential confounding factors for the primary end point that were considered included improvements in glycemic control and alterations in blood pressure or renal function during the 24 weeks of treatment in either group. As expected, linagliptin led to significant reductions in HbA1c. Adjusted mean changes in HbA1c from baseline to week 24 were −0.66% (−7.2 mmol/mol) with linagliptin compared with −0.05% (−0.5 mmol/mol) with placebo (Fig. 3A), with a between-group difference of −0.61% (−6.7 mmol/mol) in favor of linagliptin (95% CI −0.88 to −0.34% [−9.6 to −3.7 mmol/mol]; P < 0.0001). HbA1c also was stratified into quartiles based on the change from baseline to week 24. The percentage changes in the geometric mean UACR at week 24 for linagliptin across these categories were not statistically significantly different (Fig. 3B). Furthermore, significant results were seen in adjusted mean changes in FPG from baseline to week 24 (−8.88 mg/dL with linagliptin versus +16.22 mg/dL with placebo; between-group difference −25.1 mg/dL [95% CI −38.55 to −11.65]; P = 0.0003).
Figure 3.
A: Adjusted mean change in HbA1c from baseline to week 24 (■, linagliptin; □, placebo). There was a between-group difference of −0.61% (−6.7 mmol/mol) in favor of linagliptin (95% CI −0.88 to −0.34% [−9.6 to −3.7 mmol/mol]; P < 0.0001). Error bars represent SE. B: Adjusted geometric mean of percentage change in UACR by quartiles of HbA1c reduction in the linagliptin group: ■, <0.1% (<1.1 mmol/L) HbA1c reduction; □, 0.10−0.59% (1.1−6.5 mmol/L) HbA1c reduction; ▥, 0.60−0.99% (6.6−10.8 mmol/L) HbA1c reduction; ▤, ≥1.0% (≥10.9 mmol/L) HbA1c reduction; P ≥ 0.05 (ANOVA F test). Error bars represent 95% CIs. C: Adjusted mean change in SBP from baseline to last value during treatment (■, linagliptin; □, placebo). Error bars represent SE. D: Adjusted geometric mean of percentage change in UACR by categories of SBP change in the linagliptin group: ■, SBP increase >1.0 mmHg; □, −1.0 mmHg ≤ SBP change ≤ +1.0 mmHg; ▥, SBP decrease >1.0 mmHg; P ≥ 0.05 (ANOVA F test). Error bars represent 95% CIs.
We found no clinically meaningful changes in SBP from baseline to last value on treatment in subjects treated with either linagliptin or placebo (Fig. 3C). SBP was stratified into three categories based on the change from baseline to last value on treatment. The percentage changes in the geometric mean UACR at week 24 for linagliptin across these categories were not statistically significantly different (Fig. 3D). There were no relevant differences between the distribution of different RAAS inhibitors at baseline. Moreover, changes in other blood pressure–lowering medications during the study were rare and were balanced overall between the two treatment groups (data not shown).
Finally, median eGFR at baseline was similar between the linagliptin and placebo groups: 83.6 (35.8–189.7) and 87.9 (39.6–138.4) mL/min/1.73 m2, respectively (Table 1). No clinically meaningful changes were observed from baseline to the last value on treatment in either treatment group (median −1.3 and −0.2 mL/min/1.73 m2, respectively).
Safety
Among participants of this pooled analysis, linagliptin was safe and well tolerated. The overall incidence of clinical AEs was similar between the linagliptin and placebo groups (63.0 vs. 63.6%, respectively) (Supplementary Table 2). The incidence of AEs for renal and urinary disorders was similar between treatment groups (4.3 vs. 5.5%, respectively). The proportion of patients experiencing drug-related AEs was slightly higher with linagliptin than with placebo (14.2 vs. 7.3%). This difference was most likely related to a higher incidence of drug-related hypoglycemia in the linagliptin group (8.6 vs. 0.0%). Notably, all subjects in the linagliptin group who experienced investigator-reported hypoglycemia were receiving sulfonylurea background therapy. No investigator-reported hypoglycemic events were reported in patients treated with linagliptin as monotherapy or in addition to metformin. In addition, no severe hypoglycemic events (i.e., events requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions) were reported for any participant.
CONCLUSIONS
This pooled analysis of four phase III clinical trials from the clinical development program has demonstrated that linagliptin significantly reduced albuminuria from baseline by 28% compared with placebo after 24 weeks of treatment. Notably, the magnitude of the effect on albuminuria was already seen after 12 weeks of treatment. In addition, the albuminuria-lowering effect of linagliptin was consistently found in relevant patient subgroups, such as those categorized by race, baseline HbA1c, and baseline SBP. This analysis suggests that linagliptin may have direct effects on the kidney in type 2 diabetes and that prospective trials testing this hypothesis are warranted.
Several clinical factors are well known to influence urinary albumin excretion in type 2 diabetes, such as changes in SBP or loss of renal function. However, we found no clinically meaningful changes in SBP and eGFR during 24 weeks of treatment in either group, indicating both hemodynamic and renal safety of linagliptin. Moreover, for linagliptin, there was no statistically significant difference between the percentage changes in geometric mean UACR across the categories of SBP for the last value on treatment. This indicates that blood pressure does not have a significant influence on the effect of linagliptin on the UACR in our analysis.
In addition, changes in hyperglycemia are a potentially important confounder. In fact, large-scale intervention studies have demonstrated that sustained improvements in glycemic control reduce the development and progression of microvascular complications in patients with type 2 diabetes after long-term treatment (30–32). Hence, it is tempting to speculate that any reduction in albuminuria may simply mirror concomitant improvements in glucose control after short-term treatment. The evidence for a potential effect of relatively short-term glucose control (over months rather than years) on albuminuria in diabetes is, however, inconclusive. An improvement in HbA1c after 52 weeks of insulin infusion—from 9.5 to 7.3% (80.3 to 56.3 mmol/mol)—was not associated with any significant changes in renal parameters, such as GFR or urinary albumin excretion, in patients with insulin-dependent diabetes and elevated urinary albumin excretion (30–300 mg/24 h) (33). Moreover, a previous study by Tuttle et al. (34) reported that strict glycemic control did lower HbA1c levels from 8.4 to 6.9% (68 to 52 mmol/mol) after 3 weeks of intensive insulin therapy. Although renal hemodynamic responses to increased plasma amino acid concentrations were improved, the rapid HbA1c reduction did not lead to significant changes in urinary albumin excretion. In line with these findings, improvement in glycemic control did not improve microalbuminuria in an adolescent population with insulin-dependent diabetes using either intensive conventional therapy or insulin infusion up to 8 months (35). These results suggest no major influence of short-term glucose control on urinary albumin excretion. However, an exact timely separation between short- and longer-term interdependencies between glucose control and progression of renal disease in type 2 diabetes is difficult. Our analysis does not support a direct relationship between short-term glucose control and changes in UACR. We found no statistically significant difference between the percentage changes in geometric mean UACR and changes in HbA1c at week 24 for linagliptin. In fact, patients with only modest reductions in HbA1c showed similar changes in UACR compared with those having more profound reductions in HbA1c (>1.1% [12.0 mmol/mol]) after 24 weeks of treatment with linagliptin.
The mechanisms by which linagliptin may additionally improve the effects of RAAS inhibitors in the kidney remain to be fully elucidated. The hypothesis of a potential albuminuria-lowering effect of linagliptin was first raised as a result of findings from an experimental animal study assessing the renal effects of coadministration of linagliptin with telmisartan in diabetic endothelial nitric oxide synthase knockout mice (16). In this model of vascular renal damage, 11 weeks of combination therapy significantly reduced urinary albumin excretion, independent of changes in blood glucose, and the effects were greater than those seen with RAAS blockade alone. The available evidence suggests that the albuminuria-lowering effect of linagliptin may be due to inhibition of podocyte damage and myofibroblast transformation (36) as well as a consequence of improvement in renal inflammatory responses mediated by increased glucagon-like peptide-1 (GLP-1) activity (16,36) or inhibition of tumor necrosis factor-α (16). Moreover, treatment with linagliptin reduced plasma levels of osteopontin, a marker of vascular calcification and progression of renal disease (16). These results are further supported by several other experimental studies of DPP-4 inhibitors showing beneficial effects of sitagliptin and vildagliptin on albuminuria and renal function in models of diabetic nephropathy (18–20). There is also evidence from experimental research suggesting that treatment with GLP-1 receptor agonists, such as exendin-4 or liraglutide, reduces oxidative stress and urinary albumin excretion possibly via increasing GLP-1 receptor expression (22) or via protein kinase A–mediated inhibition of renal NADPH oxidase, independent of a glucose-lowering effect (17). Taken together, these preclinical findings raise the possibility of a renal class effect of DPP-4 inhibitors or even all incretin-based therapies. However, variances in experimental study designs, applied drug concentrations, and distinct pharmacological differences between agents, such as half-lives, tissue penetration, drug metabolism, and drug excretion, do not allow simple extrapolation of the results from animal studies into human clinical conditions and among compounds.
To date, the clinical renal evidence of incretin-based therapies in patients with type 2 diabetes is scarce. A recent randomized, double-blind, parallel-group study compared sitagliptin with the sulfonylurea glipizide over 54 weeks in patients with type 2 diabetes and moderate to severe renal impairment (23). Despite a significant improvement in HbA1c from baseline (by −0.8% [−8.7 mmol/mol]) at the end of the study, sitagliptin was associated with an increase in UACR from baseline by +18 and +6% after 24 and 54 weeks of treatment, respectively (23). In another study using an injectable GLP-1 analog, Ryuge et al. (24) found that liraglutide was not associated with any changes in renal function or albuminuria after 24 weeks of treatment in patients with diabetic nephropathy. However, these data must be interpreted with caution because both studies provide observational assessments of renal parameters and were not designed specifically to assess changes in albuminuria (23,24). Prospective, randomized, controlled clinical trials are now needed to assess the renal effects of incretin-based therapies in patients with type 2 diabetes. The recently initiated MARLINA (Efficacy, Safety & Modification of Albuminuria in Type 2 Diabetes Subjects with Renal Disease with LINAgliptin) trial (NCT01792518) is specifically designed to assess the albuminuria-lowering potential of linagliptin. Further evidence for the renal effects of linagliptin is expected to emerge from the CAROLINA (CARdiovascular Outcome Study of LINAgliptin Versus Glimepiride in Patients with Type 2 Diabetes) trial (NCT01243424). This study aims to evaluate the long-term effect of linagliptin on CV morbidity and mortality in direct comparison with the sulfonylurea glimepiride and will provide important insights into CV outcomes independent of any expected differences in glucose control (37). Furthermore, this trial also will allow investigators to compare progression of renal disease, including changes in albuminuria and/or eGFR, over time. Notably, markers of renal damage, such as renal impairment and/or prevalent albuminuria, are some of the predefined inclusion criteria in the CAROLINA trial. The CARMELINA (CArdiovascular Safety & Renal Microvascular OutcomE Study with LINAgliptin) trial (NCT01897532) was also recently initiated. This study will enroll more than 8,000 subjects with type 2 diabetes and renal dysfunction and aims to investigate the efficacy and safety of linagliptin versus placebo on both CV and renal microvascular outcomes.
Because of the retrospective and pooled nature of our analysis, some methodological limitations need to be considered. Although pooling data from studies allows investigators to test the primary hypothesis using an adequately sized population, our analysis relates to clinical trials that were not primarily designed to investigate the change in UACR. For this reason, results of this study should be interpreted primarily for generating a hypothesis. In addition, most patients included in the primary pooled analysis set were treated with multiple antihypertensive agents, and differences beyond stable RAAS inhibition in the background treatment may have confounded our results. However, antihypertensive therapies at screening were well balanced between the two treatment groups, and patients who required adjustments to doses of RAAS inhibitors were excluded from the primary pooled analysis set. Furthermore, changes in albuminuria did not occur concomitantly with any changes in blood pressure, indicating no relevant influence of the background antihypertensive treatment on the albuminuria-lowering effect of linagliptin. Another limitation is that UACR assessments were based on a single urine specimen; this may have reduced the precision of the results because urinary albumin excretion shows considerable intraindividual variability. However, urinary albumin was assessed at three independent time points (baseline and 12 and 24 weeks), and assays were performed in a central laboratory to minimize variability. Moreover, the results of this pooled analysis are restricted to the population of patients with type 2 diabetes and prevalent albuminuria in the range of UACR 30−3,000 mg/g Cr who were receiving stable doses of RAAS inhibitors. Therefore, these results cannot be extrapolated to all subjects with type 2 diabetes and kidney disease. Finally, although proteinuria/albuminuria is a predictor of increased CV risk in diabetes, it has been associated with only significant progression of decline in renal function and overall risk of ESRD in patients with type 2 diabetes at the overt proteinuric/albuminuric stage (38). Prevention of new-onset albuminuria, however, did not result in reduced risk for CKD in diabetes (39,40). Therefore, this analysis cannot provide conclusive evidence for improved long-term renal outcomes with linagliptin.
In summary, this pooled analysis found that linagliptin, administered in addition to stable ACEI or ARB therapy, led to a significant reduction in albuminuria after 24 weeks of treatment. Our data support a novel hypothesis that suggests an additive albuminuria-lowering effect with dual blockade of the RAAS and DPP-4 system. To substantiate these hypothesis-generating findings, further prospective clinical research has been initiated. Such efforts will address an important unmet medical need among patients with type 2 diabetes and kidney disease.
Supplementary Material
Acknowledgments
This study was funded by Boehringer Ingelheim, the manufacturer of linagliptin. Medical writing assistance during the preparation of the manuscript, provided by Audrey Koïtka-Weber, PhD, was supported by Boehringer Ingelheim.
P.-H.G. received speaker honorariums from Boehringer Ingelheim, Cebix, Eli Lilly, Genzyme, Merck Sharp & Dohme, Novartis, and Novo Nordisk; received research grants from Eli Lilly and Roche; and is an advisory member for Boehringer Ingelheim and Novartis. M.E.C. received speaker honorariums from Boehringer Ingelheim, Servier, and Eli Lilly and is an advisory member for Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Merck Sharp & Dohme, and Novo Nordisk. V.P. is supported by a Cardiovascular Research Network Fellowship from the Heart Foundation of Australia; received speaker honorariums from AstraZeneca, Merck, Roche, and Servier; serves on steering committees for trials supported by Abbott, Baxter, Boehringer Ingelheim, Janssen, and Pfizer; and is a consultant for Abbott, Astellas, Baxter, and Vitae Pharmaceuticals. A.E., H.-J.W., and M.v.E. are employed by Boehringer Ingelheim. No other potential conflicts of interest relevant to this article were reported.
P.-H.G., M.E.C., V.P., A.E., and H.-J.W. analyzed and interpreted data and critically revised the manuscript. M.v.E. conceived the design of the study, analyzed and interpreted data, and drafted the manuscript. All authors were responsible for editorial decisions about content, were involved at all stages of manuscript development, and approved the final version. P.-H.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented as a poster (no. 953-P) at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8−12 June 2012, and at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1−5 October 2012.
The authors thank the patients who took part in the clinical trials and the collaborators at each center.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0323/-/DC1.
References
- 1.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–266 [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–2047 [DOI] [PubMed] [Google Scholar]
- 3.de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004;110:921–927 [DOI] [PubMed] [Google Scholar]
- 4.Ninomiya T, Perkovic V, de Galan BE, et al. ADVANCE Collaborative Group Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG, DEMAND investigators Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006;69:2057–2063 [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Mann JF, Yi Q, et al. HOPE Study Investigators Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001;286:421–426 [DOI] [PubMed] [Google Scholar]
- 7.Matsushita K, van der Velde M, Astor BC, et al. Chronic Kidney Disease Prognosis Consortium Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association Standards of medical care in diabetes–2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 10.Lewis EJ, Hunsicker LG, Clarke WR, et al. Collaborative Study Group Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 11.de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004;65:2309–2320 [DOI] [PubMed] [Google Scholar]
- 12.Eijkelkamp WB, Zhang Z, Remuzzi G, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol 2007;18:1540–1546 [DOI] [PubMed] [Google Scholar]
- 13.Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 2005;16:3027–3037 [DOI] [PubMed] [Google Scholar]
- 14.Keane WF, Zhang Z, Lyle PA, et al. RENAAL Study Investigators Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: the RENAAL study. Clin J Am Soc Nephrol 2006;1:761–767 [DOI] [PubMed] [Google Scholar]
- 15.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 2006;116:288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alter ML, Ott IM, von Websky K, et al. DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy. Kidney Blood Press Res 2012;36:119–130 [DOI] [PubMed] [Google Scholar]
- 17.Hendarto H, Inoguchi T, Maeda Y, et al. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism 2012;61:1422–1434 [DOI] [PubMed] [Google Scholar]
- 18.Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP4 inhibitors—from preclinical development to clinical research. Kidney Blood Press Res 2012;36:65–84 [DOI] [PubMed] [Google Scholar]
- 19.Liu WJ, Xie SH, Liu YN, et al. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther 2012;340:248–255 [DOI] [PubMed] [Google Scholar]
- 20.Mega C, de Lemos ET, Vala H, et al. Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Exp Diabetes Res 2011;2011:162092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panchapakesan U, Mather A, Pollock C. Role of GLP-1 and DPP-4 in diabetic nephropathy and cardiovascular disease. Clin Sci (Lond) 2013;124:17–26 [DOI] [PubMed] [Google Scholar]
- 22.Park CW, Kim HW, Ko SH, et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol 2007;18:1227–1238 [DOI] [PubMed] [Google Scholar]
- 23.Arjona Ferreira JC, Marre M, Barzilai N, et al. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care 2013;36:1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryuge A, Minoru K, Yu K, et al. Examination of the effects of liraglutide on diabetic nephropathy. Kidney Res Clin Pract 2012;31:A70 [Google Scholar]
- 25.Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 2011;13:258–267 [DOI] [PubMed] [Google Scholar]
- 26.Haak T, Meinicke T, Jones R, Weber S, von Eynatten M, Woerle HJ. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 2012;14:565–574 [DOI] [PubMed] [Google Scholar]
- 27.Owens DR, Swallow R, Dugi KA, Woerle HJ. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med 2011;28:1352–1361 [DOI] [PubMed] [Google Scholar]
- 28.Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 2011;13:65–74 [DOI] [PubMed] [Google Scholar]
- 29.de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2010;376:1543–1551 [DOI] [PubMed] [Google Scholar]
- 30.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–117 [DOI] [PubMed] [Google Scholar]
- 31.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 32.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldt-Rasmussen B, Mathiesen ER, Hegedüs L, Deckert T. Kidney function during 12 months of strict metabolic control in insulin-dependent diabetic patients with incipient nephropathy. N Engl J Med 1986;314:665–670 [DOI] [PubMed] [Google Scholar]
- 34.Tuttle KR, Bruton JL, Perusek MC, Lancaster JL, Kopp DT, DeFronzo RA. Effect of strict glycemic control on renal hemodynamic response to amino acids and renal enlargement in insulin-dependent diabetes mellitus. N Engl J Med 1991;324:1626–1632 [DOI] [PubMed] [Google Scholar]
- 35.Ellis D, Avner ED, Kurs-Lasky M, Richards M, Becker DJ. Effects of improved glycemic control on microalbuminuria in adolescents with insulin-dependent diabetes mellitus. Int J Pediatr Nephrol 1986;7:31–38 [PubMed] [Google Scholar]
- 36.Sharkovska Y, Alter ML, Reichetzeder C, Tsuprykov O, Klein T, Hocher B. DPP-4 inhibition with linagliptin delays the progression of diabetic nephropathy in db/db mice. Diabetologia 2012;55:S20 [Google Scholar]
- 37.Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet 2012;380:475–483 [DOI] [PubMed] [Google Scholar]
- 38.Cerasola G, Cottone S, Mulè G. The progressive pathway of microalbuminuria: from early marker of renal damage to strong cardiovascular risk predictor. J Hypertens 2010;28:2357–2369 [DOI] [PubMed] [Google Scholar]
- 39.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009;361:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molitch ME, Steffes M, Sun W, et al. Epidemiology of Diabetes Interventions and Complications Study Group Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010;33:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.